Abstract
One of the most devastating environmental consequences of war is the disruption of peacetime human–microbe relationships, leading to outbreaks of infectious diseases. Indirectly, conflicts also have severe health consequences due to population displacements, with a heightened risk of disease transmission. While previous research suggests that conflicts may have accentuated historical epidemics, this relationship has never been quantified. Here, we use annually resolved data to probe the link between climate, human behavior (i.e. conflicts), and the spread of plague epidemics in pre-industrial Europe (AD 1347–1840). We find that AD 1450–1670 was a particularly violent period of Europe’s history, characterized by a mean twofold increase in conflicts. This period was concurrent with steep upsurges in plague outbreaks. Cooler climate conditions during the Little Ice Age further weakened afflicted groups, making European populations less resistant to pathogens, through malnutrition and deteriorating living/sanitary conditions. Our analysis demonstrates that warfare provided a backdrop for significant microbial opportunity in pre-industrial Europe.
Similar content being viewed by others
Introduction
Historians, scientists, and wider society have generally paid little attention to bygone epidemics, with the marked exceptions of the Black Death and the Great Plague of London (Duffy, 1977). This narrow outlook has recently changed due to the coronavirus pandemic and its profound impacts on human health, the global economy and the geography of travel. For instance, the ongoing Covid-19 crisis has sparked renewed interest in Albert Camus’ novel “The plague”, originally published in 1947. The fascist “plague” that inspired the novel may no longer be a reality, but many other varieties of “pestilence” mean that this theme still has relevance today (Franco-Paredes, 2020). Pandemics are the most dramatic manifestation of the rapid and efficient spread of infectious pathogens, capable of influencing the course of world history. Understanding why, when, and how past epidemics/pandemics spread is therefore key to contextualizing current outbreaks.
The 2019/2020 coronavirus (Covid-19) pandemic has sharpened focus on the role of human population movements in rapidly spreading pathogenic microbes from a local hotspot to the global scale (Bedford et al., 2020; Chinazzi et al., 2020). In the past 40 years, outbreaks of infectious diseases have also been underpinned by population exoduses. Scourges have often emerged in forcibly displaced populations, invariably linked to a breakdown of health and social services (Murray et al., 2002). The Office of the United Nations High Commissioner for Refugees (UNHCR) reported that the “global forced displacement population” who have escaped conflict, persecution, or human rights violations totaled ~40 million people at the end of 2016 (The UN Refugee Agency, 2016). In 2018, it is estimated that 25 people were forced to flee unsecure areas every minute (The UN Refugee Agency, 2018). War produces a multitude of opportunities for pathogenic microbes and constitutes an extremely effective way to promote microbial traffic and increase human morbidity and mortality. Migrants can act as vectors for infectious disease, leading to severe epidemics in receiving areas, where displaced populations are often housed in cramped refugee camps (see the Darfur region of Sudan; Degomme and Guha-Sapir, 2010). As early as 1995, the UNHCR stated that measles, diarrheal diseases, acute respiratory infections, and malaria account for between 60% and 80% of reported deaths in refugee camps (The UN Refugee Agency, 1995).
The health consequences of wars are nowadays circumvented by basic health care services, which alleviate the spread of epidemics, even in countries engaged in armed conflicts and where interventions are challenging (Spiegel et al., 2010; Leaning and Guha-Sapir, 2013). During the Late Middle Ages to the Early Modern Age, when persistent conflicts marred the European continent, the spread of plague (caused by the bacteria Yersinia pestis) was probably aggravated and enhanced through populations fleeing war zones, increasing the geographical range of epidemics.
Fatigue, malnutrition, wounds, and stress are known to lower immune responses in human populations. Furthermore, camp life in crowded and unsanitary conditions favors the spread of contagious diseases and creates ideal ecological niches for both native and imported parasites. It has recently been suggested that there is an urgent need for a quantitative framework for modeling modern conflicts and epidemics (Banerjee, 2019). Within this context, there is potentially much to learn from the past and historical data are key to calibrating models. Here, we quantify the fundamental link between conflicts, plagues, fatalities, and the evolution of world population for the period from the Late Middle Ages to the Early Modern Age (AD 1340–1900). We also analyze how climate deterioration aggravated past epidemics/pandemics.
Results
Plague outbreaks
Arguably the most infamous plague outbreak in human history was the second plague pandemic (AD 1346–1720 for western Europe; ending around AD 1840 in Eastern Europe, North Africa, and the Near East; Dols, 1979; Hays, 2005), which started with the Black Death (AD 1346–1353). This pandemic swept through pre-industrial Asia and Europe (Benedictow, 2006). Scholars believe that the outbreak originated in China in the early 1330s, before spreading along trade routes and reaching Europe via Mediterranean ports in the late 1340s (Herlihy, 1997; Pamuk, 2007). However, the ultimate origin of the Black Death still remains uncertain (China, Mongolia, India, central Asia, and southern Russia; Norris, 1977). Recurring plagues lingered on for centuries, particularly in cities, and, for instance, the Great Plague of London (AD 1665–66; Roberts, 1966) is blamed for around 70,000 fatalities. While numerous epidemics, probably induced by different pathogens [the Plague of Athens (430–426 BC), the Antonine plague (AD 165–180), the Cyprian plague (AD 250)] and the first plague pandemic [Plague of Justinian (AD 541–750)] severely affected Europe (Little, 2007; Cohn, 2008; McMichael, 2012; Wagner et al., 2014) or not (Mordechai et al., 2019), the second plague pandemic remains the most devastating event in human history, killing some 30–60% of Europe’s population (75–200 million individuals). A number of recent studies have focused on the geography of the second plague pandemic in pre-industrial Europe (Büntgen et al., 2012; Schmid et al., 2015; Yue and Lee, 2018), but none have looked to quantify the link between human behavior, through the lens of conflicts, and the spread of epidemics (Banerjee, 2019).
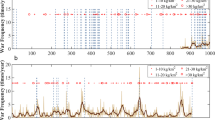
Figure 1 illustrates that conflict dynamics, which engendered population displacements, manifest a strong correlation with the spread of plagues and increased fatalities between AD 1450 and 1670. The strongest correlations, highlighted by the Mantel scalograms, frame a particularly deadly period during which all datasets follow the same trend, spanning the end of the Middle Ages and the onset of the early Modern Period (AD 1450–1670). Conflicts were frequently associated with plagues before AD 1450. The Black Death (AD 1346–1353) struck during a violent phase in European history, notably symbolized by the Hundred Years’ War (AD 1337–1453) and the Reconquista (which ended in AD 1492). The plague was so devastating that it briefly interrupted these conflicts due to the significant death toll (Nolan, 2006). During the Hundred Years’ War, the epidemic reached France in 1347 AD before striking England in the summer of AD 1348 (Cusack, 2019). The pathogen reached the British Isles through the port of Melcombe Regis in Dorset County before hitting Bristol and later London, in the autumn (Hawkins, 1990; Bolton, 1996). The Black Death (AD 1346–1353) reached England, like other countries, via the terrestrial and maritime trade routes that interlaced Asia and Europe (Schmid et al., 2015; Yue et al., 2016). After this, the disease spread rapidly amongst military populations.
The time series are shown as long-term trends (9-year and 31-year smoothing). All dates are expressed as years AD. The yellow shading highlights the main correlation phase between the three time series (based on smoothed series; 3-year smoothing) as defined by the Mantel scalograms (distance measure: Bray–Curtis).
Conflicts and plague outbreaks
The rise in conflicts played out first, with a shift in the number after AD 1450 (Fig. 2), followed, around AD 1465/1470, by a growth in towns and cities affected by the plague. The acme occurred between AD 1465 and AD 1670, when the dynamic of all signals points to multiple concurrent events (Fig. 2). Cross-correlations applied to the conflicts versus plagues and plagues versus fatalities time series show a positive correlation (Pvalue < 0.001), suggesting a chronological relationship between the three variables. A composite sequence (termed CPF, based on the sum of conflicts, plague incidences, and fatalities) further demonstrates that this period is linked to a plateau in world population figures (Fig. 3). The rate of demographic growth (50-year average) slowed from AD 1470 to 1620 and became negative during the period AD 1620–1670 before strongly increasing from the late 17th century onwards. The increasing spread of plagues during the period AD 1400–1500 (Fig. 3) is probably related to the growing population density (Supplementary Fig. 1), as the pathogen was able to reach more hosts rapidly. The connection between conflicts, plagues, population density, and fatalities is furthermore underlined by the neighbor-joining clustering (Supplementary Fig. 1), which places the conflicts at the beginning of the dynamic.
The time series are represented using long-term trends (3-year smoothing) and a sinusoidal model (Pvalue < 0.001). All dates are expressed as years AD. Each discordant period was categorized by the homogeneity tests (Pettitt, SNHT, and Buishand) and is denoted by vertical lines. Averages are shown by “mu”. The yellow shaded parts underscore the highest average for each time series.
The composite sequence, termed CPF (z-scores), is detailed with its long-term trend (3-year smoothing). A sinusoidal model was added (Pvalue < 0.001). The evolution of world population and growth rates (50-year average) are shown. The climate data are European summer temperature anomalies (Luterbacher et al., 2016) and the Palmer Drought Severity Index (Cook et al., 2015). The blue shading highlights the plateau phase in world population. All dates are expressed in years AD.
Climate and plagues
The role of climate in the reemergence of plagues in pre-industrial Europe has been previously debated in the literature (Schmid et al., 2015; Yue and Lee, 2018). Focusing on two time series of annually resolved temperature anomalies (European summer and Northern Hemisphere temperatures: Neukom et al., 2014; Luterbacher et al., 2016), it appears, in each case, that plague outbreaks mostly coincided with colder periods (Fig. 3 and Supplementary Fig. 2). The cross-correlations indicate a positive link between plague outbreaks and both climate sequences (Pvalue < 0.001) but with a higher correlation for the reconstruction based on Northern Hemisphere temperature anomalies. While variations in temperatures seem to have influenced the scourges, we find that precipitation anomalies (Cook et al., 2015) are not significant in explaining the plague data. Figure 4 summarizes (i) the link between the increase in conflicts and the rise of plague incidences in Europe, (ii) the role of cooler temperatures in the development of the plague, and (iii) the impact of conflicts and plagues on fatalities. The wavelet analyses (Fig. 5) demonstrate that the time series are characterized by equal periodicities, namely statistically significant cycles of 55 and 25 years. The associated Mantel scalograms show that while temperature anomalies are clearly associated with the CPF, drought is not a significant factor in explaining plague incidences (Figs. 3 and 5).
The climate data are Northern Hemisphere temperature anomalies (Neukom et al., 2014). Linear and polynomial models, as well as a smoothing, were added for each curve.
Wavelet transforms (scalograms) are shown for each time series. The main cycles are indicated on each graph. The cone of influence is depicted as a gray line, and the significance level (P = 0.05) as white lines. The climate data derived from the European summer temperature anomalies (Luterbacher et al., 2016) and the Palmer Drought Severity Index (Cook et al., 2015). The main zone of correlation between the time series is defined by the Mantel scalograms (distance measure: Bray–Curtis) and highlighted by yellow shading. All dates are expressed as years AD.
Discussion
Geography of population movements
In AD 1343–1347, the siege of Caffa (present-day Feodosia, Crimea) illustrates the devastating effect of the conflict–plague alliance. Caffa was the main port for Genoese merchant ships (Gardiner, 1995) and, in AD 1343, the Mongols/Tartars besieged the city (Dols, 1977). With the plague decimating the Mongol armies, they ordered the corpses to be placed in catapults and launched into the city, hoping that the disease would subsequently spread intra muros (narrative of Gabriele De’ Mussi from AD 1343–1346). The plague was transmitted to Europeans by this stream of sick corpses inside Caffa. Italians fleeing the city brought the plague to Mediterranean ports (Wheelis, 2002). For instance, the plague first broke out at Messina in October 1347 AD. Three major centers of Mediterranean contagion then developed: Sicily, Genoa, and Venice (Nolan, 2006). This view was recently revised with the addition of the Venetian community of Tana, which may have played an equally important role in the transmission of the plague as the Genoese colony of Caffa (Barker, 2021). Later, during the English Civil Wars (AD 1639–1651) outbreaks of plague in AD 1644 and 1645 largely decimated armies and cities (Slack, 1990; Jennings, 2003). Disease, and in particular typhus and plague, killed more of the local population than the fighting due to the displacement of civilians (Jennings, 2003). During the Great Northern War (1700–1721 AD), a severe plague epidemic, peaking between 1708 and 1712 AD, ravaged the Baltic region (Frandsen, 2010). Most probably introduced via Constantinople, the plague first spread to southern Poland (Pińczów), and was then introduced by the army and traders into the Baltic Sea. The plague reached the Baltic coast of Prussia in AD 1709. It spread and affected all parts of the Baltic Sea in AD 1711, reaching Hamburg in AD 1712. The plague caused many deaths in the army as well as the depopulation of towns and rural areas due to the fleeing of civilians (Frandsen, 2010). In AD 1799, during the capture of Jaffa fortress (present-day Tel Aviv, Israel), Napoleon Bonaparte’s troops contracted the bubonic plague. Following Jaffa, Napoleon Bonaparte expected to defeat the Turks at Acre (present-day Acre, Israel). While Bonaparte’s army was defeated, the plague, which had accompanied the French from Jaffa, persisted, spread, and was introduced at Acre (Harris, 2006).
Our study paints a more complete picture of the devastation wrought by conflicts and plagues. From the Late Medieval to Early Modern Era, Europe and neighboring countries were hit by several waves of conflicts (Fig. 2 and Supplementary Fig. 3), causing peaks in mortality and disease. This promoted the spread of plagues in both military and civilian populations, a trend further accentuated by a growing population density in Europe during the 15th century AD (Fig. 3) and by population displacements far beyond the initial zones of hostility. Europe suffered from the European wars of religion, but also from revolts, territorial ambitions, and great power conflicts (Nolan, 2006). The same occurred in Eurasia, before and during the Ottoman Empire (Sugar, 1977; Aksan, 2015; Varlik, 2015). Civilian populations were significantly affected because armies tended to devastate civilian areas in an effort to feed themselves, causing food shortages, deplorable sanitary conditions, diseases, and population displacement (Ramsey, 2016). This phenomenon was exacerbated by the increasing length of conflicts, such as the Thirty Years’ War (Parker, 1997) and the Eighty Years’ War (Israel, 1995), which played out in areas subjected to repeated devastation. It led to more populations being affected by conflicts, with a greater health impact and more diseases. The wars of this era were considered to be amongst the most lethal before the modern period (Ramsey, 2016). This phase was devastating because it also corresponds to the start of the widespread use of gunpowder and firearms (sometimes called “Gunpowder Empires”; McNeill, 1993). These new weapons caused greater devastation on civilians and forced populations to flee the zones of hostility (Streusand, 2011).
Throughout history, it has been shown that, in most conflicts, infectious diseases kill more soldiers and civilians than weapons (Wilson, 1995). Conflicts themselves can act as vectors of epidemics, as they result in mixing of both military and civilian populations, increasing the probability of disease (Smallman-Raynor and Cliff, 2004). Wars displace large population masses that flee conflict zones in search of refuge in more geopolitically stable areas (Kendall et al., 2013). These populations are potentially vectors of infectious diseases which can spread in host areas (Kett, 2005; Degomme and Guha-Sapir, 2010).
Spread of plagues
For the period AD 1450–1670, recurrent plague outbreaks may be explained by other scenarios. It has been suggested that reintroductions of the bacterium into European ports occurred from reservoirs located in Asia, with a delay of 15 ± 1 years, and that no permanent plague reservoirs persisted inside Europe (Schmid et al., 2015). This concept has recently been challenged, suggesting that natural reservoirs may also have been located within Europe, in the southern Alpine region (Carmichael, 2014). Rasmussen et al. (2015) has also shown that the early branching of Y. pestis appeared 5783 years ago and that the ancestor of Y. pestis strains was widely distributed across Eurasia from the Bronze Age onwards. Large-scale population movements (Allentoft et al., 2015; Haak et al., 2015) may have facilitated the first plague outbreaks (Rasmussen et al., 2015).
A second and complementary hypothesis suggests that the spread of plagues, once the bacterium reached Europe, occurred via major trade routes which maintained sufficient contagion density to sustain plague transmission (Yue et al., 2016). In addition to these hypotheses, our analysis suggests that conflicts, through population displacements from war-afflicted areas or through a plague-endemic region, favored the spread of plagues via major but also secondary routes. This scenario could explain how peripheral towns were affected by plagues (shown in Supplementary Fig. 3), and why fatalities and the plateau in world population growth are both correlated with conflicts and plague outbreaks (Figs. 3 and 4).
A final hypothesis is that Europe was primed for plague outbreaks (De Witte, 2015). Food crises were ubiquitous in Europe because of a population boom set against the backdrop of land shortages. Europe was ravaged by the Great Famine (AD 1315–1317), resulting from both torrential rains that ruined harvests and institutional factors, including market failure, and a disproportionate allocation of crop resources across different social strata (De Witte and Slavin, 2013). Famine strongly discriminated between social echelons, affecting mainly peasants and urban paupers. Other crises, such as the Great Bovine Pestilence in England (AD 1319–1320; Slavin, 2012), aggravated the situation. The gap between the poor and the elite was huge, with significant disparities in diets; peasant lives were extremely difficult. Food shortages, malnutrition, and degrading sanitary conditions paved the way for recurrent plagues.
While the usual mode of transmission of the bubonic plague to humans is via black rats (Rattus rattus) that host infective fleas (Kugeler et al., 2015), alternative modes include either pneumonic transmission (Kool, 2005) or via an intermediate human ectoparasite vector (e.g. human body louse or human fleas; Little, 2007). The pneumonic mode results from secondary involvement in bubonic cases after the spread of plague bacilli to the lungs before direct transmission (Pechous et al., 2016). The third mode of transmission, septicemic, was quite rare but highly fatal, killing within hours, before buboes had time to form (Cusack, 2019). Transmission of the bacilli also involved fleabite and produced fatal sepsis by different routes: through the lymphatic system, directly through the circulatory system, or both (Sebbane et al., 2006). The spread of conflict-linked plagues would advocate in favor of the pneumonic or human body louse/flea transmission of the bacilli. While the black rat population has been proposed as a plague reservoir in harbor cities (Keeling and Gilligan, 2000), via the fleas that it hosts (Xenopsylla cheopis and Nosopsyllus fasciatus), its role in the transmission of plague is debated (Davis, 1986; Hufthammer and Walløe, 2012). Rats were absent in large areas of northern Europe during the second plague pandemic and plague outbreaks often peaked when temperature and humidity were unfavorable for the arthropod vectors of plague to proliferate (Cohn, 2008). This would have hampered the rapid dissemination of the disease (Schmid et al., 2015). A spread from person-to-person therefore seems more plausible.
Cold climate
Another component that seems to have favored the persistence and recurrent spread of plagues is climate instability before and during the Little Ice Age (Neukom et al., 2014; Figs. 3 and 4 and Supplementary Fig. 2). It has been shown that warmer and wetter conditions favored the flea burden and host abundance (black rats or great gerbil) in the case of bubonic plague (Stenseth et al., 2006). While regional conditions may have promoted the second plague outbreak in Central Asia (Stenseth et al., 2006), the “pre” Little Ice Age (AD 1300–1550) was a wetter and cooler period in both Europe and Eurasia (Jones et al., 2001, 2006; Griggs et al., 2007; Kaniewski et al., 2011; Xoplaki et al., 2018; Lüning et al., 2019). Because rat/gerbil and flea blooms are favored by climate conditions not recorded from Eurasia to Europe (Lionello, 2012; Kushnir and Stein, 2019), and as rats were scarce in northern Europe during the plague outbreaks (Davis, 1986), the epidemics may have mostly spread via human-to-human transmission. Previous studies have mainly demonstrated that climate change has important health-related consequences because it can weaken afflicted populations by inducing food shortages, malnutrition, and even starvation (McMichael, 2012; Patz et al., 2000). These factors can alter human–microbe relationships and promote the (re)emergence of infectious diseases, stress, and respiratory illnesses. Climate change did not initiate the emergence of the scourge during the period AD 1450–1670, but our data analysis suggests that it favored subsequent outbreaks by weakening European populations before and during the Little Ice Age. The “extreme wet years” in Europe and Eurasia during the pre-Little Ice Age had a strong impact on harvests. Fluctuations in harvests are clearly linked to environmental factors, mainly an increase in rainfall regime. In southern England, the lowest grain and straw yields are observed in wet-cold as well as in wet-warm years, whereas the highest grain yields are more frequent in dry or cold years and the highest straw yields in dry or warm years (Chmielewski, Potts, 1995). Kettlewell et al. (2003) have also noted that high summer precipitation in England and Wales leads to low grain growth. The link between heavy rain and poor harvests was evoked by Beveridge (1921, 1922) as early as the 1920s, who clearly showed a correlation between the rise in grain prices and increasing precipitation in England, France, Belgium, Germany, and Austria. In ancient China, Tian et al. (2017) have suggested that cooler climate conditions indirectly accentuated the prevalence of epidemics through famines during the Little Ice Age. Munzar (1995) describes a similar situation in Finland for the years AD 1695–1697. Food crises also engulfed Europe at the same time (Appleby, 1980). Our data support previous research on temperature anomalies (Schmid et al., 2015; Yue and Lee, 2018) and imply that colder conditions and conflicts affected food-production systems and human nutrition. Furthermore, colder conditions engendered indoor crowding and a greater likelihood of human-to-human infection, either through the medium of the human louse/flea or via the pneumonic form (Little, 2007). It is worth noting that, between the 1400s and 1800s, Europe was one of the most densely populated and urbanized areas on the planet (Klein Goldewijk et al., 2010).
War–plague alliance
Our study, based on both short and long-term trends, suggests that warfare significantly transformed Europe’s human–microbe environments, and the spread of infectious disease. It is clear that conflicts may have fostered the spread of infectious diseases and, in turn, pandemics may have led to social unrest leading to conflict. All of these factors were juxtaposed and constantly influencing each other. The devastating war–plague alliance led to a strong increase in fatalities producing a plateau in world demographics. Conflicts seem to have favored the spread of plagues by the movement of military troops and by pushing civilian populations to flee in unsanitary conditions. The climate of the pre-Little Ice Age and the Little Ice Age further reinforced outbreaks due to its impact on food resources—generating malnutrition that weakened the human immune system—and on the congregation of people indoors. Plagues caused by Y. pestis have greatly reduced or disappeared thanks to the invention of quarantine and efforts to develop prevention and intervention measures, namely improved hygiene and sanitation (Bramanti et al., 2019). In Europe, the disease was superseded by smallpox (orthopox virus variola—VARV; Li et al., 2007; Duggan et al., 2016). For many centuries, smallpox devastated mankind, causing significant deaths (Behbehani, 1983; Geddes, 2006; Davenport et al., 2018). The bubonic plague has not fully disappeared and is still active in some countries (cf. Madagascar, Democratic Republic of Congo and Peru).
Concluding remarks
The 2019/2020 coronavirus pandemic has highlighted how the geography of population movements can drive the rapid escalation of infectious diseases. Within this context, recent events in Syria and Yemen show that warfare creates opportunities for pathogenic microbes to spread rapidly in displaced human populations (Abbara et al., 2020; Daw, 2020). Nonetheless, the role of armed conflict in driving recurrent plague outbreaks in the Late Middle Ages to Early Modern Age of Europe, one of the most violent in the continent’s history, is largely unknown despite its potential importance in constraining current human–microbe models of war-affected regions. Previous studies have underscored the absence of plague reservoirs in medieval Europe and have emphasized climate deterioration as a possible driver for the re-emergence of outbreaks. They have also highlighted the role of terrestrial and maritime trade routes in spreading historical outbreaks of the plague.
To the best of our knowledge, this study is the first to assess the role of conflicts in promoting the spread of plagues in pre-industrial Europe. First, we demonstrate that conflicts, plagues, and fatalities were significantly correlated during the period AD 1450–1670. Second, we find that the latter period corresponds to a plateau in world population figures. Finally, we suggest that the cooler climate conditions of the Little Ice Age affected food resources, weakening immune responses in populations through malnutrition and stress.
While recent research has focused on the role of warfare in promoting the emergence of epidemics in refugee camps, the effects of historical conflicts on human health are largely unexplored. Our study, based on high-resolution historical data from pre-industrial Europe, finds that recurrent conflicts between AD 1450 and 1670 entrained the displacement of military troops and civilian populations that acted as key vectors for disease transmission. This historical perspective reinforces the fundamental need for good healthcare in war-affected populations, in order to circumvent epidemics. Our study also demonstrates that there is potentially much to learn from the past regarding infectious disease diffusion and that such data are key to calibrating current quantitative models. These data are finally a warning for present-day overpopulation, global warming, geopolitical tensions, the constant spread of industry, escalating poverty, and our compromised diets.
Methods
We collated data for European plagues, conflicts, and climate covering the end of the medieval period to the onset of the Modern Era.
Dataset conflicts
The definition of conflicts encompasses all events that can cause population displacements, from wars to revolts, including civil wars, insurgencies, rebellions, or battles. The data for conflicts mainly derives from the Conflict Catalog compiled and curated by P. Brecke (2020). The datasets were started to be compiled in 1998 (Brecke, 1998). All conflicts were sorted by date, duration, and cities/regions/states/countries engaged in war. We summed events by year to create a linear time series covering the period AD 1347–1840 (Fig. 1). While conflicts may be missing, this dataset was controlled and used to detect general trends, to avoid uncertainties or misinterpretations.
Dataset plagues
Plagues in this study correspond to all the recorded epidemics caused by the pathogen Y. pestis (Bos et al., 2011). The initial dataset originates from the work of Biraben (1976). This seminal dataset was digitized (Atanasiu et al., 2008; Büntgen et al., 2012; Voigtländer and Voth, 2013) and improved with the additions from Russia, Constantinople, and Turkey (Schmid et al., 2015). We used this improved dataset for plague outbreaks. It has been shown that this dataset does not fully capture all historical plague activity across Europe, and that Biraben’s dataset has led to an important overestimation of plagues in cities and an underestimation in towns and villages (Rosen and Curtis, 2018). According to Rosen and Curtis (2018), the data collected by Biraben only denote the availability of sources mentioning plague and not the severity or pervasiveness of the disease in any given year. To avoid misinterpretation, we focus on overall trends, not on individual events that may be poorly expressed in the time series.
Dataset fatalities
Fatalities were gleaned from the Conflict Catalog (Brecke, 1998). We summed events by year to create a linear time series covering the period AD 1347–1840. We converted this dataset into fatalities ratioed to world population. The resulting time series was log transformed. The world population data were obtained from the United States Census Bureau (United States Census Bureau, 2020). We converted this initial matrix into an annually resolved dataset. Climate time series for the period AD 1347–1840 were obtained from three sources (Neukom et al., 2014; Cook et al., 2015; Luterbacher et al., 2016).
Statistical analyses
All data were analyzed using Xl-Stat2017 and PAST, version 2.17c. A simple smoother (smoothing transform with moving average as basic function; smoothing 9-year and 31-year) was first applied to assess long-term trends in conflicts, plague outbreaks, and fatalities (Fig. 1). Mantel scalograms (distance measure: Bray–Curtis), based on smoothed series (3-year smoothing), were subsequently used to test the similarities between the time series (Fig. 1). The apex of the triangle is the similarity between the first and last point. The base of the triangle shows similarities between pairs of consecutive points (Hammer and Harper, 2006).
We then assessed all datasets (Fig. 2) for long-term trends (3-year smoothing). Three homogeneity tests (Pettitt, SNHT, and Buishand) were applied to the time series to detect shifts in long-term dynamics. Each discordant period was categorized and its average denoted by “mu”.
We z-score transformed the time series and created a composite sequence (termed CPF) based on the sum of conflicts, plague incidences, and fatalities (Fig. 3). The results are shown with the full dataset and a long-term trend (3-year smoothing). A sinusoidal model was then applied to detect the long-term periodicities (Pvalue < 0.001). In tandem, we calculated growth rates for world population (Fig. 3), using a 50-year average (with standard deviation). The data are based on a subtraction between each consecutive data point and these differences were used to calculate 50-year averages. Climate proxies were transformed into long-term trends and a sinusoidal signal (Pvalue < 0.001) was fitted (Fig. 3).
To analyze the relationships between conflicts, plagues, fatalities, and temperature anomalies, we sorted the time series by ascending values (for the x-axis) and plotted the resulting curves with the standard error for each data point (Fig. 4). We added the results of the linear and polynomial models for each curve. A smoothing function was subsequently applied (Fig. 4).
We tested the periodicity of each time series (Fig. 5) using wavelet analyses (with Morlet as the basis function). Mantel scalograms (distance measure: Bray–Curtis; smooth 3-year) were then employed to test the similarities between the composite sequence (conflicts, plagues, and fatalities) and the climate proxies (Fig. 5).
Data availability
All the data are available at https://data.mendeley.com/datasets/ysrtx8478v/1.
References
Abbara A, Rayes D, Fahham O et al. (2020) Coronavirus 2019 and health systems affected by protracted conflict: the case of Syria. Int J Infect Dis 96:192–195
Aksan VH (2015) Ottoman wars, 1700–1870: an empire besieged. Routledge, London and New York
Allentoft ME, Sikora M, Sjögren KG et al. (2015) Population genomics of Bronze Age Eurasia. Nature 522:167–172
Appleby AB (1980) Epidemics and famine in the Little Ice Age. J Interdiscip Hist 10:643–663
Atanasiu V, Priol C, Tournieroux A et al (2008) E. Yersinia. Georeferences for places of plague occurrence in Europe 1347–1600. www.bernstein.oeaw.ac.at/atlas/yersinia-description.pdf
Banerjee S (2019) Towards a quantitative model of epidemics during conflicts. INDECS 17:598–614
Barker H (2021) Laying the corpses to rest: grain, embargoes, and Yersinia pestis in the Black Sea, 1346–1348. Speculum 96: https://doi.org/10.34055/osf.io/rqn8h
Bedford J, Enria D, Giesecke J et al (2020) COVID-19: towards controlling of a pandemic. Lancet. https://doi.org/10.1016/S0140-6736(20)30673-5
Behbehani AM (1983) The smallpox story: life and death of an old disease. Microbiol Rev 47:455–509
Benedictow OJ (2006) The Black death, 1346–1353: the complete history. Boydell Press, Woodbridge
Beveridge WH (1921) Weather and harvest cycles. Econ J 31:429–452
Beveridge WH (1922) Wheat prices and rainfall in Western Europe. J R Stat Soc 85:412–475
Biraben JN (1976) Les hommes et la peste en France et dans les pays européens et méditerranéens. Mouton, Paris
Bolton J (1996) The world upside down: plague as an agent of economic and social change. In: Ormrod M, Lindley P (eds) Black Death in England, 1348–1500, 2nd edn. Paul Watkins Publishing, Stamford, pp. 17–78
Bos KI, Schuenemann VJ, Golding GB et al. (2011) A draft genome of Yersinia pestis from victims of the Black Death. Nature 478:506–510
Bramanti B, Dean KR, Walløe L et al. (2019) The third plague pandemic in Europe. Proc R Soc B 286:20182429. https://doi.org/10.1098/rspb.2018.2429
Brecke P (1998) Finding harbingers of violent conflict: using pattern recognition to anticipate conflicts. Confl Manag Peace Sci 16:31–56
Brecke P (2020) Conflict Catalog 18 vars. https://brecke.inta.gatech.edu/research/conflict/
Büntgen U, Ginzler C, Esper J et al. (2012) Digitizing historical plague. Clin Infect Dis 55:1586–1588
Carmichael AG (2014) Plague persistence in Western Europe: a hypothesis. In: Green MN (ed) The Medieval Globe 1: pandemic disease in the medieval world rethinking the Black Death. ARC Medieval Press, Kalamazoo, Bradford, pp. 157–192
Chinazzi M, Davis JT, Ajelli M et al (2020) The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. https://doi.org/10.1126/science.aba9757
Chmielewski FM, Potts JM (1995) The relationship between crop yields from an experiment in southern England and long-term climate variations. Agric For Meteorol 73:43–66
Cohn SK (2008) Epidemiology of the Black Death and successive waves of plague. Med Hist Suppl 27:74–100
Cook ER, Seager R, Kushnir Y et al. (2015) Old World megadroughts and pluvials during the Common Era. Sci Adv 1:e1500561
Cusack CM (2019) Death, famine, war, and conquest: the Black Death, the hundred years’ war, and popular revolt in Barbara W. Tuchman’s ‘calamitous fourteenth century’. Lit Aesthet 29:141–160
Davenport RJ, Satchell M, Shaw-Taylor LMW (2018) The geography of smallpox in England before vaccination: a conundrum resolved. Soc Sci Med 206:75–85
Davis DE (1986) The scarcity of rats and the Black Death: an ecological history. J Interdiscip Hist 16:455–470
Daw MA (2020) Corona virus infection in Syria, Libya and Yemen; an alarming devastating threat. Travel Med Infect Dis. https://doi.org/10.1016/j.tmaid.2020.101652
Degomme O, Guha-Sapir D (2010) Patterns of mortality rates in Darfur conflict. Lancet 375:294–300
De Witte SN (2015) Setting the stage for medieval plague: pre-Black Death trends in survival and mortality. Am J Phys Anthropol 158:441–451
De Witte SN, Slavin P (2013) Between famine and death: England on the eve of the Black Death—evidence from paleoepidemiology and manorial accounts. J Interdiscip Hist 44:37–60
Dols MW (1977) The Black Death in the Middle East. Princeton University Press, Princeton
Dols MW (1979) The second plague pandemic and its recurrences in the Middle East: 1347–1894. J Econ Soc Hist Orient 22:162–189
Duffy J (1977) Review of Alfred W. Crosby, epidemic and peace, 1918. J Hist Med Allied Sci 32:92
Duggan AT, Perdomo MF, Piombino-Mascali D et al. (2016) 17th century variola virus reveals the recent history of smallpox. Curr Biol 26:1–6
Franco-Paredes C (2020) Albert Camus’ the Plague revisited for COVID-19. Clin Infect Dis 71:898–899
Frandsen KE (2010) The last plague in the Baltic Region, 1709–1713. Museum Tusculanum Press, Copenhagen
Gardiner R (1995) The Age of the galley: Mediterranean oared vessels since pre-classical times. Naval Institute Press, Annapolis
Geddes AM (2006) The history of smallpox. Clin Dermatol 24:152–157
Griggs C, DeGaetano A, Kuniholm P et al. (2007) A regional high-frequency reconstruction of May–June precipitation in the north Aegean from oak tree rings, A.D. 1089–1989. Int J Climatol 27:1075–1089
Haak W, Lazaridis I, Patterson N et al. (2015) Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522:207–211
Hammer O, Harper D (2006) Paleontological data analysis. Blackwell Publishing, Oxford
Harris JC (2006) Napoleon Bonaparte visiting the plague-stricken at Jaffa. Arch Gen Psychiatry 63:482–483
Hawkins D (1990) The Black Death and the new London cemeteries of 1348. Antiquity 60:637–642
Hays JN (2005) Epidemics and pandemics: their impacts on human history. ABC-CLIO, Santa Barbara
Herlihy D (1997) The Black Death & the transformation of the west. Harvard University Press, Cambridge
Hufthammer AK, Walløe L (2012) Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archaeol Sci 40:1752–1759
Israel J (1995) The Dutch Republic: its rise, greatness, and fall 1477–1806. Clarendon Press, Oxford
Jennings SB (2003) ‘A miserable, stinking, infected town’: pestilence, plague and death in a civil war garrison, Newark 1640–1649. Midl Hist 28:51–70
Jones PD, Osborn TJ, Briffa KR (2001) The evolution of climate over the last millennium. Science 292:662–667
Jones PD, Roberts CN, Leng MJ et al. (2006) A high-resolution late Holocene lake isotope record from Turkey and links to North Atlantic and monsoon climate. Geology 34:361–364
Kaniewski D, Van Campo E, Paulissen E et al. (2011) The Medieval Climate Anomaly and the Little Ice Age in coastal Syria inferred from pollen-derived palaeoclimatic patterns. Glob Planet Change 78:178–187
Keeling MJ, Gilligan CA (2000) Metapopulation dynamics of bubonic plague. Nature 407:903–906
Kendall EJ, Montgomery J, Evans JA et al. (2013) Mobility, mortality, and the Middle Ages: identification of migrant individuals in a 14th century Black Death cemetery population. Am J Phys Anthropol 150:210–222
Kett M (2005) Displaced populations and long-term humanitarian assistance. BMJ 331:98–100
Kettlewell PS, Stephenson DB, Atkinson MD et al. (2003) Summer rainfall and wheat grain quality: relationships with the North Atlantic Oscillation. Weather 58:1–9
Klein Goldewijk K, Beusen A, Janssen P (2010) Long term dynamic modeling of global population and built-up area in a spatially explicit way: HYDE 3.1. Holocene 20:565–573
Kool JL (2005) Risk of person-to-person transmission of pneumonic plague. Clin Infect Dis 40:1166–1172
Kugeler KJ, Staples JE, Hinckley AF et al. (2015) Epidemiology of human plague in the United States, 1900 to 2012. Emerg Infect Dis 21:16–22
Kushnir Y, Stein M (2019) Medieval climate in the Eastern Mediterranean: instability and evidence of solar forcing. Atmosphere 10:29. https://doi.org/10.3390/atmos10010029
Leaning J, Guha-Sapir D (2013) Natural disasters, armed conflict, and public health. N Engl J Med 369:1836–1842
Li Y, Carroll DS, Gardner SN et al. (2007) On the origins of smallpox: correlating variola phylogenics with historical smallpox records. Proc Natl Acad Sci USA 104:15787–15792
Lionello P (ed) (2012) The climate of the Mediterranean region. From the past to the future. Elsevier, London
Little LK (ed) (2007) Plague and the end of antiquity. Cambridge University Press, Cambridge
Lüning S, Schulte L, Garcés S et al. (2019) The Medieval climate anomaly in the Mediterranean region. Paleoceanogr Paleoclimatol 34:1625–1649
Luterbacher J, Werner JP, Smerdon JE et al. (2016) European summer temperatures since Roman times. Environ Res Lett 11:024001. https://doi.org/10.1088/1748-9326/11/2/024001
McMichael AJ (2012) Insights from past millennia into climatic impacts on human health and survival. Proc Natl Acad Sci USA 109:4730–4737
McNeill WH (1993) The age of gunpowder empires, 1450–1800. In: Adas M (ed) Islamic and European expansion: the forging of a global order. Temple University Press, Philadelphia, pp. 103–139
Mordechai L, Eisenberg M, Newfield TP et al. (2019) The Justinianic plague: an inconsequential pandemic? Proc Natl Acad Sci USA 116:25546–25554
Munzar J (1995) The “cold-wet” famines of the years 1695-1697 in Finland and manifestations of the Little Ice Age in Central Europe. In: Heikinheimo P (ed) International conference on past, present and future climate. Edita, Helsinki, pp. 167–170
Murray CJL, King G, Lopez AD et al. (2002) Armed conflict as a public health problem. BMJ 324:346–349
Neukom R, Gergis J, Karoly D et al. (2014) Inter-hemispheric temperature variability over the past millennium. Nat Clim Change 4:362–367
Nolan CJ (2006) The age of wars of religion, 1000–1650: an encyclopedia of global warfare and civilization. Greenwood Press, London
Norris J (1977) East or west? The geographic origin of the Black Death. Bull Hist Med 51:1–24
Pamuk S (2007) The Black Death and the origins of the ‘Great Divergence’ across Europe, 1300–1600. Eur Rev Econ Hist 11:289–317
Parker G (1997) The thirty years’ war, 2nd edn. Routledge, London and New York
Patz JA, Engelberg D, Last J (2000) The effect of changing weather on public health. Annu Rev Public Health 21:271–307
Pechous RD, Sivaraman V, Stasulli NM et al. (2016) Pneumonic plague: the darker side of Yersinia pestis. Trends Microbiol 24:190–197
Ramsey S (2016) Tools of war: history of weapons in Early Modern Times. Alpha Edition, Paris
Rasmussen S, Allentoft ME, Nielsen K et al. (2015) Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell 163:571–582
Roberts RS (1966) The place of plague in English history. J R Soc Med 59:101–105
Rosen J, Curtis DR (2018) Dangers of noncritical use of historical plague data. Emerg Infect Dis 24:103–110
Schmid BV, Büntgen U, Easterday WR et al. (2015) Climate-driven introduction of the Black Death and successive plague reintroductions into Europe. Proc Natl Acad Sci USA 112:3020–3025
Sebbane F, Jarrett CO, Gardner D et al. (2006) Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc Natl Acad Sci USA 103:5526–5530
Slack P (1990) The impact of plague in Tudor and Stuart England. Oxford University Press, Oxford
Slavin P (2012) The Great Bovine Pestilence and its economic and environmental consequences in England and Wales, 1318–50. Econ Hist Rev 65(4):1239–1266
Smallman-Raynor MR, Cliff AD (2004) War epidemics: an historical geography of infectious diseases in military conflict and civil strife, 1850–2000. Oxford University Press, Oxford
Spiegel PB, Checchi F, Colombo S et al. (2010) Health-care needs of people affected by conflict: future trends and changing frameworks. Lancet 375:341–345
Stenseth NC, Samia N, Viljugrein H et al. (2006) Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA 103:13110–13115
Streusand DE (2011) Islamic gunpowder empires: Ottomans, Safavids, and Mughals. Westview Press, Philadelphia
Sugar PF (1977) Southeastern Europe under Ottoman rule, 1354–1804. University of Washington Press, Seattle and London
The UN Refugee Agency (1995) The state of the world’s refugees 1995: in search of solutions. https://www.unhcr.org/publications/sowr/4a4c70859/state-worlds-refugees-1995-search-solutions.html
The UN Refugee Agency (2016) Global trends forced displacement in 2016. https://www.unhcr.org/statistics/unhcrstats/5943e8a34/global-trends-forced-displacement-2016.html
The UN Refugee Agency (2018) Global trends forced displacement in 2018. https://www.unhcr.org/5d08d7ee7.pdf
Tian H, Yan C, Xu L et al. (2017) Scale-dependent climatic drivers of human epidemics in ancient China. Proc Natl Acad Sci USA 114:12970–12975
United States Census Bureau. Historical estimates of world population (2020) https://www.census.gov/data/tables/time-series/demo/international-programs/historical-est-worldpop.html
Varlik N (2015) Plague and empire in the Early Modern Mediterranean world. The Ottoman experience, 1347–1600. Cambridge University Press, Cambridge
Voigtländer N, Voth HJ (2013) The three horsemen of growth: plague, war and urbanization in Early Modern Europe. Rev Econ Stud 80:774–811
Wagner DM, Klunk J, Harbeck M et al. (2014) Yersinia pestis and the Plague of Justinian 541–543 AD: a genomic analysis. Lancet Infect Dis 14:319–326
Wheelis M (2002) Biological warfare at the 1346 siege of Caffa. Emerg Infect Dis 8:971–975
Wilson ME (1995) Infectious diseases: an ecological perspective. BMJ 311:1681–1684
Xoplaki E, Luterbacher J, Wagner S et al. (2018) Modelling climate and societal resilience in the Eastern Mediterranean in the Last Millennium. Hum Ecol 46:363–379
Yue RPH, Lee HF. Pre-industrial plague transmission is mediated by the synergistic effect of temperature and aridity index. BMC Infect Dis 18:1342018. https://doi.org/10.1186/s12879-018-3045-5
Yue RPH, Lee HF, Wu CYH (2016) Navigable rivers facilitated the spread and recurrence of plague in pre-industrial Europe. Sci Rep 6:34867. https://doi.org/10.1038/srep34867
Acknowledgements
The University Paul Sabatier-Toulouse 3 and the MITI-CNRS Eco-Urbain 2019–2020 (UrbanMed program) funded this research.
Author information
Authors and Affiliations
Contributions
D.K. and N.M. gathered the data, performed the analysis, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaniewski, D., Marriner, N. Conflicts and the spread of plagues in pre-industrial Europe. Humanit Soc Sci Commun 7, 162 (2020). https://doi.org/10.1057/s41599-020-00661-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1057/s41599-020-00661-1
This article is cited by
-
In Sickness and in Health
Science & Education (2022)