Abstract
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, guidance (“Japanese Guide”) has been published by a working group of several academic societies and announced by the Ministry of Health, Labour, and Welfare. Steroids as a candidate treatment for COVID-19 were noted in the Japanese Guide. However, the prescription details for steroids, and whether the Japanese Guide changed its clinical practice, were unclear. This study aimed to examine the impact of the Japanese Guide on the trends in the prescription of steroids for COVID-19 inpatients in Japan. We selected our study population using Diagnostic Procedure Combination (DPC) data from hospitals participating in the Quality Indicator/Improvement Project (QIP). The inclusion criteria were patients discharged from hospital between January 2020 and December 2020, who had been diagnosed with COVID-19, and were aged 18 years or older. The epidemiological characteristics of cases and the proportion of steroid prescriptions were described on a weekly basis. The same analysis was performed for subgroups classified by disease severity. The study population comprised 8603 cases (410 severe cases, 2231 moderate II cases, and 5962 moderate I/mild cases). The maximum proportion of cases prescribed with dexamethasone increased remarkably from 2.5 to 35.2% in the study population before and after week 29 (July 2020), when dexamethasone was included in the guidance. These increases were 7.7% to 58.7% in severe cases, 5.0% to 57.2% in moderate II cases, and 1.1% to 19.2% in moderate I/mild cases. Although the proportion of cases prescribed prednisolone and methylprednisolone decreased in moderate II and moderate I/mild cases, it remained high in severe cases. We showed the trends of steroid prescriptions in COVID-19 inpatients. The results showed that guidance can influence drug treatment provided during an emerging infectious disease pandemic.
Similar content being viewed by others
Introduction
Coronavirus Disease 2019 (COVID-19) is an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO: World Health Organization) China Office reported a cluster of confirmed pneumonia cases in Wuhan, Hubei Province, China on December 31, 2019. The first COVID-19 case was confirmed in Japan on January 16, 2020. After the Japanese government classified COVID-19 as a designated infectious disease on February 1, 2020, the number of cases increased, and the COVID-19 epidemic has caused tremendous damage to the healthcare system, economy, and society in Japan1,2,3,4,5,6,7.
Despite the emphasis on evidence-based medicine (EBM), there is a lack of time or human resources to develop typical guidelines under an emerging infectious disease pandemic, but guidance and other information compiled by public agencies on the disease are considered important. In order for Japanese healthcare professionals to practice EBM, they need knowledge from clinically relevant research articles and reviews8.
Since the outbreak of the COVID-19 pandemic, many research papers, both clinical and non-clinical, have been published. Governments, international organizations, and academic societies in various countries have published guidance documents on the disease based on this research. In Japan, the "Clinical Management of Patients with COVID-19: a Guide for Front-line Healthcare Workers" (hereinafter, the “Japanese Guide”) was prepared and revised by the Clinical Practice Guidelines Review Committee based on the "Study on the Clinical Response in the Case of Outbreak of Class I Infectious Diseases, etc." The Japanese Guide has been disseminated by the MHLW's Novel Coronavirus Infectious Disease Control Promotion Headquarters9.
How new guidance can change a physician’s behavior during an emerging infectious disease pandemic in the United Kingdom was previously reported10. However, the impact of the guidance on prescriptions in Japan has not been clarified. Given the high likelihood of a pandemic similar to COVID-19 occurring in the future and that the probability of experiencing it in one's lifetime may double in the next few decades11, clarifying the impact of the guidance on clinical practice in Japan will help to inform policy decision and discussion of guidance development in similar situations. In addition, an analysis of the prescription patterns of potential therapeutics for COVID-19 inpatients in the United States12 and the epidemiologic characteristics and prescription patterns of COVID-19 inpatients in Japan13 has been reported. In that study in Japan, patients were classified into three subgroups by clinical procedures information and the proportions of patients administered ciclesonide or a steroid other than ciclesonide during hospitalization in each subgroup were shown. However, the impact of the guidance on prescribing and time-series trends of the proportions of steroid prescriptions in Japan are not available.
In the present study, we aimed to evaluate the impact of the Japanese Guide on the trends of prescriptions for COVID-19 inpatients in Japan and to contribute to future countermeasures against emerging infectious diseases. Therefore, we focused on steroids, which have been frequently revised in the drugs listed in the Japanese Guide (Supplementary Fig. 1).
Methods
Data sources and study population
Diagnosis Procedure Combination (DPC) data extracted from the Quality Indicator/Improvement Project (QIP) database were used for this study. The DPC/per-diem payment system (DPC/PDPS) is a case-mix patient classification system originally developed in Japan to implement a standardized electronic claims system and provide transparency of hospital performance14. The QIP project, administered by the Department of Health Economics and Quality Management, Kyoto University, collects DPC data provided by voluntarily participating acute care hospitals, including both public and private hospitals, all over Japan. As of October 2020, 317 hospitals participated in the QIP. The DPC data consist of several files, including the discharge summary (Form 1) and the EF file. Form 1 contains information on medical records including hospital identifiers, patient demographics, admission and discharge date, diagnoses classified as principal, most- and second-most-resource-intensive, or trigger, comorbidities, and complications. The EF-file contains claims data including the date, amounts, content, and reimbursement points of medical practices including surgery, tests, pharmaceuticals, and medical materials15.
The International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes were used to define the study population (Supplementary Table 1). The inclusion criteria for cases were as follows: (1) an ICD-10 code for COVID-19 (until February 2020: B34.2, after March 2020: U07.1; U07.2.) was entered for the main disease, the disease that triggered hospitalization, and the disease that most invested medical resources (excluding suspected disease); (2) patients aged ≥ 18 years, and (3) cases discharged between January 2020 and December 2020. The exclusion criteria for cases were (1) no missing EF file for each month of hospitalization and (2) pregnant woman or unknown pregnancy status.
The efficacy of steroids has been reported to vary depending on the severity of the disease16, and the fourth edition of the Guide to Clinical Practice, revised on December 4, 2020, strongly recommends the use of steroids for patients with moderate disease II or higher. Therefore, in this study, we classified cases into subgroups according to the severity of disease. In addition to the Japanese Guide and severity of disease, we also considered other guidelines, research articles, drug approvals and supplies, and medical payments that may have affected steroid prescription. The severity of disease was defined by claims codes for the medical practice that was considered to be performed on cases presenting with each severity of disease according to the severity classification described in the Japanese Guide (Supplementary Table 1). Severe cases were defined as those who underwent tracheostomy, endotracheal intubation, ventilation, or extracorporeal membrane oxygenation (ECMO); moderate II cases were defined as those who underwent oxygen administration, high-flow therapy, or non-invasive positive pressure ventilation (NPPV); moderate I and mild cases were defined as those not classified as severe cases or moderate II cases.
The proportion of prescription
The proportion of prescription was calculated by dividing the number of cases in which that particular drug was administered during a given period by the number of cases in which one or more drugs were administered during that period.
The Japanese Guide was developed by a working group involving the Japanese Society of Infectious Diseases, the Japanese Respiratory Society, the Japanese Society of Intensive Care Medicine, the Japanese Pediatric Society, the Japanese Society of Obstetrics and Gynecology, and other academic societies. It was supported by MHLW and disseminated as an administrative communication from the MHLW Headquarters for the Promotion of Countermeasures against Novel Coronavirus Infections. In this study, the steroids listed in the Japanese Guide were included in the analysis. Ciclesonide was listed in the first edition of the Japanese Guide published on March 17, 2020. Steroids were added in the second edition published on May 18, 2020. Steroids except ciclesonide were deleted and dexamethasone was added in the 2.2 edition published on July 17, 2020. “Steroids” in the Japanese Guide do not include ciclesonide and dexamethasone. Also, prednisolone and methylprednisolone were identified as steroids that could be prescribed for the treatment of COVID-19 based on efficacy and dosage form. Steroids are strongly recommended for moderate II or severe cases, while steroids should not be used in cases who do not require oxygenation in the Japanese Guide.
Cases prescribed with steroids in this study were identified using the first 7-digits of the 12-digits National Health Insurance (NHI) drug codes to represent the substance level following the previous research17 and the route of administration of steroids were restricted to oral, injection and inhalation through the discussion with medical doctors (Supplementary Table 1). The NHI drug code is a 12-digit code consisting of four digits for the drug class, three digits for the route of administration and substances, one digit for the dosage form, one digit for the standard drug class within the same class, two digits for the brand identifier within the same standard unit, and one digit for the check digit. A code is assigned to each drug listed in the NHI drug price standard. In Japan, drugs that are approved under the "Act on Quality, Efficacy, and Safety Assurance of Drugs and Medical Devices (Pharmaceuticals and Medical Devices Act)" and required for medication and dispensing by the National Health Insurance are listed in the NHI Drug Price Standards managed by MHLW.
Ethics approval
The authors conducted the present study in accordance with the "Ethical Guidelines for Life Science and Medical Research Involving Human Subjects" issued by the Ministry of Education, Culture, Sports, Science, and Technology, the Ministry of Health, Labor, and Welfare, and the Ministry of Economy, Trade, and Industry. The present study was approved by the Ethics Committee, Kyoto University Graduate School and Faculty of Medicine (approval number: R0135).
Results
Extraction of the study population
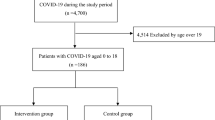
A flowchart representing the process of selecting the study population is shown in Fig. 1. The study population consisted of 8603 cases, of which 410 were severe cases, 2231 were moderate II cases, and 5962 were moderate I and mild cases.
Epidemiological characteristics of COVID-19 hospitalized patients
To determine whether the study population and subgroups classified by severity of disease had characteristics consistent with previous reports of COVID-19 patients, their epidemiological characteristics are presented in Table 1. All subgroups had a high proportion of males, and this was particularly evident in severe cases. The median age was higher in severe and moderate II cases than in moderate I and mild cases. The proportion of cases with a history of smoking was lower in severe cases; however, a higher proportion of severe cases had an unknown smoking history. Furthermore, length of hospital stay and in-hospital mortality rates increased with increasing severity of disease.
Number and proportion of cases by severity of disease
The number of cases by severity is shown in Fig. 2. The increase in the number of hospitalized cases was consistent with the increase in the number of people tested positive for SARS-CoV-2 in April, August, and November 2020, in Japan18. Although the proportion of severe/moderate II cases decreased around week 29 (July 2020), when dexamethasone was included in the Japanese Guide, the proportion of severe/moderate II cases did not change before or after that time.
Trends of steroid prescriptions
Trends of steroid prescriptions are shown in Fig. 3. As shown in Fig. 3a, in the study population as a whole, the proportion of dexamethasone prescriptions was less than 5% from January to July 2020 but increased rapidly after July 2020. The proportion of ciclesonide prescriptions reached 17.6% at Week 13 (March 2020) but declined thereafter. The highest proportion of prescriptions was 13.2% at Week 23 (June 2020) for prednisolone and 9.4% at Week 17 (April 2020) for methylprednisolone.
Trends of steroid prescriptions. (a) Study population. (b) Severe. (c) Moderate II. (d) Moderate I/mild. ① A case report of ciclesonide administration published on the Infectious Diseases Society of Japan website (March 2, 2020), ② Designation of ciclesonide as an drug subject to shipment adjustment (March 3, 2020), ③ Publication of the preprint of the Recovery Trial on the efficacy of dexamethasone (June 22, 2020), ④ Publication of the Recovery Trial in peer-reviewed journals (July 17, 2020), ⑤ Publication of the first edition of "Rapid/Living Recommendations on Drug Management for COVID‐19" (September 9, 2020), ⑥ Publication of the first edition of "The Japanese Medical Science Federation COVID-19 expert opinion" (November 20, 2020). Vertical axis represents the proportions of prescription, horizontal axis represents week number. From the fourth edition of the Japanese Guide revised on December 4, 2020, it is recommended that steroids not be administered to the patients with moderate I or lower, while dexamethasone is strongly recommended in moderate II or higher.
In the study population as a whole, several steroids such as ciclesonide, prednisolone, and methylprednisolone were prescribed prior to the publication of the Japanese Guide. However, prescription patterns stabilized after the publication and revision of the Japanese Guide, and dexamethasone became the main treatment option especially after it was included in the guidance. The proportions of prescriptions for steroids other than those listed above were lower than for ciclesonide, prednisolone, methylprednisolone, and dexamethasone.
Discussion
We analyzed nationwide administrative data to examine the impact of the Japanese Guide on the trends of steroid prescriptions for COVID-19 inpatients. The proportion of dexamethasone prescriptions was remarkably increased after July 2020 when dexamethasone was included in the guidance. Until that update, the Japanese Guide had mentioned “steroid hormones” without specifying a type.
In the past, data on the number of severe cases were reported based on each prefecture's own criteria. However, in this study, the proportions of severe, moderate II, moderate I, and mild cases among hospitalized cases were clarified in general accordance with the criteria described in the Japanese Guide. In addition, we identified changes in steroid prescriptions for COVID-19 hospitalized cases as well as changes in steroid prescriptions in accordance with continuous revisions of the Japanese Guide. The results suggest that a variety of steroids were prescribed in the early stages of the pandemic, that prescription patterns were not stable, and that the Japanese Guide had some influence on steroid prescription for COVID-19 hospitalized cases.
We analyzed patients’ demographics and epidemiological characteristics to discuss whether the selected study population was appropriate for the aims of the study. As shown in Table 1, there was a high proportion of males in all severity subgroups, and this was particularly pronounced in severe cases. In addition, the median age was higher in severe and moderate II cases than in moderate I and mild cases. It was previously reported that being male19,20 and being older than 65 years21 were prognostic factors of COVID-19. Furthermore, length of hospital stay and in-hospital mortality rate increased with the rise of severity of disease. In our study, the epidemiological characteristics of each severity subgroup were consistent with these previous reports, and the definition of severity used in this study was considered adequate to classify the patient population. Contrary to our expectations, the proportion of cases with a smoking history was lower in the severe cases, whereas the proportion of cases with an unknown smoking history was higher in the severe cases. This suggests that it may be difficult to obtain a smoking history in severe cases.
As shown in Fig. 3, all severity subgroups had a relatively high proportion of prednisolone and methylprednisolone prescriptions in early 2020, but the proportion of dexamethasone prescriptions increased markedly after it was included in the Japanese Guide. The proportions of prescriptions for prednisolone and methylprednisolone decreased in moderate II cases and in moderate I and mild cases but remained high in severe cases. The proportion of cases prescribed steroids in severe cases varied widely from week to week, which may be attributed to the small number of severe cases (410 cases). The proportion of cases prescribed steroids in moderate I and mild cases was lower than in severe and moderate II cases; however, the proportion of dexamethasone prescriptions increased after its inclusion in the Japanese Guide. Furthermore, despite the recommendation not to administer steroids to patients with moderate disease I and mild disease in the fourth edition of the Japanese Guide revised on December 4, 2020, the proportion of dexamethasone prescriptions remained around 20% after the revision. This result suggests that dexamethasone may have been administered regardless of the disease severity.
The Subcommittee on Infectious Diseases of the Health Sciences Council held on December 17, 2020 recommended that, as one of the main issues in the response against the pandemic, it was necessary to consider strengthening research on infectious diseases including collaboration between the National Institute of Infectious Diseases, the National Center for Global Health and Medical Research, and other related organizations. In the context of strengthening research, governmental interventions typically concentrate on bolstering the development on drugs and treatment. However, the means by which to effectively implement these findings into clinical practice often receive inadequate attention. Despite the emergence of efficacious drugs and treatment policies, the public's health status remains intractable unless these insights are seamlessly incorporated into clinical practice. Thus, it is imperative to parallelize research development efforts with an emphasis on the implementations of research findings such as the dissemination and compliance to guidelines encapsulating research findings. However, this study suggested that the recommendations in the Japanese Guide were not always followed adequately. Therefore, measures should be taken to promote compliance with the guidance, such as using tables and figures to communicate differences in the recommendations of drugs for each severity.
Factors other than the Japanese guide
In order to assess the impact of the Japanese Guide on steroid prescriptions, it is necessary to consider factors other than the Japanese Guide that may have influenced steroid prescription. In addition to severity of disease, such factors include guidelines, research articles, drug approval and supply, and payments including cost-sharing and reimbursement. The increase in the proportion of dexamethasone prescriptions was not likely due to changes in patient severity because the proportion of severe/moderate II cases did not change before or after Week 29 (July 2020) as shown in Fig. 2.
First editions of the "Rapid/Living Recommendations on Drug Management for COVID‐1922" and the "The Japanese Medical Science Federation COVID-19 expert opinion23", which are also the major guidelines and guidance in Japan, were published on September 9, 2020 and November 20, 2020, respectively (Fig. 3 ⑤, ⑥). Therefore, they could not have caused the increase in the proportion of dexamethasone prescriptions in July 2020. In addition, although ciclesonide attracted attention due to a case report24 posted on March 2, 2020 on the website of the Japanese Society of Infectious Diseases, the rapid increase in its demand on the following day (March 3) led to a shipment adjustment, which may have suppressed the increase in the proportion of prescription (Fig. 3 ①, ②). One of the reasons for the increase in prescriptions of prednisolone and methylprednisolone after April may have been because they are alternatives to ciclesonide, which was subject to shipment adjustments. However, because the proportion of dexamethasone prescriptions in the study population as a whole remained high despite a temporary drop during a shipment adjustment implemented around September 2020, the impact of the shipment adjustment on the proportion of dexamethasone prescriptions may not have been significant. However, it is difficult to conclude which of a report on the efficacy of dexamethasone16 (following its publication on the preprint server on June 22, 202025) or the Japanese Guide mainly affected the increase of dexamethasone prescriptions because they were released on the same day (Fig. 2 ④). Considering there was no significant increase in the proportion of dexamethasone prescriptions immediately after the publication of the preprint despite the media coverage that preceded the preprint being a hot topic in Japan, including coverage in a national newspaper26, the Japanese Guide might play the important role in the change in the proportion of dexamethasone prescriptions. Furthermore, causal inferences using quasi-experimental designs such as interrupted time-series analysis cannot be performed because a substantial number of events which may affect the proportions of steroid prescriptions simultaneously occurred in the pandemic and the key assumption that no external factors systematically affecting the trends exists was not satisfied or it is difficult to prove it is satisfied.
Regarding drug approval, steroids such as dexamethasone were indicated for the treatment of severe infectious diseases, but none were indicated for the treatment of novel coronavirus infection. Thus, no impact was expected on the proportion of prescriptions due to the switch from steroids with no indication of "novel coronavirus infection" to steroids indicated for "novel coronavirus infection". Finally, no statements were found from the MHLW notice regarding the handling of medical payments that would lead to a change in the proportion of prescriptions of a particular steroid.
Limitations of the study
The study had three limitations. First, if factors other than the Japanese Guide influenced steroid prescriptions at the same time, we could not distinguish between the influences of these factors. However, taking these factors into account as described above, it is considered that the Japanese Guide had some influence on Japanese physicians' decisions regarding prescription during the COVID-19 pandemic. Second, the data from hospitals participating in the QIP project utilized in this study may not be generalizable to the whole of Japan. Because the data utilized in this study were collected from hospitals that voluntarily participated in the QIP project, which aims to evaluate and improve the quality of medical care, they may have had a higher awareness of compliance with guidelines, etc. Thus, we may have overestimated changes in prescription in response to the Japanese Guide. However, because acute care hospitals of various sizes from all over Japan voluntarily participate in the QIP project, bias due to the region or size of the medical institution's location was considered to be small. Third, there was a possibility of misclassification of disease severity. In this study, severity of disease was defined based on the medical practices included in the DPC data. However, because severity of disease is supposed to be determined objectively by evaluating oxygen saturation, which is not included in the DPC data, it is possible that differences in classifications may have occurred. However, based on the epidemiological characteristics of each severity subgroup shown in Table 1, the differences were considered to be small.
Conclusions
The present study suggests that the Japanese Guide influenced Japanese physicians' decisions regarding prescriptions during the COVID-19 pandemic. However, contrary to the recommendations in the Japanese Guide for drug treatment according to severity, steroids were prescribed even for moderate I and mild cases. This was despite the recommendation not to administer steroids since the fourth edition of the Japanese Guide of December 4, 2020. It is possible that the recommendations in the Japanese Guide were not properly communicated.
Data availability
The information provided to waive consent from study subjects includes the list of data users. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. The other contact points are Office of Research Promotion, General Affairs and Planning Division, Kyoto University (E-mail: kikaku06@mail2.adm.kyoto-u.ac.jp; Tel: + 81-75-753-9301) and the Ethics Committee, Graduate School of Medicine, Kyoto University (e-mail: ethcom@kuhp.kyoto-u.ac.jp).
References
Katayama, Y., Kiyohara, K., Kitamura, T., Hayashida, S. & Shimazu, T. Influence of the COVID-19 pandemic on an emergency medical service system: A population-based, descriptive study in Osaka, Japan. Acute Med. Surg. https://doi.org/10.1002/ams2.534 (2020).
Fujita, K. et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thoracic Cancer. 11(10), 2983–2986. https://doi.org/10.1111/1759-7714.13615 (2020).
Oda, J. et al. JAAM Nationwide Survey on the response to the first wave of COVID-19 in Japan Part II: How the medical institutions overcame the first wave and how to prepare in future?. Acute Med. Surg. https://doi.org/10.1002/ams2.592 (2020).
Bank of Japan. The Impact of COVID-19 on the Japanese Economy and the Bank of Japan’s Response (2020).
Miyakawa, D., Oikawa, K. & Ueda, K. Firm exit during the COVID-19 pandemic: Evidence from Japan. J. Jpn. Int. Econ. 59, 101118. https://doi.org/10.1016/j.jjie.2020.101118 (2021).
Bank of Japan. COVID-19 and the Global Economy : Impact and Challenges (2020).
Okubo, T. Spread of COVID-19 and telework: Evidence from Japan. Covid Econ. 32, 1–25 (2020).
Sakai, Y., Sato, Y., Sato, M. & Watanabe, M. Clinical usefulness of library and information services in Japan: The detailed use and value of information in clinical settings. PLoS ONE 13(6), 1–18. https://doi.org/10.1371/journal.pone.0199944 (2018).
Ministry of Health, Labour and Welfare. Information for medical institutions. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00111.html (Accessed 14 Nov 2021).
Curtis, H. J. et al. OpenSAFELY: Impact of national guidance on switching anticoagulant therapy during COVID-19 pandemic. Open Heart. 8(2), 1–10. https://doi.org/10.1136/openhrt-2021-001784 (2021).
Marani, M., Katul, G. G., Pan, W. K. & Parolari, A. J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. USA 118(35), 0–3. https://doi.org/10.1073/pnas.2105482118 (2021).
Watanabe, J. H. et al. Medication use patterns in hospitalized patients with COVID-19 in California during the pandemic. JAMA Netw. Open 4(5), 20–22. https://doi.org/10.1001/jamanetworkopen.2021.10775 (2021).
Matsunaga, N. et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 REGISTRY Japan. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa1470 (2020).
Yasunaga, H., Matsui, H., Horiguchi, H., Fushimi, K. & Matsuda, S. Clinical epidemiology and health services research using the diagnosis procedure combination database in Japan. Asian Pac. J. Dis. Manag. 7(1–2), 19–24 (2013).
Hayashida, K., Murakami, G., Matsuda, S. & Fushimi, K. History and profile of diagnosis procedure combination (DPC): Development of a real data collection system for acute inpatient care in Japan. J. Epidemiol. 31(1), 1–11. https://doi.org/10.2188/jea.JE20200288 (2021).
The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N. Engl. J. Med. https://doi.org/10.1056/nejmoa2021436 (2020).
Nomura, K. et al. Identifying drug substances of screening tool for older persons’ appropriate prescriptions for Japanese. BMC Geriatr. 18(1), 1–15. https://doi.org/10.1186/s12877-018-0835-y (2018).
Ministry of Health, Labour and Welfare. COVID-19 Open Data. https://www.mhlw.go.jp/stf/covid-19/open-data.html (Accessed 10 Nov 2021).
Peckham, H. et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 11(1), 1–10. https://doi.org/10.1038/s41467-020-19741-6 (2020).
Patel, U. et al. Age-adjusted risk factors associated with mortality and mechanical ventilation utilization amongst COVID-19 hospitalizations—a systematic review and meta-analysis. SN Compr. Clin. Med. https://doi.org/10.1007/s42399-020-00476-w (2020).
Du, R. H. et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARSCoV- 2: A prospective cohort study. Eur. Respir. J. https://doi.org/10.1183/13993003.00524-2020 (2020).
Special Committee of The Japanese Clinical Practice Guidelines For The Management Of Sepsis And Septic Shock 2020 (J-SSCG 2020) The COVID-19 Task Force. Rapid/Living Recommendations on Drug Management for COVID-19. 1st ed. (2020).
The Japanese Medical Science Federation. The Japanese Medical Science Federation COVID-19 Expert Opinion. 1st ed. (2020).
Iwabuchi, K. et al. Three cases of improvement with ciclesonide inhalation in early to mid-stage of COVID-19 pneumonia (Japanese). The Japanese Association for Infectious Disease. (2020). https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_200302_02.pdf (Accessed 3 Feb 2022).
Horby, P. et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. medRxiv. https://doi.org/10.1101/2020.06.22.20137273 (2020).
Hirose, M. Anti-inflammatory drug “dexamethasone” effective in reducing COVID-19 mortality...widely used and inexpensive. Yomiuri Shimbun Online. https://www.yomiuri.co.jp/world/20200617-OYT1T50168/. (2020).
Acknowledgements
We gratefully acknowledge the participating hospitals in the QIP and their staff.
Funding
This study was supported by JSPS KAKENHI Grant Number JP19H01075 to Yuichi Imanaka and JP21K21136 to Jung-ho Shin from the Japan Society for the Promotion of Science, and Health Labour Sciences Research Grant Numbers JPMH20HA2003, JPMH21IA1005, JPMH23HA2002 and JPMH23HA2011 by the Ministry of Health, Labour and Welfare, Japan to Yuichi Imanaka. The funders played no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.H. conceptualized the study, selected methods, analyzed and interpreted the data, wrote the original draft, reviewed and edited the manuscript, and visualized data. T.H. was a major contributor in writing the manuscript. J.S. conceptualized the study, interpreted the data, developed the programming codes for analyzing data, validated and curated the data, reviewed and edited the manuscript, and contributed the funding acquisition. D.T., T.M. conceptualized the study, interpreted the data, developed the programming codes for analyzing data, validated and curated the data, and reviewed and edited the manuscript. S.K., Y.I. conceptualized the study, interpreted the data, validated and curated the data, and reviewed and edited the manuscript. Y.I. is the guarantor of the article. Y.I. supervised and administrated the research project, and contributed the funding acquisition. All authors read and approved the final manuscript. Consent from study subjects was waived by providing required information to study subjects according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labour and Welfare, Japan (a provisional translation is available from https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Higuchi, T., Shin, Jh., Takada, D. et al. The Japanese Guide affected the prescription of steroids for COVID-19 inpatients during the COVID-19 epidemic in Japan. Sci Rep 13, 9041 (2023). https://doi.org/10.1038/s41598-023-36199-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36199-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.