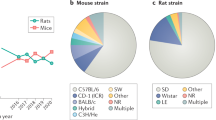
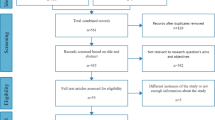
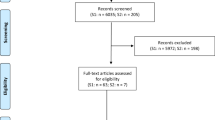
Abstract
Translating basic pain research from rodents to humans has proven to be a challenging task. Efforts have been made to develop preclinical large animal models of pain, such as the pig. However, no consistent overview and comparison of pig models of pain are currently available. Therefore, in this review, our primary aim was to identify the available pig models in pain research and compare these models in terms of intensity and duration. First, we systematically searched Proquest, Scopus and Web of Science and compared the duration for which the pigs were significantly sensitized as well as the intensity of mechanical sensitization. We searched models within the specific field of pain and adjacent fields in which pain induction or assessment is relevant, such as pig production. Second, we compared assessment methodologies in surrogate pain models in humans and pigs to identify areas of overlap and possible improvement. Based on the literature search, 23 types of porcine pain models were identified; 13 of which could be compared quantitatively. The induced sensitization lasted from hours to months and intensities ranged from insignificant to the maximum attainable. We also found a near to complete overlap of assessment methodologies between human and pig models within the area of peripheral neurophysiology, which allows for direct comparison of results obtained in the two species. In spite of this overlap, further development of pain assessment methodologies is still needed. We suggest that central nervous system electrophysiology, such as electroencephalography, electrocorticography or intracortical recordings, may pave the way for future objective pain assessment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Breivik, H., Eisenberg, E. & O’Brien, T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 13, 1229 (2013).
Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research (National Academies Press, 2011).
Reid, K. J. et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr. Med. Res. Opin. 27, 449–462 (2011).
Hill, R. NK1 (substance P) receptor antagonists—why are they not analgesic in humans? Trends Pharmacol. Sci. 21, 244–246 (2000).
Mogil, J. S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294 (2009).
Burma, N. E., Leduc-Pessah, H., Fan, C. Y. & Trang, T. Animal models of chronic pain: advances and challenges for clinical translation. J. Neurosci. Res. 95, 1242–1256 (2017).
Percie du Sert, N. & Rice, A. S. C. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br. J. Pharmacol. 171, 2951–2963 (2014).
Taxonomy Working Group. Classification of Chronic Pain (IASP Press, 2011).
Melzack, R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1, 277–299 (1975).
Rose, J. D. & Woodbury, C. J. Animal models of nociception and pain. in Sourcebook of Models for Biomedical Research (ed. Conn, P. M.) 333–340 (Humana Press, 2008).
Haroutounian, S. et al. How central is central poststroke pain? The role of afferent input in poststroke neuropathic pain: a prospective, open-label pilot study. Pain 159, 1317–1324 (2018).
Le Bars, D., Dickenson, A. H. & Besson, J. M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6, 283–304 (1979).
Woolf, C. J. Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688 (1983).
Liu, X.-G. & Sandkühler, J. Characterization of long-term potentiation of C-fiber–evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J. Neurophysiol. 78, 1973–1982 (1997).
Sandkühler, J. & Liu, X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury: LTP in spinal cord induced by noxious stimulation. Eur. J. Neurosci. 10, 2476–2480 (1998).
Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 89, 707–758 (2009).
Campbell, J. N. & Meyer, R. A. Mechanisms of neuropathic pain. Neuron 52, 77–92 (2006).
Decosterd, I. & Woolf, C. J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Jaggi, A. S., Jain, V. & Singh, N. Animal models of neuropathic pain. Fundam. Clin. Pharmacol. 25, 1–28 (2011).
Casals-Díaz, L., Vivó, M. & Navarro, X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp. Neurol. 217, 84–95 (2009).
Shields, S. D. et al. Insensitivity to pain upon adult-onset deletion of Nav1.7 or its blockade with selective inhibitors. J. Neurosci. 38, 10180–10201 (2018).
Tappe-Theodor, A., King, T. & Morgan, M. M. Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci. Biobehav. Rev. 100, 335–343 (2019).
Dostrovsky, J. & Craig, A. Ascending projection systems. in Wall & Melzack’s Textbook of Pain 182–197 (Elsevier Health Sciences, 2013).
McIntyre, P. et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br. J. Pharmacol. 132, 1084–1094 (2001).
Whiteside, G. T., Adedoyin, A. & Leventhal, L. Predictive validity of animal pain models? A comparison of the pharmacokinetic–pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54, 767–775 (2008).
Henze, D. A. & Urban, M. O. Large animal models for pain therapeutic development. in Translational Pain Research: From Mouse to Man (CRC Press/Taylor & Francis, 2010).
Cobianchi, L. et al. Pain assessment in animal models: do we need further studies? J. Pain Res. https://doi.org/10.2147/JPR.S59161 (2014).
Sauleau, P., Lapouble, E., Val-Laillet, D. & Malbert, C.-H. The pig model in brain imaging and neurosurgery. Animal 3, 1138–1151 (2009).
Schmidt, V. Comparative anatomy of the pig brain—an integrative magnetic resonance imaging (MRI) study of the porcine brain with special emphasis on the external morphology of the cerebral cortex (VVB Laufersweiler, 2015).
Schomberg, D. T. et al. Translational relevance of swine models of spinal cord injury. J. Neurotrauma 34, 541–551 (2017).
Lynn, B., Faulstroh, K. & Pierau, F.-K. The classification and oroperties of nociceptive afferent units from the skin of the anaesthetized pig. Eur. J. Neurosci. 7, 431–437 (1995).
Ohta, T., Komatsu, R., Imagawa, T., Otsuguro, K. & Ito, S. Molecular cloning, functional characterization of the porcine transient receptor potential V1 (pTRPV1) and pharmacological comparison with endogenous pTRPV1. Biochem. Pharmacol. 71, 173–187 (2005).
Dusch, M. et al. Comparison of electrically induced flare response patterns in human and pig skin. Inflamm. Res. 58, 639–648 (2009).
Castel, D., Sabbag, I., Brenner, O. & Meilin, S. Peripheral neuritis trauma in pigs: a neuropathic pain model. J. Pain 17, 36–49 (2016).
Ottoboni, T. et al. Mechanism of action of HTX-011: a novel, extended-release, dual-acting local anesthetic formulation for postoperative pain. Reg. Anesth. Pain Med. 45, 117–123 (2020).
Viscusi, E. et al. HTX-011 reduced pain intensity and opioid consumption versus bupivacaine HCl in herniorrhaphy: results from the phase 3 EPOCH 2 study. Hernia 23, 1071–1080 (2019).
Sutherland, M. Welfare implications of invasive piglet husbandry procedures, methods of alleviation and alternatives: a review. N. Z. Vet. J. 63, 52–57 (2015).
Dzikamunhenga, R. S. et al. Pain management in the neonatal piglet during routine management procedures. Part 1: a systematic review of randomized and non-randomized intervention studies. Anim. Health Res. Rev. 15, 14–38 (2014).
O’Connor, A. et al. Pain management in the neonatal piglet during routine management procedures. Part 2: grading the quality of evidence and the strength of recommendations. Anim. Health Res. Rev. 15, 39–62 (2014).
O’Connor, A. et al. Review: Assessment of completeness of reporting in intervention studies using livestock: an example from pain mitigation interventions in neonatal piglets. Animal 10, 660–670 (2016).
Ison, S. H., Clutton, R. E., Di Giminiani, P. & Rutherford, K. M. D. A review of pain assessment in pigs. Front. Vet. Sci. 3, 108 (2016).
Herskin, M. S. & Di Giminiani, P. Pain in pigs. in Advances in Pig Welfare 325–355 (Elsevier, 2018). https://doi.org/10.1016/B978-0-08-101012-9.00011-3
Noonan, G. J., Rand, J. S., Priest, J., Ainscow, J. & Blackshaw, J. K. Behavioural observations of piglets undergoing tail docking, teeth clipping and ear notching. Appl. Anim. Behav. Sci. 39, 203–213 (1994).
Swindle, M. M., Makin, A., Herron, A. J., Clubb, F. J. & Frazier, K. S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49, 344–356 (2012).
Reyes, L. Observer-blinded comparison of two nonopioid analgesics for postoperative pain in piglets. Pharmacol. Biochem. Behav. 73, 521–528 (2002).
Harvey-Clark, C. J., Gilespie, K. & Riggs, K. W. Transdermal fentanyl compared with parenteral buprenorphine in post-surgical pain in swine: a case study. Lab. Anim. 34, 386–398 (2000).
Malavasi, L. M., Nyman, G., Augustsson, H., Jacobson, M. & Jensen-Waern, M. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab. Anim. 40, 16–27 (2006).
Lykkegaard, K., Lykkesfeldt, J., Lauritzen, B. & Svendsen, O. Morphine reduces spinal c-fos expression dose-dependently during experimental laparotomy in pigs: A combined pharmacokinetic and surgical study. Res. Vet. Sci. 84, 457–464 (2008).
Lykkegaard, K., Lauritzen, B., Tessem, L., Weikop, P. & Svendsen, O. Local anaesthetics attenuates spinal nociception and HPA-axis activation during experimental laparotomy in pigs. Res. Vet. Sci. 79, 245–251 (2005).
Castel, D., Willentz, E., Doron, O., Brenner, O. & Meilin, S. Characterization of a porcine model of post-operative pain: A pig model of incisional pain. Eur. J. Pain 18, 496–505 (2014).
Castel, D., Sabbag, I. & Meilin, S. The effect of local/topical analgesics on incisional pain in a pig model. J. Pain Res. 10, 2169–2175 (2017).
Wilsey, J. T. & Block, J. Sustained analgesic effect of clonidine co-polymer depot in a porcine incisional pain model. J. Pain Res. 11, 693–701 (2018).
Obreja, O. et al. NGF enhances electrically induced pain, but not axon reflex sweating. Pain 152, 1856–1863 (2011).
Hirth, M. et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain 154, 2500–2511 (2013).
Rukwied, R. et al. Nerve growth factor-evoked nociceptor sensitization in pig skin in vivo. J. Neurosci. Res. https://doi.org/10.1002/jnr.22351 (2010).
Petersson, M. E. et al. Differential axonal conduction patterns of mechano-sensitive and mechano-insensitive nociceptors—a combined experimental and modelling study. PLoS One 9, e103556 (2014).
Di Giminiani, P., Petersen, L. J. & Herskin, M. S. Characterization of nociceptive behavioural responses in the awake pig following UV-B-induced inflammation: UV-B induced hyperalgesia in the pig. Eur. J. Pain 18, 20–28 (2014).
Rukwied, R., Dusch, M., Schley, M., Forsch, E. & Schmelz, M. Nociceptor sensitization to mechanical and thermal stimuli in pig skin in vivo. Eur. J. Pain 12, 242–250 (2008).
Sandercock, D. A. et al. Development of a mechanical stimulator and force measurement system for the assessment of nociceptive thresholds in pigs. J. Neurosci. Methods 182, 64–70 (2009).
Vergara, D. M. et al. Establishment of a novel porcine model to study the impact of active stretching on a local carrageenan-induced inflammation. Am. J. Phys. Med. Rehabil. 99, 1012–1019 (2020).
Di Giminiani, P., Petersen, L. J. & Herskin, M. S. Capsaicin-induced neurogenic inflammation in pig skin: A behavioural study. Res. Vet. Sci. 96, 447–453 (2014).
Rice, F. L. et al. Human-like cutaneous neuropathologies associated with a porcine model of peripheral neuritis: A translational platform for neuropathic pain. Neurobiol. Pain 5, 100021 (2019).
Castel, D., Sabbag, I., Nasaev, E., Peng, S. & Meilin, S. Open field and a behavior score in PNT model for neuropathic pain in pigs. J. Pain Res. 11, 2279–2293 (2018).
Goel, S. A., Varghese, V. & Demir, T. Animal models of spinal injury for studying back pain and SCI. J. Clin. Orthop. Trauma 11, 816–821 (2020).
Bellampalli, S. S. & Khanna, R. Towards a neurobiological understanding of pain in neurofibromatosis type 1: mechanisms and implications for treatment. Pain 160, 1007–1018 (2019).
Pairis-Garcia, M. et al. Measuring the efficacy of flunixin meglumine and meloxicam for lame sows using nociceptive threshold tests. Anim. Welf. 23, 219–229 (2014).
Fosse, T. K. et al. Ketoprofen in piglets: enantioselective pharmacokinetics, pharmacodynamics and PK/PD modelling: Pharmacokinetics and pharmacodynamics of ketoprofen in piglets. J. Vet. Pharmacol. Ther. 34, 338–349 (2011).
Mohling, C. M. et al. Evaluation of mechanical and thermal nociception as objective tools to measure painful and nonpainful lameness phases in multiparous sows. J. Anim. Sci. 92, 3073–3081 (2014).
Tapper, K. R. et al. Pressure algometry and thermal sensitivity for assessing pain sensitivity and effects of flunixin meglumine and sodium salicylate in a transient lameness model in sows. Livest. Sci. 157, 245–253 (2013).
Pairis-Garcia, M. et al. Behavioural evaluation of analgesic efficacy for pain mitigation in lame sows. Anim. Welf. 24, 93–99 (2015).
Unger, M. D. et al. Clinical magnetic resonance-enabled characterization of mono-iodoacetate-induced osteoarthritis in a large animal species. PLoS One 13, e0201673 (2018).
LaVallee, K. T. et al. Quantitation of gait and stance alterations due to monosodium -idoacetate–induced knee osteoarthritis in Yucatan swine. Comp. Med. 70, 248–257 (2020).
Khanna, R. et al. Assessment of nociception and related quality-of-life measures in a porcine model of neurofibromatosis type 1. Pain 160, 2473–2486 (2019).
Royal, J. M. et al. Assessment of postoperative analgesia after application of ultrasound-guided regional anesthesia for surgery in a swine femoral fracture model. J. Am. Assoc. Lab. Anim. Sci. 52, 12 (2013).
Sutherland, M. A., Davis, B. L., Brooks, T. A. & McGlone, J. J. Physiology and behavior of pigs before and after castration: effects of two topical anesthetics. Animal 4, 2071–2079 (2010).
Taylor, A. A., Weary, D. M., Lessard, M. & Braithwaite, L. Behavioural responses of piglets to castration: the effect of piglet age. Appl. Anim. Behav. Sci. 73, 35–43 (2001).
Weary, D. M., Braithwaite, L. A. & Fraser, D. Vocal response to pain in piglets. Appl. Anim. Behav. Sci. 56, 161–172 (1998).
Leidig, M. S., Hertrampf, B., Failing, K., Schumann, A. & Reiner, G. Pain and discomfort in male piglets during surgical castration with and without local anaesthesia as determined by vocalisation and defence behaviour. Appl. Anim. Behav. Sci. 116, 174–178 (2009).
Kluivers-Poodt, M. et al. Effects of a local anaesthetic and NSAID in castration of piglets, on the acute pain responses, growth and mortality. Animal 6, 1469–1475 (2012).
Taylor, A. A. & Weary, D. M. Vocal responses of piglets to castration: identifying procedural sources of pain. Appl. Anim. Behav. Sci. 70, 17–26 (2000).
Marx, G., Horn, T., Thielebein, J., Knubel, B. & von Borell, E. Analysis of pain-related vocalization in young pigs. J. Sound Vib. 266, 687–698 (2003).
Hansson, M., Lundeheim, N., Nyman, G. & Johansson, G. Effect of local anaesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet. Scand. 53, 34 (2011).
Llamas Moya, S., Boyle, L. A., Lynch, P. B. & Arkins, S. Effect of surgical castration on the behavioural and acute phase responses of 5-day-old piglets. Appl. Anim. Behav. Sci. 111, 133–145 (2008).
Sutherland, M. A., Davis, B. L., Brooks, T. A. & Coetzee, J. F. The physiological and behavioral response of pigs castrated with and without anesthesia or analgesia. J. Anim. Sci. 90, 2211–2221 (2012).
Lonardi, C., Scollo, A., Normando, S., Brscic, M. & Gottardo, F. Can novel methods be useful for pain assessment of castrated piglets? Animal 9, 871–877 (2015).
Keita, A., Pagot, E., Prunier, A. & Guidarini, C. Pre–emptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet. Anaesth. Analg. 37, 367–374 (2010).
Hay, M., Vulin, A., Génin, S., Sales, P. & Prunier, A. Assessment of pain induced by castration in piglets: behavioral and physiological responses over the subsequent 5 days. Appl. Anim. Behav. Sci. 82, 201–218 (2003).
Van Beirendonck, S., Driessen, B., Verbeke, G. & Geers, R. Behavior of piglets after castration with or without carbon dioxide anesthesia. J. Anim. Sci. 89, 3310–3317 (2011).
Di Giminiani, P. et al. The assessment of facial expressions in piglets undergoing tail docking and castration: toward the development of the piglet grimace scale. Front. Vet. Sci. 3, 100 (2016).
Luna, S. P. L. et al. Validation of the UNESP-Botucatu pig composite acute pain scale (UPAPS). PLoS One 15, e0233552 (2020).
Tenbergen, R., Friendship, R. & Haley, D. Investigation of the use of meloxicam for reducing pain associated with castration and tail docking and improving performance in piglets. J. Swine Health Prod. 22, 7 (2014).
Bates, J. L. et al. Impact of transmammary-delivered meloxicam on biomarkers of pain and distress in piglets after castration and tail docking. PLoS One 9, e113678 (2014).
Torrey, S., Devillers, N., Lessard, M., Farmer, C. & Widowski, T. Effect of age on the behavioral and physiological responses of piglets to tail docking and ear notching1. J. Anim. Sci. 87, 1778–1786 (2009).
Sutherland, M. A., Davis, B. L. & McGlone, J. J. The effect of local or general anesthesia on the physiology and behavior of tail docked pigs. Animal 5, 1237–1246 (2011).
Herskin, M. S., Di Giminiani, P. & Thodberg, K. Effects of administration of a local anaesthetic and/or an NSAID and of docking length on the behaviour of piglets during 5 h after tail docking. Res. Vet. Sci. 108, 60–67 (2016).
Sutherland, M. A., Bryer, P. J., Krebs, N. & McGlone, J. J. Tail docking in pigs: acute physiological and behavioural responses. Animal 2, 292–297 (2008).
Di Giminiani, P. et al. Characterization of short- and long-term mechanical sensitisation following surgical tail amputation in pigs. Sci. Rep. 7, 4827 (2017).
Sandercock, D. A. et al. Transcriptomics analysis of porcine caudal dorsal root ganglia in tail amputated pigs shows long-term effects on many pain-associated genes. Front. Vet. Sci. 6, 314 (2019).
Leslie, E., Hernández-Jover, M., Newman, R. & Holyoake, P. Assessment of acute pain experienced by piglets from ear tagging, ear notching and intraperitoneal injectable transponders. Appl. Anim. Behav. Sci. 127, 86–95 (2010).
Ison, S. H., Jarvis, S., Hall, S. A., Ashworth, C. J. & Rutherford, K. M. D. Periparturient behavior and physiology: further insight into the farrowing process for primiparous and multiparous sows. Front. Vet. Sci. 5, 122 (2018).
Ison, S. H., Jarvis, S. & Rutherford, K. M. D. The identification of potential behavioural indicators of pain in periparturient sows. Res. Vet. Sci. 109, 114–120 (2016).
Viitasaari, E. et al. The effect of ketoprofen on post-partum behaviour in sows. Appl. Anim. Behav. Sci. 158, 16–22 (2014).
Navarro, E., Mainau, E. & Manteca, X. Development of a facial expression scale using farrowing as a model of pain in sows. Animals 10, 2113 (2020).
Nalon, E. et al. Mechanical nociception thresholds in lame sows: Evidence of hyperalgesia as measured by two different methods. Vet. J. 198, 386–390 (2013).
Meijer, E., van Nes, A., Back, W. & van der Staay, F. J. Clinical effects of buprenorphine on open field behaviour and gait symmetry in healthy and lame weaned piglets. Vet. J. 206, 298–303 (2015).
Larsen, T., Kaiser, M. & Herskin, M. S. Does the presence of shoulder ulcers affect the behaviour of sows? Res. Vet. Sci. 98, 19–24 (2015).
Schouenborg, J. & Kalliomiki, J. Functional organization of the nociceptive withdrawal reflexes. Exp. Brain Res. 83, 67–78 (1990).
Obreja, O. et al. Nerve growth factor locally sensitizes nociceptors in human skin. Pain 159, 416–426 (2018).
Rukwied, R., Weinkauf, B., Main, M., Obreja, O. & Schmelz, M. Axonal hyperexcitability after combined NGF sensitization and UV-B inflammation in humans: Axonal hyperexcitability after combined NGF and UV-B. Eur. J. Pain 18, 785–793 (2014).
Lo Vecchio, S. et al. Interaction between ultraviolet B-induced cutaneous hyperalgesia and nerve growth factor-induced muscle hyperalgesia. Eur. J. Pain 20, 1058–1069 (2016).
Sørensen, L. B., Gazerani, P. & Graven‐Nielsen, T. Nerve growth factor‐induced muscle hyperalgesia facilitates ischaemic contraction‐evoked pain. Eur. J. Pain 23, 1814–1825 (2019).
Schabrun, S. M., Christensen, S. W., Mrachacz-Kersting, N. & Graven-Nielsen, T. Motor cortex reorganization and impaired function in the transition to sustained muscle pain. Cereb. Cortex 26, 1878–1890 (2016).
Enax-Krumova, E. K., Pohl, S., Westermann, A. & Maier, C. Ipsilateral and contralateral sensory changes in healthy subjects after experimentally induced concomitant sensitization and hypoesthesia. BMC Neurol. 17, (2017).
Costa, Y. M. et al. Masseter corticomotor excitability is decreased after intramuscular administration of nerve growth factor. Eur. J. Pain 23, 1619–1630 (2019).
De Martino, E., Zandalasini, M., Schabrun, S., Petrini, L. & Graven-Nielsen, T. Experimental muscle hyperalgesia modulates sensorimotor cortical excitability, which is partially altered by unaccustomed exercise. Pain 159, 2493–2502 (2018).
Sørensen, L. B., Boudreau, S. A., Gazerani, P. & Graven-Nielsen, T. Enlarged areas of pain and pressure hypersensitivity by spatially distributed intramuscular injections of low-dose nerve growth factor. J. Pain 20, 566–576 (2019).
Exposto, F., Masuda, M., Castrillon, E. & Svensson, P. Effects of nerve growth factor experimentally-induced craniofacial muscle sensitization on referred pain frequency and number of headache days: A double-blind, randomized placebo-controlled study. Cephalalgia 38, 2006–2016 (2018).
Munkholm, T. K. & Arendt-Nielsen, L. The interaction between NGF-induced hyperalgesia and acid-provoked pain in the infrapatellar fat pad and tibialis anterior muscle of healthy volunteers. Eur. J. Pain 21, 474–485 (2017).
Liu, M., Max, M. B., Robinovitz, E., Gracely, R. H. & Bennett, G. J. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction. J Pain Symptom Manage. 16, 10–20 (1998).
Furman, A. J. et al. Cerebral peak alpha frequency reflects average pain severity in a human model of sustained, musculoskeletal pain. J. Neurophysiol. 122, 1784–1793 (2019).
McLennan, K. M. et al. Conceptual and methodological issues relating to pain assessment in mammals: The development and utilisation of pain facial expression scales. Appl. Anim. Behav. Sci. 217, 1–15 (2019).
Haga, H. A. & Ranheim, B. Castration of piglets: the analgesic effects of intratesticular and intrafunicular lidocaine injection. Vet. Anaesth. Analg. 32, 1–9 (2005).
Karlsson, P., Hincker, A. M., Jensen, T. S., Freeman, R. & Haroutounian, S. Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: a systematic review. Pain 160, 286–297 (2019).
Khanna, R. et al. Sex-dependent differences in pain and sleep in a porcine model of Neurofibromatosis type 1. Preprint at bioRxiv https://doi.org/10.1101/495358 (2018).
De Briyne, N., Berg, C., Blaha, T., Palzer, A. & Temple, D. ‘Phasing out pig tail docking in the EU—present state, challenges and possibilities’. Porc. Health Manag. 4, 27 (2018).
Klein, T., Magerl, W. & Treede, R.-D. Perceptual correlate of nociceptive long-term potentiation (LTP) in humans shares the time course of early-LTP. J. Neurophysiol. 96, 3551–3555 (2006).
Magerl, W., Hansen, N., Treede, R.-D. & Klein, T. The human pain system exhibits higher-order plasticity (metaplasticity). Neurobiol. Learn. Mem. 154, 112–120 (2018).
Henrich, F., Magerl, W., Klein, T., Greffrath, W. & Treede, R.-D. Capsaicin-sensitive C- and A-fibre nociceptors control long-term potentiation-like pain amplification in humans. Brain 138, 2505–2520 (2015).
Haga, H. A., Tevik, A. & Moerch, H. Electroencephalographic and cardiovascular indicators of nociception during isoflurane anaesthesia in pigs. Vet. Anaesth. Analg. 28, 126–131 (2001).
Zheng, Z., Gibson, S. J., Khalil, Z., Helme, R. D. & McMeeken, J. M. Age-related differences in the time course of capsaicin-induced hyperalgesia. Pain 85, 51–58 (2000).
Rolke, R. et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 123, 231–243 (2006).
Prunier, A. et al. Identifying and monitoring pain in farm animals: a review. Animal 7, 998–1010 (2013).
Bilsborrow, K., Seddon, Y. M., Brown, J., Waldner, C. & Stookey, J. M. An investigation of a novel behavioural test to assess pain in piglets following castration. Can. J. Anim. Sci. 96, 376–385 (2016).
Deuis, J. R., Dvorakova, L. S. & Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 10, 284 (2017).
Matsumiya, L. C. et al. Using the Mouse Grimace Scale to Reevaluate the Efficacy of Postoperative Analgesics in Laboratory Mice. J. Am. Assoc. Lab. Anim. Sci. 51, 8 (2012).
Ängeby Möller, K. et al. Gait analysis and weight bearing in pre-clinical joint pain research. J. Neurosci. Methods 300, 92–102 (2018).
Nordquist, R. E., Meijer, E., van der Staay, F. J. & Arndt, S. S. Pigs as model species to investigate effects of early life events on behavioral and neurological function. in Animal models for the study of human disease. Academic Press, 1003–1030 (2017).
Zebunke, M., Kreiser, M., Melzer, N., Langbein, J. & Puppe, B. Better, not just more—contrast in qualitative aspects of reward facilitates impulse control in pigs. Front. Psychol. 9, 2099 (2018).
Kennedy, W. R. et al. A randomized, controlled, open-label study of the long-term fffects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J. Pain 11, 579–587 (2010).
Landmann, G. et al. Short lasting transient effects of a capsaicin 8% patch on nociceptor activation in humans. Eur. J. Pain 20, 1443–1453 (2016).
Lo Vecchio, S., Andersen, H. H. & Arendt-Nielsen, L. The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Exp. Brain Res. 236, 2231–2244 (2018).
Nielsen, T. A., Eriksen, M. A., Gazerani, P. & Andersen, H. H. Psychophysical and vasomotor evidence for interdependency of TRPA1 and TRPV1-evoked nociceptive responses in human skin: an experimental study. Pain 159, 1989–2001 (2018).
Koppert, W., Brueckl, V., Weidner, C. & Schmelz, M. Mechanically induced axon reflex and hyperalgesia in human UV-B burn are reduced by systemic lidocaine. Eur. J. Pain 8, 237–244 (2004).
Sycha, T. et al. Rofecoxib attenuates both primary and secondary inflammatory hyperalgesia: a randomized, double blinded, placebo controlled crossover trial in the UV-B pain model. Pain 113, 316–322 (2005).
Weinkauf, B., Main, M., Schmelz, M. & Rukwied, R. Modality-specific nociceptor sensitization following UV-B irradiation of human skin. J. Pain 14, 739–746 (2013).
Lötsch, J. et al. Quantitative sensory testing response patterns to capsaicin- and ultraviolet-B–induced local skin hypersensitization in healthy subjects: a machine-learned analysis. Pain 159, 11–24 (2018).
Rössler, B. et al. Central origin of pinprick hyperalgesia adjacent to an UV-B induced inflammatory skin pain model in healthy volunteers. Scand. J. Pain 4, 40–45 (2013).
Andresen, T. et al. Intradermal injection with nerve growth factor: a reproducible model to induce experimental allodynia and hyperalgesia. Pain Pract. 16, 12–23 (2016).
Papagianni, A., Siedler, G., Sommer, C. & Üçeyler, N. Capsaicin 8% patch reversibly reduces A-delta fiber evoked potential amplitudes. PAIN Rep. 3, e644 (2018).
Doll, R. J. et al. Responsiveness of electrical nociceptive detection thresholds to capsaicin (8 %)-induced changes in nociceptive processing. Exp. Brain Res. 234, 2505–2514 (2016).
Maihöfner, C., Ringler, R., Herrndobler, F. & Koppert, W. Brain imaging of analgesic and antihyperalgesic effects of cyclooxygenase inhibition in an experimental human pain model: a functional MRI study: Functional imaging of COX inhibition. Eur. J. Neurosci. 26, 1344–1356 (2007).
Acknowledgements
The Center for Neuroplasticity and Pain (CNAP) is supported by the Danish National Research Foundation (DNRF121).
Author information
Authors and Affiliations
Contributions
S. Meijs conceived the original work, carried out data acquisition, analysis and interpretation, wrote the draft and revised the manuscript. M.S and S. Meijlin revised the manuscript according to their expertise. W.J. contributed to conceiving the work, interpreting data, writing the draft and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Lab Animal thanks Suzanne Millman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1.
Rights and permissions
About this article
Cite this article
Meijs, S., Schmelz, M., Meilin, S. et al. A systematic review of porcine models in translational pain research. Lab Anim 50, 313–326 (2021). https://doi.org/10.1038/s41684-021-00862-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-021-00862-4