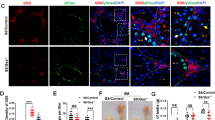
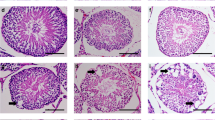
Abstract
Oxidative stress in spermatozoa is a major contributor to male subfertility, which makes it an informed choice to generate animal models of male subfertility with targeted modifications of the antioxidant systems. However, the critical male germ cell-specific antioxidant mechanisms have not been well defined yet. Here we identify LanCL1 as a major male germ cell-specific antioxidant gene, reduced expression of which is related to human male infertility. Mice deficient in LanCL1 display spermatozoal oxidative damage and impaired male fertility. Histopathological studies reveal that LanCL1-mediated antioxidant response is required for mouse testicular homeostasis, from the initiation of spermatogenesis to the maintenance of viability and functionality of male germ cells. Conversely, a mouse model expressing LanCL1 transgene is protected against high-fat-diet/obesity-induced oxidative damage and subfertility. We further show that germ cell-expressed LanCL1, in response to spermatogenic reactive oxygen species, is regulated by transcription factor specific protein 1 (SP1) during spermatogenesis. This study demonstrates a critical role for the SP1–LanCL1 axis in regulating testicular homeostasis and male fertility mediated by redox balance, and provides evidence that LanCL1 genetically modified mice have attractive applications as animal models of male subfertility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Agarwal, A., Mulgund, A., Hamada, A. & Chyatte, M. R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 13, 37 (2015).
Inoue, S. et al. TAp73 is required for spermatogenesis and the maintenance of male fertility. Proc. Natl Acad. Sci. USA 111, 1843–1848 (2014).
Bisht, S., Faiq, M., Tolahunase, M. & Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 14, 470 (2017).
Hermann, B. P. et al. The mammalian spermatogenesis single-cell transcriptome, from spermatogonial stem cells to spermatids. Cell Rep. 25, 1650–1667 (2018).
Rato, L. et al. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 9, 330–338 (2012).
Endo, T., Freinkman, E., de Rooij, D. G. & Page, D. C. Periodic production of retinoic acid by meiotic and somatic cells coordinates four transitions in mouse spermatogenesis. Proc. Natl Acad. Sci. USA 114, E10132–E10141 (2017).
Morimoto, H. et al. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell 12, 774–786 (2013).
Guerriero, G., Trocchia, S., Abdel-Gawad, F. K. & Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regulation. Front. Endocrinol. 5, 56 (2014).
Lenzi, A. et al. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 6, 226–231 (2000).
WS, O., Chen, H. & Chow, P. H. Male genital tract antioxidant enzymes–their ability to preserve sperm DNA integrity. Mol. Cell. Endocrinol. 250, 80–83 (2006).
Myatt, S. S., Brosens, J. J. & Lam, E. W.-F. Sense and sensitivity: FOXO and ROS in cancer development and treatment. Antioxid. Redox Signal. 14, 675–687 (2011).
Dalton, T. P., Shertzer, H. G. & Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 39, 67–101 (1999).
Jaiswal, A. K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 36, 1199–1207 (2004).
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S. & Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 5, 9–19 (2012).
Gong, S., San Gabriel, M. C., Zini, A., Chan, P. & O’Flaherty, C. Low amounts and high thiol oxidation of peroxiredoxins in spermatozoa from infertile men. J. Androl. 33, 1342–1351 (2012).
Wu, W. et al. GSTM1 and GSTT1 null polymorphisms and male infertility risk: an updated meta-analysis encompassing 6934 subjects. Sci. Rep. 3, 2258 (2013).
Naghavi, A., Fazeli, F., Salimi, S. & Nemati, B. M. Glutathione-S-transferase P1 Ile105Val polymorphism and idiopathic male infertility. Eur. Urol. Suppl. 12, e1133, S1125 (2013).
Faure, C. et al. Are superoxide dismutase 2 and nitric oxide synthase polymorphisms associated with idiopathic infertility? Antioxid. Redox Signal. 21, 565–569 (2014).
Ji, G. et al. Genetic variants in antioxidant genes are associated with sperm DNA damage and risk of male infertility in a Chinese population. Free Radic. Biol. Med. 52, 775–780 (2012).
Ozkosem, B., Feinstein, S. I., Fisher, A. B. & O’Flaherty, C. Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice. Redox Biol. 5, 15–23 (2015).
Ishii, T. et al. Accelerated impairment of spermatogenic cells in SOD1-knockout mice under heat stress. Free Radic. Res. 39, 697–705 (2005).
Smith, T. B., Baker, M. A., Connaughton, H. S., Habenicht, U. & Aitken, R. J. Functional deletion of Txndc2 and Txndc3 increases the susceptibility of spermatozoa to age-related oxidative stress. Free Radic. Biol. Med. 65, 872–881 (2013).
Iuchi, Y. et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem. J. 419, 149–158 (2009).
Tsunoda, S., Kawano, N., Miyado, K., Kimura, N. & Fujii, J. Impaired fertilizing ability of superoxide dismutase 1-deficient mouse sperm during in vitro fertilization. Biol. Reprod. 87, 121 (2012).
Ho, Y. S. et al. Reduced fertility in female mice lacking copper–zinc superoxide dismutase. J. Biol. Chem. 273, 7765–7769 (1998).
Carlsson, L. M., Jonsson, J., Edlund, T. & Marklund, S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl Acad. Sci USA. 92, 6264–6268 (1995).
Ho, Y. S. et al. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 272, 16644–16651 (1997).
Neumann, C. A. et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424, 561 (2003).
Argyropoulou, V. et al. Peroxiredoxin-5 as a novel actor in inflammation and tumor suppression. Free Radical Biol. Med. 100, S92 (2016).
Henderson, C. J. et al. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc. Natl Acad. Sci USA. 95, 5275–5280 (1998).
Engle, M. R. et al. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 194, 296–308 (2004).
Lim, C. E. et al. Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases. Am. J. Pathol. 165, 679–693 (2004).
Huang, C. et al. Developmental and activity-dependent expression of LanCL1 confers antioxidant activity required for neuronal survival. Dev. Cell 30, 479–487 (2014).
Mayer, H., Bauer, H., Breuss, J., Ziegler, S. & Prohaska, R. Characterization of rat LANCL1, a novel member of the lanthionine synthetase C-like protein family, highly expressed in testis and brain. Gene 269, 73–80 (2001).
Chung, C. H. et al. Identification of lanthionine synthase C-like protein-1 as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochemistry 46, 3262–3269 (2007).
Tan, H. et al. LanCL1 promotes motor neuron survival and extends the lifespan of amyotrophic lateral sclerosis mice. Cell Death Differ. https://doi.org/10.1038/s41418-019-0422-6 (2019).
Nielsen, J. E. et al. Germ cell differentiation-dependent and stage-specific expression of LANCL1 in rodent testis. Eur. J. Histochem. 47, 215–222 (2003).
Hanley, J. A. & McNeil, B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36 (1982).
Ernst, C., Eling, N., Martinez-Jimenez, C. P., Marioni, J. C. & Odom, D. T. Staged developmental mapping and X chromosome transcriptional dynamics during mouse spermatogenesis. Nat. Commun. 10, 1251 (2019).
Griswold, M. D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 96, 1–17 (2016).
Conrad, M., Ingold, I., Buday, K., Kobayashi, S. & Angeli, J. P. ROS, thiols and thiol-regulating systems in male gametogenesis. Biochim. Biophys. Acta 1850, 1566–1574 (2015).
World-Health-Organization. WHO laboratory manual for the examination and processing of human semen. (2010).
Gallardo, T., Shirley, L., John, G. B. & Castrillon, D. H. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417 (2007).
Morimoto, H. et al. ROS amplification drives mouse spermatogonial stem cell self-renewal. Life Sci. Alliance 2, https://doi.org/10.26508/lsa.201900374 (2019)
Morimoto, H., Kanatsu-Shinohara, M. & Shinohara, T. ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells. Biol. Reprod. 92, 147 (2015).
Takashima, S. et al. Rac mediates mouse spermatogonial stem cell homing to germline niches by regulating transmigration through the blood–testis barrier. Cell Stem Cell 9, 463–475 (2011).
Manova, K., Nocka, K., Besmer, P. & Bachvarova, R. F. Gonadal expression of c-kit encoded at the W locus of the mouse. Development 110, 1057–1069 (1990).
Kanatsu-Shinohara, M., Toyokuni, S. & Shinohara, T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol. Reprod. 70, 70–75 (2004).
Johnsen, S. G. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1, 2–25 (1970).
Osuru, H. P. et al. The acrosomal protein SP-10 (Acrv1) is an ideal marker for staging of the cycle of seminiferous epithelium in the mouse. Mol. Reprod. Dev. 81, 896–907 (2014).
Du Plessis, S. S., Cabler, S., McAlister, D. A., Sabanegh, E. & Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 7, 153 (2010).
Khan, A. et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46, D260–D266 (2018).
Farré, D. et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 31, 3651–3653 (2003).
Saffer, J. D., Jackson, S. P. & Annarella, M. B. Developmental expression of Sp1 in the mouse. Mol. Cell. Biol. 11, 2189–2199 (1991).
Ryu, H. et al. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J. Neurosci. 23, 3597–3606 (2003).
Thomas, K. et al. Identification, characterization, and functional analysis of sp1 transcript variants expressed in germ cells during mouse spermatogenesis. Biol. Reprod. 72, 898–907 (2005).
Sleiman, S. F., Langley, B. C., Basso, M. & Berlin, J. Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J. Neurosci. 31, 6858–6870 (2011).
Nakamura, B. N. et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med. 49, 1368–1379 (2010).
Fujimoto, K. et al. Generation and functional characterization of mice with a disrupted glutathione S-transferase, theta 1 gene. Drug Metab. Dispos. 35, 2196–2202 (2007).
Fujimoto, K. et al. Characterization of phenotypes in Gstm1-null mice by cytosolic and in vivo metabolic studies using 1,2-dichloro-4-nitrobenzene. Drug Metab. Dispos. 34, 1495–1501 (2006).
Ilic, Z., Crawford, D., Vakharia, D., Egner, P. A. & Sell, S. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1. Toxicol. Appl. Pharmacol. 242, 241–246 (2010).
Imai, H. et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 284, 32522–32532 (2009).
Ursini, F. et al. Dual function of the selenoprotein PHGPx during sperm maturation. Science 285, 1393–1396 (1999).
Deshpande, S. S., Nemani, H., Pothani, S. & Balasinor, N. H. Altered endocrine, cytokine signaling and oxidative stress: a plausible reason for differential changes in testicular cells in diet-induced and genetically-inherited - obesity in adult rats. Reprod. Biol. 19, 303–308 (2019).
Erdemir, F. et al. The effect of diet induced obesity on testicular tissue and serum oxidative stress parameters. Actas Urol. Esp. 36, 153–159 (2012).
Zhao, J., Zhai, L., Liu, Z., Wu, S. & Xu, L. Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue. Oxid. Med. Cell Longev. 2014, 190945 (2014).
Atilgan, D. et al. Weight loss and melatonin reduce obesity-induced oxidative damage in rat testis. Adv. Urol. 2013, 836121 (2013).
Marin, M., Karis, A., Visser, P., Grosveld, F. & Philipsen, S. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89, 619–628 (1997).
Hensley, K., Olcott, M., Downey, A., Spector, D. & Munsell, A. CRISPR–Cas9 knockout of lanthionine synthase-like protein-1 (LanCL1) in HeLa cells renders the cells hypersensitive to oxidative stress despite inducing an upregulation of glutathione-dependent antioxidant defense mechanisms. Free Radic. Biol. Med. 128, S131 (2018).
Wang, J. et al. LanCL1 protects prostate cancer cells from oxidative stress via suppression of JNK pathway. Cell Death Dis. 9, 1–12 (2018).
Xie, Z. et al. LanCL1 attenuates ischemia-induced oxidative stress by Sirt3-mediated preservation of mitochondrial function. Brain Res. Bull. 142, 216–223 (2018).
Downey, A. et al. Stable knockout of lanthionine synthase C-like protein-1 (LanCL1) from HeLa cells indicates a role for LanCL1 in redox regulation of deubiquitinating enzymes. Free Radic. Biol. Med. 161, 115–124 (2020).
Lai, K.-Y. et al. LanCLs add glutathione to dehydroamino acids generated at phosphorylated sites in the proteome. Cell 184, 2680–2695 (2021).
Ravina, C. G. et al. A role for tachykinins in the regulation of human sperm motility. Hum. Reprod. 22, 1617–1625 (2007).
Preece, C. et al. Replacement of surgical vasectomy through the use of wild-type sterile hybrids. Lab Anim. 50, 49–52 (2021).
Rio, D. C., Ares, M., Hannon, G. J. & Nilsen, T. W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protoc. 2010, pdb. prot5439 (2010).
Liu, Z. Q., Mahmood, T. & Yang, P. C. Western blot: technique, theory and trouble shooting. N. Am. J. Med. Sci. 6, 160 (2014).
Gallo-Oller, G., Ordonez, R. & Dotor, J. A new background subtraction method for Western blot densitometry band quantification through image analysis software. J. Immunol. Methods 457, 1–5 (2018).
Biggers, J., Whitten, W. & Whittingham, D. in Methods in Mammalian Embryology (ed. Daniel, J. C. Jr) 86–116 (WH Freeman Co., 1971).
Chang, Y.-F., Lee-Chang, J. S., Panneerdoss, S., MacLean, J. A. & Rao, M. K. Isolation of Sertoli, Leydig, and spermatogenic cells from the mouse testis. Biotechniques 51, 341–342 (2011).
Sherf, B. A., Navarro, S. L., Hannah, R. R. & Wood, K. V. Dual-luciferase reporter assay: an advanced co-reporter technology integrating firefly and Renilla luciferase assays. Promega Notes 57, 2–8 (1996).
Zaffagnini, M. et al. Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: a proteomic survey. Mol. Cell Proteomics 11, M111 014142 (2012).
Butturini, E., Boriero, D., Carcereri de Prati, A. & Mariotto, S. Immunoprecipitation methods to identify S-glutathionylation in target proteins. MethodsX 6, 1992–1998 (2019).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31501200, 31871179 and 32071161 to C.H.), Key Research Project from Sichuan Provincial Department of Education, China (16ZA0029 to C.H.), Disciplines and Talents Support Program of Sichuan Agricultural University (2015–2019 to C.H.) and in part by the National Key Technology Support Program of China (2014BAI03B01 to Z.C). We thank P. Zhong for helping with illustration drawing.
Author information
Authors and Affiliations
Contributions
C.H., Z.C. and B.X. designed the experiments and interpreted the data; C.Y. performed most of the staining experiments with the help of H.G.; D.P. did most of the in vitro studies and biochemistry assays with the help of C.L.; Y.C. and M.C. generated the LanCL1 KO and transgenic mice, while X. Cao initiated this project, generated the conditional LanCL1 KO/KI mice and performed studies with human samples; X.H. collected the seminal samples; X. Chen generated the LanCL1 antibody; B.M., W.L. and Q.L. executed the other experiments; C.H. and Z.C. supervised the project; C.H. and B.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Lab Animal thanks Elizabeth Bromfield and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Table 1.
Rights and permissions
About this article
Cite this article
Huang, C., Yang, C., Pang, D. et al. Animal models of male subfertility targeted on LanCL1-regulated spermatogenic redox homeostasis. Lab Anim 51, 133–145 (2022). https://doi.org/10.1038/s41684-022-00961-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-022-00961-w