Abstract
Individuals who consume alcohol have a higher chance of contracting tuberculosis (TB) due to their social mixing patterns. We aimed to study the social mixing patterns of TB patients who consume alcohol on a regular basis using a quantitative social network approach. In a high-TB prevalence context in India, a social network survey of 300 newly diagnosed pulmonary drug-sensitive TB patients was done. The survey found 52 (17%) male TB patients who shared alcohol on a regular basis with 106 (4%) of their first-degree social contacts. Alcohol sharing happened in 16 neighborhood venues. When compared to contacts who did not use alcohol, a higher proportion of contacts with regular alcohol use were diagnosed with TB (12.3%; 95% CI: 6.6–20.00 vs. 3.5%; 95% CI: 2.8–4.3). Social network analysis showed that the network consisting of patients and contacts was less dense and less connected (with density ratio of 0.009, and degree centrality of 1.3, and betweenness centrality of 0.5), indicating weaker transmission potential of the network. Comparatively the network consisting of patients, contacts and their alcohol sharing venues was more dense and more connected (with density ratio of 0.018, higher degree centrality of 3.1 and betweenness centrality of 154.2) indicating stronger transmission potential of the network. Regular alcohol sharing in four venues created a giant network component, that linked a higher proportion of contacts without TB (72.3%) to a higher proportion of TB patients (67.3%) and their contacts with TB (38.4%). When examined from a network perspective, the pooled TB transmission exposure of contacts with regular alcohol use grew by a factor of 10, which helped explain the unfavorable social mixing of patients and contacts with regular alcohol use.
Similar content being viewed by others
Introduction
According to the global tuberculosis (TB) report, alcohol use disorders were attributed for 2,33,000 new TB cases detected in India. Alcohol use accounted for 8.9% of all incident TB cases in India (WHO, 2020). Individuals with alcohol use disorders are more likely to develop active TB and are more prone to re-infection, due to the impact of alcohol on their immune system and underlying alcohol-related diseases. (Lonnrot K, 2008). TB patients with alcohol use disorder are more likely to have poor treatment outcomes, as alcohol use potentially alters the pharmacokinetics of TB medicines (Rehm et al., 2009). Studies have also emphasized the importance of social mixing patterns of individuals who consume alcohol in congregate settings, which puts them in a disadvantageous position with a higher chance of transmission (Classen et al., 1999). Previous research had suggested that alcohol sharing groups and drinking venues are important in amplifying TB transmission outside of families (Classen et al., 1999; Diel et al., 2002). However, from a quantitative standpoint, there is still a lack of understanding of the alcohol-driven social mixing of TB patients. Quantifying social mixing patterns necessitates individual relational data and an adequate analytical tool for analyzing such relational data.
We attempted to undertake a primary study among TB patients who reported regular alcohol usage, through a social network-based approach. The social mixing of TB patients and their contacts was meticulously followed and investigated using social network analysis tools to obtain standard network metrics and quantitative insights. Through this primary social network study, we attempted to collect and explore a difficult-to-obtain data on alcohol-driven social mixing of TB patients.
Methods
Study setting
This investigation of TB patients who regularly consumed alcohol was undertaken as part of a wider social network study in Chennai, India, which tracked the potential extra-household contacts of TB patients (Nagarajan et al., 2021). As of 2019, Chennai has a disproportionately high TB burden, accounting for 14370 (13.7%) of the state’s total cases (CTD, 2020). The National Tuberculosis Elimination Program (NTEP) of Chennai has established several case finding strategies to address gaps in early TB case identification and effective tracking of vulnerable contacts of TB patients. Efforts were also undertaken to identify the hotspots of TB with high transmission potential in the city. The present study was conducted in this background of identifying social mixing patterns in congregate settings that could drive transmission of TB in the locality. The sample catchment area for this study represented the population of 24 geographically adjacent Designated Microscopy Center’s (DMCs) of the NTEP in the norther part of Chennai with high incidence of TB.
Sample size and participant recruitment
Between February 2018 and June 2019, a total of 713 newly diagnosed TB patients were consecutively screened in 24 geographically adjacent DMCs of the NTEP in the northern part of Chennai. Of the total screened, 300 patients were identified to be eligible for the study as they were newly diagnosed adult pulmonary (drug-sensitive) TB patients who had lived in the study area for at least one year and agreed to give information about their social mixing outside of their homes.
Study process
An exploratory cross-sectional personal social network survey (ego-centric survey) was conducted among the eligible TB patients (Nagarajan and Das, 2019). A tested social network questionnaire was used to probe the study subjects to generate a list of first-degree social network members with whom the TB patients had consistently lived, socialized, worked, spent time together during their pre-TB diagnostic period (6-month period before their diagnosis). Name generator method was used to list the network members and social mixing venues of the TB patients. The utility of personal social network methods in eliciting the closer social relationships of TB patients, as well as methodological specifics and quality issues, have already been discussed (Nagarajan and Das, 2019). A thorough participant recruitment process has already been published (Nagarajan et al., 2021).
Study definitions
Consistent social relation
Lived or social mixed or worked together for three or more days in a week and for two to four hours during the pre-diagnostic reference period.
First degree social network contacts or contacts
Those individuals with whom the TB patients had consistent social relation (lived, social mixed, worked) during their pre-TB diagnostic reference period.
Patients with regular alcohol use
Patients who had alcohol sharing for three or more days in a week during the pre-diagnostic reference period with his first-degree social network contact in a neighborhood venue. Henceforth termed as “patients with RAU”.
Contacts with regular alcohol use
First degree contacts who regularly shared alcohol with a patient for three or more days in a week during the pre-diagnostic reference period. Henceforth termed as “contacts with RAU”.
Contacts without regular alcohol use
First-degree contacts who did not share alcohol with a patient on a regular basis during the pre-diagnostic reference period. Henceforth termed as “contacts without RAU”.
Regular alcohol use venues
The neighborhood venues or places in which the patients and contacts with RAU had social mixing during the pre-diagnostic reference period. Henceforth termed as “venues”.
TB diagnosis
TB diagnostic definition used for patients and contacts with RAU were based on the guidelines of NTEP in India (Nagarajan et al., 2021).
Attribute and relationship characteristics
Attribute characteristics of patients and contacts with RAU were collected which included age, sex, occupation status, TB diagnostic status, and alcoholic social mixing during their pre-diagnostic reference period. TB diagnostic status of the contacts with RAU were tracked and confirmed through documentation verification at health facilities. Relationship types of contacts with RAU were classified into family, extended family, friendship, neighborhood, and occupational contacts.
Statistical analysis
Descriptive statistics were used to characterize attributes of patient’s and contacts with RAU. Standard deviations and confidence intervals were used to assess the difference in the proportion of contacts with RAU who had been diagnosed with TB. STATA software version 15.1 was used for the analysis.
Network generation and analysis
Social network analysis methods were used to explore the social mixing patterns of the patients and contacts with RAU. Using relational data, a ‘Patient-contact’ network was created, with each patient and contact with RAU considered a ‘node’. Alcoholic sharing relation between a patient and a contact was considered an ‘edge’ or ‘relational tie’. A relational dataset was used to develop another ‘Patient-contact-venue’ network, which included alcohol venues as a ‘node’ and regular alcoholic sharing at that venue as an ‘edge.’ Such approach was considered to compare the positional importance of patients and contacts within their respective networks, as well as their positional importance in relation to the venues they shared as a network.
Both relational datasets from respective networks were loaded into Gephi Program (Version 0.9.2) for separate network analysis. An undirected network analysis perspective was used to construct network metrics indicative of node transmission potentials (Nagarajan et al., 2020; Salathé and Jones, 2010). Both networks were visualized and interpreted using network graphs or sociograms. The network graphs were shown using Gephi software’s force atlas and Fruchterman Reingold layouts (Bastian et al., 2009).
Network metrics
We used conventional network measures such as degree centrality, betweenness centrality, network density, and network components to quantify the social mixing patterns. A more in-depth description of network measurements can be found elsewhere. Mean differences were used to compare summary metrics from both the ‘Patient-contact’ network and the ‘Patient-contact-venue’ network.
Definition of network metrics
Degree centrality
The number of direct relational linkages that a node has with other nodes in the network (patients, contacts with RAU, and their venue). Within the ‘Patient-contact’ and ‘Patient-contact-venue’ networks, mean degree centrality shows a node’s immediate transmission potential (Science Direct, 2022a).
Betweenness centrality
Indicates the number of times a given node acts as a bridge between other nodes in the network. Mean betweenness centrality indicates the power of nodes with high transmission potential within the ‘Patient-contact’ network and ‘Patient-contact-venue’ network (Science Direct, 2022b).
Density
Indicates the total number of relational ties present between the nodes divided by the total possible number of relational ties within the networks. Density highlights on how connected the nodes are when compared to how connected they could be.
Network component
Connected sub-structures composed of patients, contacts and their venues with at least one direct or indirect relation between them.
Ethical considerations
This study was approved by the Institutional Ethics Committee of National Institute for Research in Tuberculosis (NIRT), Indian Council of Medical Research (ICMR), Chennai (IEC No. 2017018). The study was conducted as per the Ethical Guidelines for Biomedical Research on Human Participants, 2017. All respondents received written and oral explanations of the study and signed an informed consent form before the interview.
Results
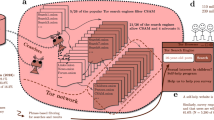
Of the enrolled 300 TB patients, 52 (17%) were identified as patients with RAU, i.e., who regularly shared alcohol with their 106 (4% of 2544), first degree contacts (contacts with RAU) (Table 1). Alcohol was shared at 16 overcrowded and poorly ventilated neighborhood venues (Fig. 1). Patients with RAU (n = 106) and contacts with RAU were all male with majority being friends, neighbors or occupational relations (96.2%).
During the pre-diagnostic reference period or after the TB diagnosis of patients with RAU, a high proportion of contacts with RAU were also diagnosed with TB (12.3%; 95% CI: 6.6–20.00 vs. 3.5%; 95% CI: 2.8–4.3). (Table 2). ‘Patient-contact network’ was found less dense (ratio = 0.009) with sparsely connected components (n = 52). Mean degree centrality showed that one patient with RAU had 1.3 immediate contacts with RAU in this network. The nodes in this network had a low mean betweenness centrality (0.5), indicating that their transmission potential was limited (Figs. 2 and 3; Tables 3 and 4).
Left figure depicts 52 patients and 106 contacts with RAU as circular nodes, the lines between nodes represent alcohol social mixing ties. Node size is proportional to the betweenness centrality measure representing its transmission potential. Sparsely connected 52 components are depicted which lack ties between them. Color depiction  Patients with RAU;
Patients with RAU;  Contacts with RAU but without TB
Contacts with RAU but without TB  Contacts with RAU and TB. Right figure depicts 52 patients, 106 contacts with RAU and 8 alcohol venues as circular nodes, the lines between nodes represent alcohol social mixing ties. Node size is proportional to the betweenness centrality measure representing its transmission potential. Of the 10 components the giant component in the central consists of 4 alcoholic venues nodes connecting 67% of index, 38% contacts with TB and 72% contacts without TB. Color depiction
Contacts with RAU and TB. Right figure depicts 52 patients, 106 contacts with RAU and 8 alcohol venues as circular nodes, the lines between nodes represent alcohol social mixing ties. Node size is proportional to the betweenness centrality measure representing its transmission potential. Of the 10 components the giant component in the central consists of 4 alcoholic venues nodes connecting 67% of index, 38% contacts with TB and 72% contacts without TB. Color depiction  Alcoholic venue
Alcoholic venue  Patient with RAU
Patient with RAU Contacts with RAU but without TB
Contacts with RAU but without TB  Contacts with RAU and TB.
Contacts with RAU and TB.
Socio gram of ‘Patient-contact’ network labeled with component IDs Numbers in the circular nodes depict component IDs as shown in Table 4.
‘Patient-contact-venue’ network was relatively denser by 66.7% (ratio = 0.018) with strongly connected components (n = 10) and each patient with RAU had 3.1 immediate contacts with RAU. The transmission potential of nodes increased by 198% in this network as indicated by the higher mean (154.2) betweenness centrality. A giant network component was identified which comprised of 67.3% of patients with RAU, 38.4% of contacts with RAU and TB and 72.3% of contacts with RAU but without TB. (Figs. 2 and 4, Tables 3 and 5).
Socio gram of ‘Patient-contact-venue’ network labeled with component IDs Numbers in the circular nodes depict component IDs as shown in Table 5.
Discussion
For the first time, we used a social network-based method to examine the social mixing patterns of TB patients and their connections who regularly consume alcohol in an environment where TB is more prevalent (Thomas et al., 2017). The study findings provide preliminary empirical evidence on how TB patients and their contacts with regular alcohol use are at a disadvantage in terms of increased TB transmission risk. The study identified that contacts with RAU had higher proportion of TB when compared to contacts without alcohol sharing. The proportion of TB was especially found to be higher among contacts with RAU, who were friends or neighbors of the patient users. This emphasizes the findings of earlier molecular epidemiological studies that non-household or community contacts contribute to the major proportion of TB transmission than the household contacts (Nagarajan et al., 2021).
Findings show that contacts with RAU had a higher proportion of TB, than contacts who did not share alcohol. The proportion of those who had TB was found to be higher among contacts with RAU (especially friends or neighbors). This finding of the study strengthens the earlier molecular epidemiological findings which highlighted that non-household or community contacts could relatively contribute more to TB transmission than household contacts (Nagarajan et al., 2021).
The study also found that contacts with RAU had a greater proportion of TB, which holds implications for TB contact tracing in countries like India, where higher levels of alcohol consumption and TB burden go hand in hand. Currently, India’s TB contact tracing strategy is primarily limited to household contacts and does not include extra household contacts of TB patients (Velayutham et al., 2020). In this context, India and similar countries could prioritize contact tracing among the individuals who regularly share alcohol with the TB patients. This could lead to early identification of new TB occurrences which are driven by social mixing of regular alcohol users.
The social mixing patterns happened in a way that two-thirds of all contacts with RAU but without TB were exposed to multiple other patients and contacts with RAU and TB. Their mutually frequented settings for regular alcohol sharing were the source of this exposure. The findings highlight that the pooled transmission exposure for contacts with RAU increased manifold when assessed from a network perspective and helps explain the disadvantageous social mixing driven by alcohol use in key venues. This finding holds implication for the TB contact tracing strategy in countries like India. At present contact tracing strategy in general emphasize active case finding in congregation settings (prisons, mines, hospitals, etc.) which are likely to have higher transmission potential (CTD, 2020). For the first time our study underscores the importance of venues (places where index and contact users regularly shared alcohol) in amplifying the TB transmission from a network analysis perspective. Thus, prioritizing venues where alcohol being shared by patients could be a potential opportunity for TB contact screening interventions to yield more cases and also to implement preventive measures for reducing transmission (Velayutham et al., 2020).
In the past, molecular epidemiology approaches have shown TB transmission clusters among people who share alcohol. These methodologies defined “clusters” from an epidemiological standpoint, but they lacked information and fine details on the underlying social mixing patterns among cluster members. There has been a dearth of research in terms of systematically analyzing the alcohol-driven social mixing patterns of TB patients and their contacts. Such systematic approach would require a quantitative methods and analysis and also necessitates primary social mixing data of TB patients and alcohol users which is rare to obtain. Our study filled in this gap by gathering primary social network data from TB patients who were regular alcohol users, as well as exploring and understanding it by using appropriate network analysis methods. As a result, the current study advances our understanding of social mixing patterns of regular alcohol users and its implications in the context of tuberculosis transmission.
The present study holds some limitation. The study did not assess the social mixing patterns of TB patients in their non-alcohol consumption venues in order to explain TB transmission. We found that non-alcohol venue based social mixing of patients and contacts identified in our study were sparse to conduct a network analysis. We also found that non–alcohol (or casual) social mixing took place mostly in open public spaces, which would be less appropriate to compare to social mixing that took place in closed and crowded locations where alcohol was shared.
Conclusion
The present study had explored the social mixing patterns of TB patients with regular alcohol use in the context of TB transmission by using the social network data and analysis methods. The findings highlight those contacts who regularly shared alcohol with TB patients had increased proportion of TB when compared to contacts who didn’t share alcohol with TB patients. The alcohol sharing venues served as important nodes in the network of TB patients and their contacts. Alcohol sharing venues also increased the TB transmission exposure by manifold for the contacts. The findings have implications for the contact tracing strategy of TB and highlights the need for prioritizing contacts with alcohol use and venues in which such sharing occurred.
Data availability
Data cannot be shared publicly because of patient confidentiality who participated in this study. Data are available from the Institutional Ethics Committee of ICMR-NIRT for researchers who meet the criteria for access to confidential data. Corresponding author could be communicated in this regard.
References
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open-source software for exploring and manipulating networks. Association for the Advancement of Artificial Intelligence. In: International AAAI conference on Weblogs and Social Media.
Classen CN, Warren R, Richardson M, Hauman JH, Gie RP, Ellis JH, van Helden PD, Beyers N (1999) Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax 54(2):136–140
CTD (2020) National strategic plan for tuberculosis elimination. Ministry of Health and Family Welfare, New Delhi https://tbcindia.gov.in/WriteReadData/NSPDraft2020.02.2017%201.pdf
Diel R, Schneider S, Meywald-Walter K, Ruf CM, Rüsch-Gerdes S, Niemann S (2002) Epidemiology of tuberculosis in Hamburg, Germany: long-term population-based analysis applying classical and molecular epidemiological techniques. J Clin Microbiol 40(2):532–539. https://doi.org/10.1128/JCM.40.2.532-539.2002
Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C (2008) Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health 8:289. https://doi.org/10.1186/1471-2458-8-289
Nagarajan K, Das B (2019) Exploratory, cross-sectional social network study to assess the influence of social networks on the care-seeking behaviour, treatment adherence and outcomes of patients with tuberculosis in Chennai, India: a study protocol. BMJ Open 9(5):e025699
Nagarajan K, Muniyandi M, Palani B, Sellappan S (2020) Social network analysis methods for exploring SARS-CoV-2 contact tracing data. BMC Med Res Methodol 20(1):233. https://doi.org/10.1186/s12874-020-01119-3
Nagarajan K, Muniyandi M, Palani B, Sellappan S (2021) Tracing the potential extra-household contacts of TB patients: findings from a personal social network survey in a high TB burden setting in India. Trans R Soc Trop Med Hyg trab099 https://doi.org/10.1093/trstmh/trab099
Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lönnroth K, Patra J, Poznyak V, Popova S (2009) The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 9:450
Salathé M, Jones JH (2010) Dynamics and control of diseases in networks with community structure. PLoS Comput Biol 6(4):e1000736. https://doi.org/10.1371/journal.pcbi.1000736
Science Direct (2022a) Centrality metric. https://www.sciencedirect.com/topics/computer-science/centrality-metric
Science Direct (2022b) Betweenness centrality—an overview https://www.sciencedirect.com/topics/computer-science/betweenness-centrality
Thomas B, Watson B, Senthil EK, Deepalakshmi A, Balaji G, Chandra S, Manogaran C, Nagarajan K, Ovung S, Jayabal L, Swaminathan S (2017) Alcohol intervention strategy among tuberculosis patients: a pilot study from South India. Int J Tuber Lung Dis 21(8):947–952
Velayutham B, Jayabal L, Watson B, Jagadeesan S, Angamuthu D, Rebecca P et al. (2020) Tuberculosis screening in household contacts of pulmonary tuberculosis patients in an urban setting. PLoS ONE 15(10):e0240594
WHO (2020) Global tuberculosis report 2020. https://www.who.int/publications/i/item/9789240013131
Acknowledgements
The authors would like to acknowledge the TB patients and their social network contacts for their participation and contribution in this study. The authors are thankful to the National Tuberculosis Elimination Program (NTEP) Staff of Greater Chennai Corporation, Tamil Nadu, India, for their extensive support in conducting this challenging study for the first time in India. The authors would like to thank UNOPS and STOP TB for their financial support in conducting this study. The study was supported by United States Agency for International Development (USAID) through Tuberculosis Health Action and Learning initiative (THALI) grant/ STOP TB Partnership/ United Nations Office for Project Services (UNOPS) under the TB free Chennai initiative (UNOPS/400/401/2017/00052). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: NK, MM; Methodology: KK, MM; Formal analysis: NK, MM, PB, JB; Draft preparation: NK, MM, LJ; Review and editing: PB, JB, LJ; Supervision: NK, MM, LJ; Administration: PB, JB, LJ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Ethics Committee of National Institute for Research in Tuberculosis (NIRT), Indian Council of Medical Research (ICMR), Chennai (IEC No: 2017018). The study was conducted as per the Ethical Guidelines for Biomedical Research on Human Participants, 2017.
Informed consent
All respondents received written and oral explanations of the study and signed an informed consent form before the interview.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagarajan, K., Palani, B., Basha, J. et al. A social networks-driven approach to understand the unique alcohol mixing patterns of tuberculosis patients: reporting methods and findings from a high TB-burden setting. Humanit Soc Sci Commun 9, 171 (2022). https://doi.org/10.1057/s41599-022-01191-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1057/s41599-022-01191-8