Abstract
Home use tests to monitor hormone trends during the menstrual cycle have been available over-the-counter for a long time. However, these tests often depend upon manual readouts and hence may lead to false analysis. Furthermore, a lot of these tests are also not quantitative. The aim of this study was to evaluate the accuracy of the quantitative home-based fertility monitor, Inito Fertility Monitor (IFM) and to use it to identify novel hormone trends in natural menstrual cycles. There were two aspects to our analysis: (i) Evaluating the efficacy of Inito Fertility Monitor in the measurement of urinary Estrone-3-glucuronide (E3G), Pregnanediol glucuronide (PdG) and Luteinizing hormone (LH), and (ii) A retrospective study of patients' hormone profiles using IFM. To evaluate the efficacy, the recovery percentage of the three hormones from IFM was evaluated using standard spiked solutions, the accuracy of measurement was calculated and the correlation between reproducible values from IFM and ELISA was established. During the validation of IFM, novel hormone trends were also observed. In order to reinforce the observations, a second group of 52 women was recruited. Assessment of the accuracy of IFM and evaluation of the volunteer urine samples was performed in a laboratory. Home assessment of hormone analysis was carried out using IFM. For the validation study, 100 women aged 21–45 years with cycle lengths ranging from 21 to 42 days were recruited. The participants had no previously diagnosed infertility conditions and their cycle lengths did not vary for more than 3 days from the expected cycle length. Daily first morning urine samples were collected from these 100 women. For the second group, 52 women were selected meeting the same criteria set for the validation study and IFM was provided to these women for testing at home. Coefficient of variation and recovery percentage of IFM with respect to laboratory based ELISA. Percentage occurrence of novel hormone trends and AUC analysis of a novel criteria identified for confirming ovulation. We observed that with all three hormones, IFM had an accurate recovery percentage. We found that the assay has an average CV of 5.05% in PdG measurement, 4.95% in E3G measurement and 5.57% in LH measurement. Furthermore, in predicting the concentration of E3G, PdG and LH in urine samples, we show that IFM has a high correlation with ELISA. In this study, we could also reproduce hormones trends across the menstrual cycle that have been observed by previous studies. We also identified a novel criterion for earlier confirmation of ovulation which could accurately distinguish ovulatory from anovulatory cycles with 100% specificity and had an area under the ROC curve of 0.98. In addition, we identified a new hormone trend which could be observed in 94.5% of the ovulatory cycles. The Inito Fertility Monitor is an effective tool for calculating the urinary concentrations of E3G, PdG and LH and can also be used to provide accurate fertility scores and confirm ovulation. We show that certain hormone trends associated with urinary E3G, PdG and LH could be accurately captured using IFM. In addition, we report a novel criterion for earlier confirmation of ovulation compared to existing criteria. Finally, we present a novel hormone pattern associated with most of the menstrual cycles by examining hormone profiles from the volunteers recruited for the clinical trial.
Trial registration: The trial is registered at the current controlled trials ISRCTN registry #ISRCTN15534557.
Similar content being viewed by others
Introduction
For couples seeking to conceive, the timing of intercourse is a critical question. Almost 45–50 percent of women do not know their fertile window1,2. Calendar based methods cannot accurately predict the fertile window. During the fertile window, having intercourse increases the likelihood of becoming pregnant3. Based on different factors, such as age, diet, genetic predisposition and any infertility disorder, the fertile window may vary.
The pituitary hormones, FSH and LH, and steroid hormones, Estrogen and Progesterone, regulate follicle formation, egg release and uterine preparation for implantation. The predictable behavior of these hormones in a regular menstrual cycle can be exploited to forecast fertile days (Fig. 1a). Lateral flow assay based luteinizing hormone tests at home have widely been used to estimate fertile days. These tests, however, provide a window of 1–2 fertile days(s) near the ovulation day. Previous studies have concluded that the fertile window usually lasts for 6 days and involves the ovulation date4,5,6. Measuring estrone-3-glucuronide (E3G) along with LH can potentially boost the fertility window from 2 to 6 days. Predicting the fertility status by evaluating the levels of LH and oestrogen is also highly correlated with transvaginal ultrasound ovulation prediction7. Fertile window estimation, however, is not a guarantee of ovulation. In fact, about 26–37% of natural cycles are anovulatory8. This may lead to frustration for couples trying to conceive. Therefore, the confirmation of ovulation is sought after by both clinicians and patients and an additional measurement of Pregnanediol-3-glucuronide (PdG) can be used to confirm ovulation9,10.
Behaviour of urinary hormones E3G, PdG and LH in a normal menstrual cycle and the prediction of high fertility, peak fertility and ovulation confirmation based on hormone levels (a), location of testing regions in the Inito fertility test strips (b) and schematic of usage of the Inito Fertility Monitor at home (c).
Women use many home-use tests to predict fertile days in the menstrual cycle. Canonical over-the-counter ovulation test kits only assess urinary LH and thus skip a series of fertile days. Other home-use devices available on the market forecast fertile days by tracking LH and E3G. Getting a full fertile window can increase the chances of conception by around 89%3. These home-use techniques, however, have low sensitivity and a narrow detection range. Most of these tests are either visual or provide a binary digital result (positive or negative)26,27. However, the hormone patterns are more complex and need quantification. Furthermore, physicians and users are keen on visualizing their hormone trends. Therefore, there’s a need for a more sensitive, quantitative home-based test to account for person-to-person and cycle-to-cycle variability while predicting fertile days in women.
We introduce the Inito Fertility Monitor (IFM) (Fig. 1b,c), a mobile mounted, app-connected home based device that predicts the fertile days and also confirms ovulation by measuring E3G, LH and PdG simultaneously in urine. The test strip for IFM contains two lateral flow assays: one assay is multiplexed to measure E3G and PdG in a competitive ELISA format and the other assay measures LH in a sandwich ELISA format. IFM captures the image of the test strip using the Inito mobile application and processes the image to yield the optical density (OD) which corresponds to the concentration of the metabolite. IFM uses a multi-scale algorithm to detect the device and eliminate the variations in resolution and aspect ratio due to smartphone variability11. A recent study has also shown that urine metabolite measurements by IFM are well correlated with their respective serum hormones30.
In this study, we compare urinary hormone measurements by IFM with laboratory-based ELISA in order to assess the accuracy of reproduced urinary hormone values. We also measure the CV across multiple measurements using the same standard solution in order to investigate the internal variations in IFM. Further, in order to validate measurement from IFM in the context of a menstrual cycle, we compare the data obtained from the volunteers for the study, with previously reported hormone trends. In addition, by observing trends in the urinary PdG rise after the LH peak, we propose a novel criteria to confirm ovulation earlier in women and also evaluate the accuracy of the criteria by ROC analysis as well as by using a test volunteer group. Also, we present a previously unobserved occurrence of PdG rise before the LH surge congruent with progesterone surge before LH reported previously.
Materials and methods
Testing with IFM and estimation of concentration from calibration curve
Test strips were analyzed and qualified using the image processing algorithms and AI algorithms used in Inito Fertility Monitor (Supplementary Method 1). Calibration curve for each batch of Inito Fertility test strips was generated using standard solutions prepared in spiked urine. The ODs obtained from standard solutions were plotted against concentration and this plot was used to reproduce concentrations in all further experiments. For testing, the test strips were dipped in the urine samples for 15 s. The strips were then inserted into the Inito Fertility Monitor attached to mobile and values of E3G, PdG and LH were obtained along with the fertility ratings for each day. In the Inito Fertility test strip, E3G and PdG are measured in a competitive ELISA assay format where the intensity of respective test lines decreases with increasing concentration and LH is measured in a sandwich ELISA format where the intensity of the test line increases with increasing concentration.
Sample preparation for characterization of Inito Fertility Monitor
Samples used for precision studies, linearity of reproduction of concentration and cross-reactivity studies were prepared by spiking male urine samples with target concentrations of the metabolites. The male urine samples were tested with ELISA to confirm negligible concentrations of the respective metabolites. Purified metabolites for all studies were obtained from Sigma-Aldrich chemicals as described in supplementary table 1.
Testing with ELISA kits
The same user samples were also used in an ELISA to measure the exact concentration. E3G and PdG were measured using the Arbor Estrone-3-Glucuronide EIA kit (K036-H5) and the Arbor Pregnanediol-3-Glucuronide EIA kit (K037-H5) ELISA kits. Urinary LH was measured using the DRG LH (urine) ELISA kit (EIA-1290). For all runs, solutions of fixed concentration (provided along with the respective kits) were used to generate the standard curve and the concentration of metabolites in urine samples were calculated from the standard curve generated. All samples were measured in triplicates and the average value was considered for comparison.
Interference analysis
Potential interfering substances were identified. LH (L6420, Sigma Aldrich), E3G (E2127, Sigma Aldrich), PdG (903620, Sigma Aldrich), hCG (230734, Sigma Aldrich), progesterone (P0130, Sigma Aldrich), acetaminophen (A3035, Sigma Aldrich), ascorbic acid (A7506, Sigma Aldrich), caffeine (C0750, Sigma Aldrich), glucose (Y0001745, Sigma Aldrich), ampicillin (A9518, Sigma Aldrich), ketone (179124, Sigma Aldrich), acetylsalicylic acid (A3160, Sigma Aldrich), hemoglobin (ERMAD500, Sigma Aldrich), tetracycline (31741, Sigma Aldrich), nitrite (1.06549, Sigma Aldrich), phenothiazine (46624, Sigma Aldrich), ethanol (02870, Sigma Aldrich) and albumin (A2153, Sigma Aldrich) were obtained and redissolved to prepare final concentrations as per Supplementary Table 4. 120 μL of each interfering agent solution was added to the test strips and the test was observed for presence or absence of a test line as detected by IFM.
Study participants
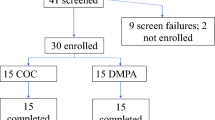
The study design was approved by the Institutional Review Board (IRB) of Sparsh Hospital (EC approval number: CLIN/INI/001). Two groups of women were recruited for the study. The first group of women was shortlisted from a list of students/nurses working in different hospitals near the research facility who volunteered to participate in this trial. The shortlisted participants were further screened and selected based on the exclusion criteria. The second group consisted of women who were looking to try out novel methods for monitoring fertility at home and registered to try Inito at home on the Inito website. Women in the second group were then provided the IFM and the Inito Fertility Test strips to be tested at home. The study protocol was approved by the appropriate institutional review boards (ISRCTN15534557). Informed consent forms were obtained from all volunteers. Women aged between 21 and 45 years of age were recruited for the study.
Women were included in the study based if all of the following criteria were met:
-
1.
Age of the participant must be between 21 and 45 years at the time of registration for the trial.
-
2.
Cycle length of the participants must be between 21 and 42 days.
-
3.
Cycle length of the participants must not vary by more than ± 3 days from one menstrual cycle to the other.
Women were excluded if:
-
a.
They have been diagnosed with any infertility conditions and are on infertility medications or hormone replacement therapy containing hCG or LH.
-
b.
They were using hormonal contraceptives, including oral, emergency oral, implants, patches, transdermal injections, vaginal ring and progesterone intrauterine systems (IUS).
-
c.
Were consuming clomiphene citrate or other ovulation induction drugs.
-
d.
Have recently been pregnant, miscarried or breastfeeding.
Reasons for data exclusion were irregularities in testing leading to insufficient data points for any conclusion and failure to meet the selection criteria. The characteristics of those who were considered for the study are summarized in Table 1.
Research ethics
The study protocol was approved by the Institutional Review Board at Sparsh Hospital, Bangalore. The approval number assigned was CLIN/INI/001. Informed consent forms were obtained from all participants before being enrolled for the study and all studies were carried out in accordance with the Declaration of Helsinki.
Study design
Urine samples collected from women in the first group were frozen, transported to the testing site and tested with the IFM on the same day. Freeze–thaw has been previously shown to have no effect on the concentration of urinary hormones12,13. For the second group, IFM and Inito Fertility test strips were sent to participants to perform the test at home. Standard instructions for use were provided with the IFM box and the test strips. All participants had the option to contact the manufacturer’s helpline number for any additional information concerning the IFM. Women began using the IFM at the start of the menstrual cycle after admission into the study. Participants were instructed to take tests on specific cycle days when instructed by the application. Hormone data from all participants was collected via the Inito application. Although women were recruited for one cycle each, participants were excluded if they did not take tests on the required days The study design is summarized in Fig. 2.
Definitions
Cycle day 1 is defined as the day of bleeding.
Day of ovulation is the day of peak LH value. An event of LH surge is defined as any day where the LH value exceeds by 5 mIU/ml from the baseline value in urine. A cycle with multiple LH surges is any cycle that has smaller LH surges before the actual LH peak.
A normal E3G surge is the rise in E3G expected to be seen at least 5–6 days before the LH peak value and any surge before that is considered to be premature.
Surge of E3G is defined as a rise in E3G by at least 1.5 × from the previous day.
For confirming ovulation, the canonical method implements measurement of urinary PdG at 7 days after the LH peak. If the urinary PdG value obtained is greater than 5 µg/ml, the cycle is classified ovulatory16,17.
Results
Inito fertility monitor accurately predicts the concentration of urine metabolites and is specific to the metabolites of interest
Accuracy of the Inito Fertility test strips was assessed by reproducing the concentrations of E3G, PdG and LH in standard solutions prepared in male urine not containing any of the metabolites. Six spiked solutions containing all the three metabolites were prepared from respective stock solutions as per Supplementary Table 2. Each solution was tested with five different Inito fertility test strips and the recovery percentage was calculated. We found that the average recovery percentage across five tests was 100.16% for E3G, 104.63% for PdG and 104.74% for LH. The observed concentration obtained from IFM was also highly correlated to the expected concentration in the spiked male urine (Fig. 3a–c) implying a high accuracy in predicting the concentrations.
Since Inito Fertility test strips are manufactured in a batch wise fashion, we wanted to ensure that the accuracy is maintained across lots. Therefore, we chose to test with 2 strips each from 5 different batches manufactured on different dates accounting for a total of ten strips from different lots. The test strips were tested with four different concentrations of E3G, PdG and LH and the coefficient of variation (CV) was calculated. All three assays were found to have a CV of less than 10% across different lots (Supplementary Table 3). The E3G assay had an average CV of 4.95%, the PdG assay was found to have an average CV of 5.05% and the LH assay was observed to have an average CV of 5.57% indicating that the recovery percentage does not vary significantly from test strip to test strip.
Next, we wanted to analyze the specificity of Inito fertility test strips towards the metabolites of interest. We identified the components of human urine that could potentially interfere with the test and spiked negative male urine with these compounds. The concentration of each metabolite was selected to encompass normal and pathological conditions. We tested four strips from different batches with the spiked male urine and found that the test strips did not cross-react with any urine metabolite at even the highest possible physiological condition (Supplementary Table 4).
Urinary hormone concentrations estimated by IFM and ELISA are strongly correlated
Enzyme linked-immunosorbent assay (ELISA) is a standard laboratory procedure for measuring concentrations of chemical compounds. Therefore, we wanted to compare the efficacy of estimation by Inito with respect to ELISA. For this, we chose all the urine samples collected by 3 random women (out of the 100) over one complete cycle. The cycle lengths of these three women were 26 days, 32 days and 22 days accounting for a total of 80 samples that were tested with both Inito and ELISA and the correlation was calculated. We found that results obtained from ELISA and Inito are highly similar (r2 = 0.96 for E3G, r2 = 0.99 for PdG, and r2 = 0.99 for LH) and are linearly correlated (Fig. 3d–f).
Incidence of premature E3G surge and multi-phasic LH surge maybe higher in women than expected
Our first idea was to observe how well the 6-day fertile window predicted by the IFM correlates with occurrence of ovulatory cycles. We observed that on average a 6-day fertile window correlates the best with the occurrence of an ovulatory cycle compared to lower fertile days or higher (Supplementary Fig. 4).
Our study design allowed us to monitor urinary E3G, LH and PdG on all days of the follicular phase and the luteal phase including the day of ovulation. Therefore, we decided to observe the occurrence of aberrant E3G and LH patterns in the volunteer menstrual cycles. Specifically, we investigated the prevalence of premature E3G surge and multi-phasic LH surge (multiple LH peaks). We observed that among the cycles which displayed LH rise and ovulation (confirmed using urinary PdG), 39 cycles recorded multiple surges of LH before the actual peak that caused ovulation and 16 cycles recorded a single dominant LH surge. 39 cycles were anovulatory and had no LH surge. However, 6 cycles were anovulatory despite a single surge of LH, a phenomenon previously described as luteinized anovulation. While the occurrence of multi-phasic LH is coincident with previous studies14,15, the percentage of cycles with a single LH surge leading to ovulation may actually be lower than what had been previously assumed. With respect to E3G, we observed that 20 cycles showed a significantly high E3G (> 100 ng/ml) in the early part of the cycle, typically more than 6 days before the LH peak and before the expected E3G surge (right before LH surge) the occurrence of which is coincident, albeit slightly higher than previously reported studies15.
Monitoring continuous rise of urinary PdG after LH surge provides early confirmation of ovulation
Serum progesterone (P4) concentration is widely considered as a confirmatory marker for ovulation. Typically, a mid-luteal phase value of > 3 ng/mL has been used to confirm ovulation. Recently, it has been shown that mid-luteal phase measurement of urinary PdG (a threshold value of > 5 µg/mL) has a good correlation with serum P4 behaviour and can also be used to confirm ovulation16,17. However, both these methods require the users/patients to wait for almost 7 days after the LH peak value.
For our analysis, to classify a menstrual cycle as ovulatory or anovulatory, we selected the same urinary PdG threshold values as previously discussed and this was regarded as the actual positive or negative value for further evaluation of the novel criteria. The baseline urinary PdG was determined before the LH surge as the average of the PdG levels and compared with the PdG levels 2–3 days after the LH surge. We found that the distribution of fold change in ovulatory cycles was substantially different from the fold change observed in the anovulatory cycles during the first three days after the LH peak (Fig. 4a,b). From this observation, we inquired if measuring a particular fold rise within 3 days after the LH peak could be used as a criteria to predict ovulation earlier. We decided to judge this criteria by performing an ROC analysis. We found that a fold change of 2.75 × within 3 days could distinguish ovulatory from anovulatory cycles. The criteria had a specificity and sensitivity of 100% in detecting ovulation and was significant in differentiating the ovulatory from anovulatory cycles (p < 0.0001, AUC: 0.981, Fig. 4c,d and Table 2a). In addition, we applied this criterion to the set of users who used the IFM at home to predict ovulation. We found that the new criteria could differentiate the 38 ovulatory cycles from the 14 anovulatory cycles with a 92.2% precision (Table 2b).
(a) Hormone chart showing multi-phasic LH surges with each event of LH surge marked (black arrows), (b) Cumulative graph of all volunteers with median values and 95th Centile showing PdG rise before the LH surge (green arrow) and the gradual rise in PdG after LH peak (blue arrow) for confirming ovulation as opposed to a single test mid-luteal phase, (c) Comparison of fold rise in PdG levels within 3 days after LH surge in ovulatory versus anovulatory cycles (****p < 0.0001 and (d) ROC analysis to evaluate the accuracy of the proposed criteria to predict ovulatory and anovulatory cycles.
Luteinizing hormone peak is preceded by a distinct rise in the urinary PdG
A rise in estradiol levels prior to the LH surge is observed in canonical menstrual cycles. Progesterone, however, is thought to be the baseline during the follicular phase (Fig. 1a). Our study design allowed us to monitor urinary PdG prior to LH surge as well. Furthermore, the sensitivity of the Inito Fertility test strips allowed the smallest changes in the urinary PdG to be visualised. We observed that urinary PdG increased in Group I prior to the LH surge by an average of 3.16 ± 0.7 fold in 52/55 cycles from the baseline stage (in cycles where there was a prominent LH surge). This surge was found to occur on average 2.87 ± 0.9 days before the day on which LH peaked. Just 2 cycles illustrated this phenomenon in the 6 anovulatory cycles of Group I, where there was an LH surge. In Group II, we found the frequency of this phenomenon in 43/52 cycles by re-evaluating this observation. Although we remain unsure of the source of this urinary PdG, the phenomenon appears to be highly conserved during menstrual cycles.
Discussion
Optimal intercourse timing has been established as a key parameter influencing the frequency of pregnancy in women18. Previous studies have addressed the existence of a 6-day fertile window as the optimum fertile window correlated with an increased probability of pregnancy19,20. In addition, the fertile window prediction is well-correlated with the concentrations of urinary hormones, specifically the growth of E3G and LH. Nonetheless, about 26–37 percent of natural cycles are anovulatory8 and may occur despite normal hormonal conduct for instance in case of luteinized unruptured follicle21,22. Thus, to ensure ovulation, the estimation of the fertile window is not adequate and therefore may not be well associated with pregnancy results as well.
The Inito Fertility Monitor (IFM) is a home-based diagnostic device which measures urinary E3G, PdG and LH concentrations. Measurement of these three hormones enables confirmation of ovulation after prediction of a potential fertile window for women. The performance analysis of IFM proves that it is a very sensitive, accurate and laboratory equivalent device for monitoring urinary hormone profiles of women. While in our study, we found a high correlation between ELISA estimated values and hormone values measured by Inito Fertility Monitor, the study was performed using urine samples throughout the menstrual cycle from only three different subjects. Although the data points take into account different phases of the menstrual cycle, the limited number of subjects may undermine the individual-to-individual variation. In addition, all the subjects for this study were from the same race. Therefore, it is possible that while the correlation may hold good, the correlation factor may vary depending on the population, age, race and other demographic factors.
From a population analysis of women who volunteered to collect urine over one menstrual cycle and women who used IFM at their homes, our system classified 40.2% of the menstrual cycles as anovulatory in the random trial we conducted which is similar albeit slightly higher than what has been observed in previous studies8. Since this study was performed in a different group of women compared to the previous study (different populations), it can be inferred that the severity of this subfertility class may be similar across different populations and hence a generic regime can be implemented for different women assuming that the extent of this problem is the same.
The occasional cases of luteinized anovulation could also be captured, the frequency of which often coincides with what was previously recorded22. From the hormone data obtained, we propose a novel paradigm for faster confirmation of ovulation by virtue of continuous monitoring of urinary PdG. This new threshold can now be applied to the AI integrated with the Inito Fertility Monitor in order to alert users once the rise is observed and to confirm ovulation earlier than mid-luteal phase. This paradigm could encompass all cycles that were classified as ovulatory according to the existing thresholds and methods of confirmation proving that the measurement of mid-luteal phase progesterone may be redundant and that the rise in progesterone could be captured much earlier to confirm ovulation. Since pre-implantation intercourse diminishes fecundability by almost 26%29, application of this method to confirm ovulation may help in increasing the fecundability since women can decide not to have intercourse once they receive a confirmation of ovulation.
From the hormone trends, we also show that urinary PdG rises in a number of cycles before the LH surge. Previously, the occurrence a pre-ovulatory progesterone rise has been reported by Hoff et al.23 The role of this pre-ovulatory progesterone has been proposed to be triggering the LH surge and a premature rise in this has been indicated in the pathology of poly-cystic ovarian syndrome (PCOS)24,25. The source of this progesterone has been hypothesized to be follicular and the surge is supposed to occur 12–24 h before the expected LH surge. The progesterone surge observed by us happens much prior to this period and hence may be a separate phenomena possibly correlated to a particular developmental phase of the follicle. However, since the occurrence is highly conserved, it is likely that this feature may be an indicator of a common feature associated with normal natural cycles and could help in differentiating between other infertility related conditions.
Conclusion
The results from this study indicate that IFM is an accurate home-based fertility monitor to help women determine their fertile window as well as to confirm ovulation. Since the Inito Fertility Monitor provides the hormone charts to the users, they can monitor the effects of changes in lifestyle on their hormones and select regimes that contribute to the best hormone behavior.
While IFM is currently designed for use at home, the sensitivity and repeatability of results allow it to be used as a device for basic understanding of hormone trends in women as well28. This suggests that in future this platform could be used by physicians as well for monitoring hormone profiles of their patients. A remote monitoring platform like this would make the path to diagnosis and treatment of subfertility conditions faster since women can use it at home, obtain hormone charts and share them with their doctors. In addition, using IFM, doctors could also visualize the effects of treatments on their patients and modify them in order to obtain the best possible output in terms of fecundity.
Additionally more clinical trials involving women using IFM at home can open avenues of the device’s use in handling cycle related factors such as PMS and monitoring pregnancy health. Furthermore, monitoring hormone patterns of specific groups of infertile women can aid in formulating algorithms that can help in a basic prognosis of these infertility conditions at home.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. The corresponding author has access to all the data and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
Zinaman, M., Johnson, S., Ellis, J. & Ledger, W. Accuracy of perception of ovulation day in women trying to conceive. Curr. Med. Res. Opin. https://doi.org/10.1185/03007995.2012.681638 (2012).
Kudesia, R., Chernyak, E. & McAvey, B. Low fertility awareness in United States reproductive-aged women and medical trainees: Creation and validation of the Fertility & Infertility Treatment Knowledge Score (FIT-KS). Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2017.07.1158 (2017).
Robinson, J. E., Wakelin, M., Ellis, J. E. Increased pregnancy rate with use of the Clearblue Easy Fertility Monitor. Fertil. Steril. (2007).
Barrett, J. C. & Marshall, J. The risk of conception on different days of the menstrual cycle. Popul. Stud. (NY) https://doi.org/10.1080/00324728.1969.10405297 (1969).
Wilcox, A. J., Weinberg, C. R. & Baird, D. D. Timing of sexual intercourse in relation to ovulation. Obstet. Gynecol. Surv. https://doi.org/10.1097/00006254-199606000-00016 (1996).
Royston, P. Identifying the fertile phase of the human menstrual cycle. Stat. Med. https://doi.org/10.1002/sim.4780100207 (1991).
Roos, J. et al. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur. J. Contracept. Reprod. Health Care. https://doi.org/10.3109/13625187.2015.1048331 (2015).
Prior, J. C., Naess, M., Langhammer, A. & Forsmo, S. Ovulation prevalence in women with spontaneous normal-length menstrual cycles—A population-based cohort from HUNT3, Norway. PLoS ONE https://doi.org/10.1371/journal.pone.0134473 (2015).
O’Connor, K. A. et al. Ovulation detection methods for urinary hormones: Precision, daily and intermittent sampling and a combined hierarchical method. Hum. Reprod. https://doi.org/10.1093/humrep/dei497 (2006).
Santoro, N. et al. Assessing menstrual cycles with urinary hormone assays. Am. J. Physiol. Endocrinol. Metab. https://doi.org/10.1152/ajpendo.00381.2002 (2003).
Thakur, R. et al. Development of smartphone-based lateral flow device for the quantification of LH and E3G hormones. IEEE Sens. J. https://doi.org/10.1109/jsen.2020.3008566 (2020).
O’Connor, K. A. et al. Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin. Chem. https://doi.org/10.1373/49.7.1139 (2003).
Brindle, E. et al. Urinary beta-luteinizing hormone and beta-follicle stimulating hormone immunoenzymometric assays for population research. Clin. Biochem. https://doi.org/10.1016/j.clinbiochem.2006.08.009 (2006).
Park, S. J., Goldsmith, L. T., Skurnick, J. H., Wojtczuk, A. & Weiss, G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2007.01.045 (2007).
Alliende, M. E. Mean versus individual hormonal profiles in the menstrual cycle. Fertil. Steril. https://doi.org/10.1016/S0015-0282(02)03167-9 (2002).
Ecochard, R. et al. Use of urinary pregnanediol 3-glucuronide to confirm ovulation. Steroids https://doi.org/10.1016/j.steroids.2013.06.006 (2013).
Leiva, R., McNamara-Kilian, M., Niezgoda, H., Ecochard, R. & Bouchard, T. Pilot observational prospective cohort study on the use of a novel home-based urinary pregnanediol 3-glucuronide (PDG) test to confirm ovulation when used as adjunct to fertility awareness methods (FAMs) stage 1. BMJ Open https://doi.org/10.1136/bmjopen-2018-028496 (2019).
Stanford, J. B., White, G. L. & Hatasaka, H. Timing intercourse to achieve pregnancy: Current evidence. Obstet. Gynecol. https://doi.org/10.1016/S0029-7844(02)02382-7 (2002).
Royston, P. & Ferreira, A. A new approach to modeling daily probabilities of conception. Biometrics https://doi.org/10.1111/j.0006-341X.1999.01005.x (1999).
Dunson, D. B., Baird, D. D., Wilcox, A. J. & Weinberg, C. R. Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation. Hum. Reprod. https://doi.org/10.1093/humrep/14.7.1835 (1999).
Killick, S. & Elstein, M. Pharmacologic production of luteinized unruptured follicles by prostaglandin synthetase inhibitors. Fertil. Steril. https://doi.org/10.1016/S0015-0282(16)59163-8 (1987).
Qublan, H. et al. Luteinized unruptured follicle syndrome: Incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum. Reprod. https://doi.org/10.1093/humrep/del113 (2006).
Hoff, J. D., Quigley, M. E. & Yen, S. S. C. Hormonal dynamics at midcycle: A reevaluation. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/jcem-57-4-792 (1983).
Dozortsev, D., Pellicer, A. & Diamond, M. P. Progesterone is a physiological trigger of ovulatory gonadotropins. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2019.12.024 (2020).
Dozortsev, D. I., Pellicer, A. & Diamond, M. P. Premature progesterone rise as a trigger of polycystic ovarian syndrome. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2020.07.007 (2020).
Bouchard, T. P., Fehring, R. J. & Schneider, M. Pilot evaluation of a new urine progesterone test to confirm ovulation in women using a fertility monitor. Front. Public Health https://doi.org/10.3389/fpubh.2019.00184 (2019).
Behre, H. M. et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan® Fertility Monitor: Comparison with transvaginal ultrasound scans and serum hormone measurements. Hum. Rep. https://doi.org/10.1093/humrep/15.12.2478 (2000).
Dozortsev, D. I., Pellicer, A. & Diamond, M. P. Oscillations of estradiol and gonadotropins are a missing link to solving the mystery of mono-ovulation in humans. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2021.05.107 (2021).
Steiner, A. Z. et al. Peri-implantation intercourse lowers fecundability. Fertil. Steril. https://doi.org/10.1016/j.fertnstert.2014.03.017 (2014).
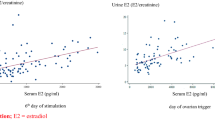
Pattnaik, S. et al. Predicting serum hormone concentration by estimation of urinary hormones through a home-use device. Hum. Reprod. Open 2023(1), 058 (2023).
Author information
Authors and Affiliations
Contributions
S.P. and V.A.V. designed the study. S.P. planned the study procedure and conducted the clinical trials. S.P. and D.D. performed the laboratory analysis and established the equivalence with ELISA. S.P. analyzed the data. S.P. and V.A.V. analyzed user hormone trends. S.P. wrote the manuscript. S.P. and V.A.V. were involved in editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.P. heads the research and development division of Samplytics Technologies Pvt. Ltd. which is a forwarder for Inito Inc., USA. D.D. is employed as the clinical research scientist at Samplytics Technologies Pvt. Ltd. V.A.V. is the co-founder of Inito Inc., USA.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pattnaik, S., Das, D. & Venkatesan, V.A. Validation of urinary reproductive hormone measurements using a novel smartphone connected reader. Sci Rep 13, 9227 (2023). https://doi.org/10.1038/s41598-023-36539-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36539-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.