Abstract
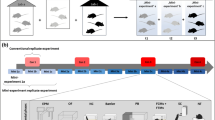
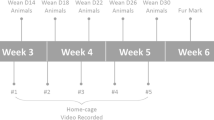
Low replicability of animal experiments is perceived as a major hurdle in the field of biomedicine. Attempts to enhance the replicability and to reduce the variability in basic research has led to the recommendation to use isogenic mice. The C57BL/6 strain has evolved as a gold standard strain for this purpose. However, C57BL/6 mice are maintained as substrains by multiple vendors. Evidence exists that the subtle differences between these mouse lines have not been systematically investigated and are often ignored. In the present study, we characterized the female mice of two closely related substrains (C57BL/6J and C57BL/6N) from three vendors in Europe (Charles River Laboratories, Envigo, Janvier Labs) in a battery of behavioral tests. Our data show and confirm substantial behavioral differences between the C57BL/6J and C57BL/6N mice. Importantly, the substrain differences were largely affected by the origin of the animals, as a significant effect of vendor or interaction between the substrain and vendor occurred in all tests. This work highlights the importance of adhering to precise international nomenclature in all publications reporting animal experiments. Moreover, the generalization of research findings from a single mouse substrain can be seriously limited due to genetic drift and environmental variables occurring at different vendors. However, heterogenization of samples, by including animals of different substrains, can enhance generalizability. These issues need to be seriously addressed to improve reproducibility, replicability, and the translational potential of the mouse models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Collins, F. S., Rossant, J. & Wurst, W. A mouse for all reasons. Cell 128, 9–13 (2007).
Gerlai, R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 19, 177–181 (1996).
Crawley, J. N. et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132, 107–124 (1997).
Silva, A. J. et al. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron 19, 755–759 (1997).
Ayadi, A. et al. Mouse large-scale phenotyping initiatives: overview of the European Mouse Disease Clinic (EUMODIC) and of the Wellcome Trust Sanger Institute Mouse Genetics Project. Mamm. Genome 23, 600–610 (2012).
Brown, S. D. & Moore, M. W. The international mouse phenotyping consortium: past and future perspectives on mouse phenotyping. Mamm Genome 23, 632–640 (2012).
Pettitt, S. J. et al. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat. Methods 6, 493–495 (2009).
Bradley, A. et al. The mammalian gene function resource: the International Knockout Mouse Consortium. Mamm. Genome 23, 580–586 (2012).
Doetschman, T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol. Biol. 530, 423–433 (2009).
Bourdi, M., Davies, J. S. & Pohl, L. R. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chemical research in toxicology 24, 794–796 (2011).
Wotjak, C. T. C57BLack/BOX? The importance of exact mouse strain nomenclature. Trends Genet. 19, 183–184 (2003).
Kiselycznyk, C. & Holmes, A. All (C57BL/6) mice are not created equal. Front. Neurosci 5, 10 (2011).
Bryant, C. D. The blessings and curses of C57BL/6 substrains in mouse genetic studies. Ann. N Y Acad. Sci. 1245, 31–33 (2011).
Fontaine, D. A. & Davis, D. B. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes 65, 25–33 (2016).
Kafkafi, N. et al. Reproducibility and replicability of rodent phenotyping in preclinical studies. Neurosci. Biobehav. Rev. 87, 218–232 (2018).
Baker, M. 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454 (2016).
Bespalov, A. & Steckler, T. Lacking quality in research: Is behavioral neuroscience affected more than other areas of biomedical science? J. Neurosci. Methods 300, 4–9 (2018).
Perrin, S. Preclinical research: Make mouse studies work. Nature 507, 423–425 (2014).
Begley, C. G. & Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature 483, 531–533 (2012).
Festing, M. F. Warning: the use of heterogeneous mice may seriously damage your research. Neurobiol. Aging 20, 237–244 (1999). discussion 245–236.
Festing, M. F. Inbred strains should replace outbred stocks in toxicology, safety testing, and drug development. Toxicologic pathology 38, 681–690 (2010).
Rivera, J. & Tessarollo, L. Genetic background and the dilemma of translating mouse studies to humans. Immunity 28, 1–4 (2008).
Sittig, L. J. et al. Genetic Background Limits Generalizability of Genotype-Phenotype Relationships. Neuron 91, 1253–1259 (2016).
Tuttle, A. H., Philip, V. M., Chesler, E. J. & Mogil, J. S. Comparing phenotypic variation between inbred and outbred mice. Nat. Methods 15, 994–996 (2018).
Mills, M. C. & Rahal, C. A scientometric review of genome-wide association studies. Commun. Biol 2, 9 (2019).
Haga, S. B. Impact of limited population diversity of genome-wide association studies. Genet. Med 12, 81–84 (2010).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412 (2010).
Steward, O. & Balice-Gordon, R. Rigor or mortis: best practices for preclinical research in neuroscience. Neuron 84, 572–581 (2014).
Mogil, J. S. & Macleod, M. R. No publication without confirmation. Nature 542, 409–411 (2017).
Smith, A. J., Clutton, R. E., Lilley, E., Hansen, K. E. A. & Brattelid, T. PREPARE: guidelines for planning animal research and testing. Lab Anim 52, 135–141 (2017).
Ioannidis, J. P. A. The proposal to lower P value thresholds to .005. Jama 319, 1429–1430 (2018).
Steckler, T. et al. The preclinical data forum network: A new ECNP initiative to improve data quality and robustness for (preclinical) neuroscience. Eur. Neuropsychopharmacol. 25, 1803–1807 (2015).
Crabbe, J. C., Wahlsten, D. & Dudek, B. C. Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672 (1999).
Wurbel, H. Behaviour and the standardization fallacy. Nat. Genet. 26, 263 (2000).
Richter, S. H., Garner, J. P. & Wurbel, H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat. Methods 6, 257–261 (2009).
Voelkl, B., Vogt, L., Sena, E. S. & Wurbel, H. Reproducibility of preclinical animal research improves with heterogeneity of study samples. PLoS Biol. 16, e2003693 (2018).
Voelkl, B. & Wurbel, H. Reproducibility crisis: Are we ignoring reaction norms? Trends Pharmacol. Sci. 37, 509–510 (2016).
Bailoo, J. D., Reichlin, T. S. & Wurbel, H. Refinement of experimental design and conduct in laboratory animal research. ILAR J. 55, 383–391 (2014).
Karp, N. A. Reproducible preclinical research-Is embracing variability the answer? PLoS Biol. 16, e2005413 (2018).
Ehrenreich, H. The impact of environment on abnormal behavior and mental disease: To alleviate the prevalence of mental disorders, we need to phenotype the environment for risk factors. EMBO Rep 18, 661–665 (2017).
Sundberg, J. P. & Schofield, P. N. Living inside the box: environmental effects on mouse models of human disease. Dis. Model Mech. 11, 1–7 (2018).
Editorial. Considerations for experimental design of behavioral studies using model organisms. J.Neurosci 39, 1–2 (2019).
Editorial. Building a better mouse test. Nat. Methods 8, 697–697 (2011).
Editorial. Troublesome variability in mouse studies. Nat. Neurosci. 12, 1075 (2009).
Garner, J. P. The significance of meaning: why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J. 55, 438–456 (2014).
Garner, J. P., Gaskill, B. N., Weber, E. M., Ahloy-Dallaire, J. & Pritchett-Corning, K. R. Introducing Therioepistemology: the study of how knowledge is gained from animal research. Lab Anim (NY) 46, 103–113 (2017).
Matsuo, N. et al. Behavioral profiles of three C57BL/6 substrains. Front. Behav. Neurosci. 4, 29 (2010).
Mekada, K. et al. Genetic differences among C57BL/6 substrains. Exp. Anim. 58, 141–149 (2009).
Zurita, E. et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 20, 481–489 (2011).
Mattapallil, M. J. et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest. Ophthalmol Vis Sci 53, 2921–2927 (2012).
Huang, T. T. et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum. Mol. Genet. 15, 1187–1194 (2006).
Specht, C. G. & Schoepfer, R. Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2, 11 (2001).
Zeldovich, L. Genetic drift: the ghost in the genome. Lab Anim (NY) 46, 255–257 (2017).
Taft, R. A., Davisson, M. & Wiles, M. V. Know thy mouse. Trends Genet. 22, 649–653 (2006).
Simon, M. M. et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 14, R82 (2013).
Fritz, A. K., Amrein, I. & Wolfer, D. P. Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am. J. Med. Genet. C Semin. Med. Genet 175, 380–391 (2017).
Prendergast, B. J., Onishi, K. G. & Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 40, 1–5 (2014).
Deacon, R. M. Housing, husbandry and handling of rodents for behavioral experiments. Nat. Protoc. 1, 936–946 (2006).
Kappel, S., Hawkins, P. & Mendl, M. T. To group or not to group? Good practice for housing male laboratory mice. Animals 7, pii: E88 (2017).
Paylor, R., Spencer, C. M., Yuva-Paylor, L. A. & Pieke-Dahl, S. The use of behavioral test batteries, II: Effect of test interval. Physiol. Behav. 87, 95–102 (2006).
McIlwain, K. L., Merriweather, M. Y., Yuva-Paylor, L. A. & Paylor, R. The use of behavioral test batteries: Effects of training history. Physiol. Behav. 73, 705–717 (2001).
Crawley, J. N. & Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 31, 197–211 (1997).
Bryant, C. D. et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J. Neurogenet. 22, 315–331 (2008).
Hager, T. et al. Display of individuality in avoidance behavior and risk assessment of inbred mice. Front. Behav. Neurosci. 8, 314 (2014).
Kumar, V. et al. C57BL/6N mutation in Cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342, 1508–1512 (2013).
Mulligan, M. K. et al. Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes Brain Behav. 7, 677–689 (2008).
Radulovic, J., Kammermeier, J. & Spiess, J. Generalization of fear responses in C57BL/6N mice subjected to one-trial foreground contextual fear conditioning. Behav. Brain Res. 95, 179–189 (1998).
Stiedl, O. et al. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav. Brain Res. 104, 1–12 (1999).
Sturm, M., Becker, A., Schroeder, A., Bilkei-Gorzo, A. & Zimmer, A. Effect of chronic corticosterone application on depression-like behavior in C57BL/6N and C57BL/6J mice. Genes Brain Behav 14, 292–300 (2015).
Swallow, J. et al. Guidance on the transport of laboratory animals. Lab Anim. 39, 1–39 (2005).
Baumans, V. & Van Loo, P. L. How to improve housing conditions of laboratory animals: The possibilities of environmental refinement. Vet. J. 195, 24–32 (2013).
Kulesskaya, N., Rauvala, H. & Voikar, V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS ONE 6, e24755 (2011).
Gaskill, B. N. et al. Impact of nesting material on mouse body temperature and physiology. Physiol Behav 110-111C, 87–95 (2013).
Richter, S. H. et al. Effect of population heterogenization on the reproducibility of mouse behavior: a multi-laboratory study. PLoS ONE 6, e16461 (2011).
Harro, J. Animals, anxiety, and anxiety disorders: How to measure anxiety in rodents and why. Behav. Brain Res. 352, 81–93 (2018).
Siegmund, A., Langnaese, K. & Wotjak, C. T. Differences in extinction of conditioned fear in C57BL/6 substrains are unrelated to expression of alpha-synuclein. Behav. Brain Res. 157, 291–298 (2005).
Labots, M., Zheng, X., Moattari, G., Ohl, F. & van Lith, H. A. Effects of light regime and substrain on behavioral profiles of male C57BL/6 mice in three tests of unconditioned anxiety. J. Neurogenet. 30, 306–315 (2016).
Pinheiro, B. S. et al. Dyadic social interaction of C57BL/6 mice versus interaction with a toy mouse: conditioned place preference/aversion, substrain differences, and no development of a hierarchy. Behav. Pharmacol. 27, 279–288 (2016).
Grottick, A. J. et al. Neurotransmission- and cellular stress-related gene expression associated with prepulse inhibition in mice. Brain Res. Mol. Brain Res. 139, 153–162 (2005).
Martin, A. L. & Brown, R. E. The lonely mouse: Verification of a separation-induced model of depression in female mice. Behav. Brain Res. 207, 196–207 (2010).
Schipper, L., Harvey, L., van der Beek, E. M. & van Dijk, G. Home alone: a systematic review and meta-analysis on the effects of individual housing on body weight, food intake and visceral fat mass in rodents. Obesity Reviews 19, 614–637 (2018).
Wahlsten, D., Bachmanov, A., Finn, D. A. & Crabbe, J. C. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci. U S A 103, 16364–16369 (2006).
Gulinello, M. et al. Rigor and reproducibility in rodent behavioral research. Neurobiol. Learn. Mem. in press, https://doi.org/10.1016/j.nlm.2018.01.001 (2018).
Tanila, H. Testing cognitive functions in rodent disease models: Present pitfalls and future perspectives. Behav. Brain Res. 352, 23–27 (2018).
Boleij, H., Salomons, A. R., van Sprundel, M., Arndt, S. S. & Ohl, F. Not all mice are equal: welfare implications of behavioural habituation profiles in four 129 mouse substrains. PLoS ONE 7, e42544 (2012).
Cook, M. N., Bolivar, V. J., McFadyen, M. P. & Flaherty, L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav. Neurosci. 116, 600–611 (2002).
Sittig, L. J. et al. Phenotypic instability between the near isogenic substrains BALB/cJ and BALB/cBy. J. Mamm. Genome 25, 564–572 (2014).
Olfe, J., Domanska, G., Schuett, C. & Kiank, C. Different stress-related phenotypes of BALB/c mice from in-house or vendor: alterations of the sympathetic and HPA axis responsiveness. BMC Physiol. 10, 2 (2010).
Meehan, T. F. et al. Disease model discovery from 3,328 gene knockouts by The international mouse phenotyping consortium. Nat. Genet. 49, 1231–1238 (2017).
Kafkafi, N. et al. Addressing reproducibility in single-laboratory phenotyping experiments. Nat. Methods 14, 462–464 (2017).
Ashworth, A. et al. Comparison of neurological function in males and females from two substrains of C57BL/6 Mice. Toxics 3, 1–17 (2015).
Kirkpatrick, S. L. et al. Cytoplasmic FMR1-interacting protein 2 is a major genetic factor underlying binge eating. Biol. Psychiatry 81, 757–769 (2017).
Lister, R. G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92, 180–185 (1987).
Crawley, J. N. & Goodwin, F. K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 13, 167–170 (1980).
Moy, S. S. et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 3, 287–302 (2004).
Golden, S. A., Covington, H. E. 3rd, Berton, O. & Russo, S. J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 6, 1183–1191 (2011).
Deacon, R. M. Assessing nest building in mice. Nat. Protoc. 1, 1117–1119 (2006).
Van der Heyden, J. A., Zethof, T. J. & Olivier, B. Stress-induced hyperthermia in singly housed mice. Physiol. Behav 62, 463–470 (1997).
Acknowledgements
This study was supported by Jane and Aatos Erkko foundation (VV), Biocenter Finland and Helsinki Institute of Life Science. Professors Iiris Hovatta and Jaan-Olle Andressoo are greatly acknowledged for their valuable comments and discussions during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Åhlgren, J., Voikar, V. Experiments done in Black-6 mice: what does it mean?. Lab Anim 48, 171–180 (2019). https://doi.org/10.1038/s41684-019-0288-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41684-019-0288-8
This article is cited by
-
Importing genetically altered animals: ensuring quality
Mammalian Genome (2022)
-
Inflammatory responses of C57BL/6NKorl mice to dextran sulfate sodium-induced colitis: comparison between three C57BL/6 N sub-strains
Laboratory Animal Research (2021)
-
The development of nonalcoholic steatohepatitis is subjected to breeder dependent variation in guinea pigs
Scientific Reports (2021)
-
Inbred lab mice are not isogenic: genetic variation within inbred strains used to infer the mutation rate per nucleotide site
Heredity (2021)
-
Improving reproducibility in animal research by splitting the study population into several ‘mini-experiments’
Scientific Reports (2020)