Abstract
The designer receptor exclusively activated by designer drugs (DREADD) system is one of the most widely used chemogenetic techniques to modulate the activity of cell populations in the brains of behaving animals. DREADDs are activated by acute or chronic administration of their ligand, clozapine-N-oxide (CNO). There is, however, a current lack of a non-invasive CNO administration technique that can control for drug timing and dosing without inducing substantial distress for the animals. Here, we evaluated whether the recently developed micropipette-guided drug administration (MDA) method, which has been used as a non-invasive and minimally stressful alternative to oral gavages, may be applied to administer CNO orally to activate DREADDs in a dosing- and timing-controlled manner. Unlike standard intraperitoneal injections, administration of vehicle substances via MDA did not elevate plasma levels of the major stress hormone, corticosterone, and did not attenuate exploratory activity in the open field test. At the same time, however, administration of CNO via MDA or intraperitoneally was equally efficient in activating hM3DGq-expressing neurons in the medial prefrontal cortex, as evident by time-dependent increases in mRNA levels of neuronal immediate early genes (cFos, Arc and Zif268) and cFos-immunoreactive neurons. Compared to vehicle given via MDA, oral administration of CNO via MDA was also found to potently increase locomotor activity in mice that express hM3DGq in prefrontal neurons. Taken together, our study confirms the effectiveness of CNO given orally via MDA and provides a novel method for non-stressful, yet well controllable CNO treatments in mouse DREADD systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. U. S. A. 104, 5163–5168 (2007).
Whissell, P. D., Tohyama, S. & Martin, L. J. The use of DREADDs to deconstruct behavior. Front. Genet. 7, 70 (2016).
Zhan, J., Komal, R., Keenan, W. T., Hattar, S. & Fernandez, D. C. Non-invasive strategies for chronic manipulation of DREADD-controlled neuronal activity. J. Vis. Exp. (150), e59439 (2019).
Machholz, E., Mulder, G., Ruiz, C., Corning, B. F. & Pritchett-Corning, K. R. Manual restraint and common compound administration routes in mice and rats. J. Vis. Exp. (67), e2771 (2012).
Meijer, M. K., Spruijt, B. M., van Zutphen, L. F. & Baumans, V. Effect of restraint and injection methods on heart rate and body temperature in mice. Lab. Anim. 40, 382–391 (2006).
Stuart, S. A. & Robinson, E. S. Reducing the stress of drug administration: implications for the 3Rs. Sci. Rep. 5, 14288 (2015).
Lewis, R. E., Kunz, A. L. & Bell, R. E. Error of intraperitoneal injections in rats. Lab. Anim. Care 16, 505–509 (1966).
Morton, D. B. et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 35, 1–41 (2001).
Turner, P. V., Brabb, T., Pekow, C. & Vasbinder, M. A. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613 (2011).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18 (2018).
Urban, D. J. et al. Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41, 1404–1415 (2016).
Jain, S. et al. Chronic activation of a designer G(q)-coupled receptor improves beta cell function. J. Clin. Invest. 123, 1750–1762 (2013).
Scarborough, J. et al. Preclinical validation of the micropipette-guided drug administration (MDA) method in the maternal immune activation model of neurodevelopmental disorders. Brain Behav. Immun. 88, 461–470 (2020).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Notter, T. et al. Neuronal activity increases translocator protein (TSPO) levels. Mol. Psychiatry Forthcoming (2020).
Campbell, E. J. & Marchant, N. J. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 175, 994–1003 (2018).
Jansen van’t Land, C. & Hendriksen, C. F. Change in locomotor activity pattern in mice: a model for recognition of distress? Lab. Anim. 29, 286–293 (1995).
Matson, D. J., Broom, D. C. & Cortright, D. N. Locomotor activity in a novel environment as a test of inflammatory pain in rats. Methods Mol. Biol. 617, 67–78 (2010).
Gronli, J. et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol. Behav. 84, 571–577 (2005).
Shoji, H. & Miyakawa, T. Differential effects of stress exposure via two types of restraint apparatuses on behavior and plasma corticosterone level in inbred male BALB/cAJcl mice. Neuropsychopharmacol. Rep. 40, 73–84 (2020).
Tuli, J. S., Smith, J. A. & Morton, D. B. Stress measurements in mice after transportation. Lab. Anim. 29, 132–138 (1995).
Malisch, J. L. et al. Acute restraint stress alters wheel-running behavior immediately following stress and up to 20 hours later in house mice. Physiol. Biochem. Zool. 89, 546–552 (2016).
National Research Council Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals. (National Academies Press (US), Washington, DC, USA, 2009).
Belzung, C. & Griebel, G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 125, 141–149 (2001).
Zimprich, A. et al. A robust and reliable non-invasive test for stress responsivity in mice. Front. Behav. Neurosci. 8, 125 (2014).
Salvi, S. S. et al. Acute chemogenetic activation of CamKIIα-positive forebrain excitatory neurons regulates anxiety-like behaviour in mice. Front. Behav. Neurosci. 13, 249 (2019).
Lukas, G., Brindle, S. D. & Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 178, 562–564 (1971).
Gomez, J. L. et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357, 503–507 (2017).
Fernandez, J. W., Grizzell, J. A., Philpot, R. M. & Wecker, L. Postpartum depression in rats: differences in swim test immobility, sucrose preference and nurturing behaviors. Behav. Brain Res. 272, 75–82 (2014).
Wyvell, C. L. & Berridge, K. C. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward ‘wanting’ without enhanced ‘liking’ or response reinforcement. J. Neurosci. 20, 8122–8130 (2000).
Donato, F., Jacobsen, R. I., Moser, M. B. & Moser, E. I. Stellate cells drive maturation of the entorhinal-hippocampal circuit. Science 355, eaai8178 (2017).
Mahler, S. V. et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 17, 577–585 (2014).
Lichtenberg, N. T. et al. Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J. Neurosci. 37, 8374–8384 (2017).
Arakawa, H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 341, 98–108 (2018).
Lukkes, J. L., Watt, M. J., Lowry, C. A. & Forster, G. L. Consequences of post-weaning social isolation on anxiety behavior and related neural circuits in rodents. Front. Behav. Neurosci. 3, 18 (2009).
Mueller, F. S., Polesel, M., Richetto, J., Meyer, U. & Weber-Stadlbauer, U. Mouse models of maternal immune activation: mind your caging system! Brain Behav. Immun. 73, 643–660 (2018).
Soden, M. E. et al. Genetic isolation of hypothalamic neurons that regulate context-specific male social behavior. Cell Rep. 16, 304–313 (2016).
Farrell, M. S. et al. A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38, 854–862 (2013).
Richetto, J. et al. Genome-wide DNA methylation changes in a mouse model of infection-mediated neurodevelopmental disorders. Biol. Psychiatry 81, 265–276 (2017).
Weber-Stadlbauer, U. et al. Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Mol. Psychiatry 22, 102–112 (2017).
Notter, T., Panzanelli, P., Pfister, S., Mircsof, D. & Fritschy, J. M. A protocol for concurrent high-quality immunohistochemical and biochemical analyses in adult mouse central nervous system. Eur. J. Neurosci. 39, 165–175 (2014).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ CT method. Methods 25, 402–408 (2001).
Richetto, J., Polesel, M. & Weber-Stadlbauer, U. Effects of light and dark phase testing on the investigation of behavioural paradigms in mice: relevance for behavioural neuroscience. Pharmacol. Biochem. Behav. 178, 19–29 (2019).
Notter, T. et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatry 23, 323–334 (2018).
Acknowledgements
This study was supported by a Postdoc Mobility grant (grant no. P2ZHP3_174868) awarded to T.N. by the Swiss National Science Foundation. Additional financial support was received from the Swiss National Science Foundation (grant no. 310030_188524 awarded to U.M.; grant no. PZ00P3_18009/1 awarded to J.R.). We would like to thank the Hodge Foundation UK, as well as the Viral Vector Facility (VVF) of the Neuroscience Center Zurich (ZNZ). Imaging was performed with equipment maintained by the Center for Microscopy and Image Analysis, University of Zurich.
Author information
Authors and Affiliations
Contributions
S.M.S. designed and performed research, analyzed data and contributed to the preparation of the manuscript. F.S.M., J.S., J.R. and U.W.-S. performed research. U.M. designed research and contributed to the preparation of the manuscript. T.N. designed and performed research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
Unrelated to the present study, U.M. has received financial support from Boehringer Ingelheim Pharma GmbH & Co. and from Wren Therapeutics Ltd. All authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
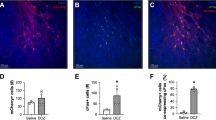
Extended Data Fig. 1 Immediate early gene expression in the mPFC after CNO (1 mg/kg) or VEH administration via IP injection or MDA in the absence of the modified human muscarinic M3 G-protein-coupled receptor (DREADD).
The scatter bar blots represent mRNA levels of cFos, Arc and Zif268 in the mPFC of adult (12-week-old) C57Bl6/N mice 60 min after treatment with VEH or CNO given via MDA or IP injections; n(VEH/IP) = 7, n(CNO/IP) = 7, n(VEH/MDA) = 7, n(CNO/MDA) = 7. Each dot in the scatter bar plot represents an individual animal, and error bars represent s.e.m.
Supplementary information
Rights and permissions
About this article
Cite this article
Schalbetter, S.M., Mueller, F.S., Scarborough, J. et al. Oral application of clozapine-N-oxide using the micropipette-guided drug administration (MDA) method in mouse DREADD systems. Lab Anim 50, 69–75 (2021). https://doi.org/10.1038/s41684-021-00723-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-021-00723-0
This article is cited by
-
Serotonin modulates excitatory synapse maturation in the developing prefrontal cortex
Nature Communications (2024)
-
Towards more translatable research: Exploring alternatives to gavage as the oral administration route of vaccines in rodents for improved animal welfare and human relevance
Lab Animal (2023)
-
Chemogenetic rectification of the inhibitory tone onto hippocampal neurons reverts autistic-like traits and normalizes local expression of estrogen receptors in the Ambra1+/- mouse model of female autism
Translational Psychiatry (2023)
-
A review on particle assembly in standing wave acoustic field
Journal of Nanoparticle Research (2022)
-
On the road to refinement
Lab Animal (2021)