Abstract
This study aimed to investigate the relationship between serum ferritin level and prognosis in sepsis. It also explored the potential prognostic value of serum ferritin for predicting outcomes in sepsis based on a large public database. Sepsis patients in MIMIC-IV database were included. Different models including crude model (adjusted for none), model I (adjusted for age and gender) and model II (adjusted for all potential confounders) were performed. Smooth fitting curves were constructed for exploring the relationships between serum ferritin and mortalities of 28-day, 90-day, 180-day and 1-year. Receiver operator characteristic (ROC) curve analysis was utilized for assessing the predictive value of serum ferritin. 1947 sepsis patients were included. The mortalities of 28-day, 90-day, 180-day and 1-year were 20.18% (n = 393), 28.35% (n = 552), 30.30% (n = 590) and 31.54% (n = 614), respectively. In Model II (adjusted for all potential confounders), for every 1000 ng/ml increment in serum ferritin, the values of OR in mortalities of in 28-day, 90-day, 180-day and 1-year were 1.13 (95% CI 1.07–1.19, P < 0.0001), 1.15 (95% CI 1.09–1.21, P < 0.0001), 1.16 (95% CI 1.10–1.22, P < 0.0001) and 1.17 (95% CI 1.10–1.23, P < 0.0001), respectively. The relationships between serum ferritin level and outcomes were non-linear. The areas under the ROC curve (AUC) of ferritin for predicting mortalities of 28-day, 90-day, 180-day and 1-year were 0.597 (95% CI 0.563–0.629), 0.593 (95% CI 0.564–0.621), 0.595 (95% CI 0.567–0.623) and 0.592 (95% CI 0.564–0.620), respectively. The non-linear relationships between serum ferritin and clinical outcomes in sepsis were found. Serum ferritin had a predictive value for short-term and long-term outcomes in sepsis.
Similar content being viewed by others
Introduction
Ferritin, as an iron storge protein, is broadly distributed in the cells, organs and circulation1. It has a significant role in maintaining iron homeostasis and helps to transport oxygen, produce energy and red blood cells2. In serum, ferritin level is usually regulated by various factors including iron imbalance, hormones and inflammatory cytokines3. Researches showed that serum ferritin not only could be an indicator for differentiating anemia4, but also was associated with numerous other diseases including malignant tumor5, metabolic syndrome6, digestive disease7 and even COVID-198.
Ferritin, as an indispensable factor of the immune system, is capable of indicating the cellular defensive response to inflammation9. Increased ferritin was identified as both an acute phase reactant and a mediator of immune dysregulation due to infection10. Serum ferritin was a marker of cellular injury and related with prognosis in infectious diseases11,12. In critically ill patients, elevated levels of serum ferritin were associated with risk factors of worse outcomes including thrombocytopenia, hypoalbuminemia and life support managements13. Sepsis, as dysregulated organ dysfunction due to infection, usually stimulates high levels of inflammatory cytokines, which might raise serum ferritin14.
Since ferritin was closely related to infection, we aimed to investigate the relationship between serum ferritin level and prognosis and explored the prognostic value of serum ferritin for predicting short-term and long-term outcomes in sepsis based on a large public database.
Methods
Database
This retrospective study was performed on the basis of the data from the Medical Information Mart for Intensive Care IV(MIMIC-IV) database (https://mimic.mit.edu/iv/). MIMIC-IV is a U.S. public database and contains the clinical information of critical ill patients admitted in intensive care unit (ICU) of Beth Israel Deaconess Medical Center of Boston between 2008 and 201915,16,17. The corresponding author (N.D.) passed the Protecting Human Research Participants exam (No.32900964) and had the permission for utilizing the MIMIC-IV database.
Study design
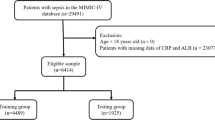
We enrolled the septic patients from MIMIC-IV for data analysis. The definition of Sepsis 3.0 was applied for confirming the sepsis, which indicated that sepsis was diagnosed with infection and sequential organ failure assessment (SOFA) score ≥ 2 points18. Patients who met the criteria as follow were excluded: (1) missing data of serum ferritin within 24 h after admission; (2) missing data > 5% individual variables; (3) < 18 years old.
Information and variables
Variables were extracted within 24 h after admission and only the first record of each variable was utilized.
The following information and variables were extracted: age, gender, marital status, ethnicity, comorbidities, organ dysfunction (acute kidney injury (AKI), septic shock), managements (the utility of vasopressors and ventilator, renal replacement therapy (RRT)), scores of SOFA and chronic health evaluation (APACHEII), length of stay (LOS) in ICU and hospital, prognosis (28-day mortality, 90-day mortality, 180-day mortality and 1-year mortality), vital signs(systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), respiratory rate (RR)), ferritin, total calcium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), thrombin time (TT), creatinine, urea nitrogen, red blood cell (RBC), white blood cell (WBC), hemoglobin, hematocrit, red blood cell distribution width (RDW), platelet (PLT), anion gap (AG), bicarbonate, chloride and sodium.
Statistical analysis
EmpowerStats (http://www.empowerstats.com) and the software packages R (http://www.R-project.org) were applied for statistical analysis. Statistically significant was considered when the P value was less than 0.05.
The septic patients were divided into four group (Q1 (≤ 244 ng/ml, n = 486), Q2 (245–542 ng/ml, n = 485), Q3 (543–1124 ng/ml, n = 488), Q4 (≥ 1125 ng/ml, n = 488)) based on the quartiles of serum ferritin level (Table 1). Different variables were expressed as follow: medians for continuous variables, and percentages or frequencies for categories variables. Chi-squared test and Mann–Whitney U-test were applied for variables analysis between four groups. Univariate analysis for clinical outcomes including 28-day mortality, 90-day mortality, 180-day mortality and 1-year mortality was performed. Associations between serum ferritin and outcomes were investigated in three models: crude model (adjusted for none), model I (adjusted for age and gender) and model II (adjusted for all potential confounders). Covariates were included as potential confounders in the final models if they changed the estimates of ferritin on 1-year mortality in sepsis by more than 10% or were significantly associated with clinical outcomes in sepsis19,20. The calculating steps were showed in Supplementary materials. The following covariates were selected a priori on the basis of established associations and/or plausible biological relations and tested: age; ALT; AG; total calcium; creatinine; hematocrit; hemoglobin; PLT; PT; TT; RDW; RBC; urea nitrogen; renal disease; APAHCEII; SOFA. In addition, gender, as a common confounder in many previous studies21,22, was also added to be adjusted in the final models.
In addition, two models including model A (linear model) and model B (two-segment nonlinear model) were utilized for comparison and the better one was selected based on the P value. If < 0.05, the nonlinear model was the better and the turning point of serum ferritin was calculated. The smooth fitting curves were performed for indicating the relationships between serum ferritin level and outcomes. Kaplan–Meier analysis for survival probability in four groups (Q1–Q4) was constructed. Subgroup analysis was done for investigating the stability of the results. The receiver-operator characteristic (ROC) analysis of serum ferritin for predicting outcomes were performed. The different performances of ferritin, SOFA score and APAHCEII score including specificity, sensitivity and cut-off value were analyzed.
Ethical approval
This study was conducted in accordance with Declaration of Helsinki 2002. MIMIC-IV was an anonymized public database. To apply for access to the database, we passed the Protecting Human Research Participants exam (No.32900964). The project was approved by the institutional review boards of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC) and was given a waiver of informed consent.
Results
Description of the sepsis cohort and comparison of variables
1947 sepsis patients were included in our study (Supplementary Fig. 1 and Supplementary Table 1). The median age was 64 years old and the proportion of males was 55.47% (n = 867). 40.16% of the patients were married and 65.07% were White. The in-hospital incidences of AKI and septic shock were 75.76% and 43.40%, respectively. Of the sepsis patients, 22.85% had RRT use, 26.50% had vasopressors use and 83.82% had ventilation supply. The mortalities of 28-day, 90-day, 180-day and 1-year were 20.18% (n = 393), 28.35% (n = 552), 30.30% (n = 590) and 31.54% (n = 614), respectively.
In Table 1, we compared the variables between four groups based on the quartiles of serum ferritin level. Significant differences were showed in age (P < 0.001), gender (P < 0.001), renal disease (P < 0.001), diabetes (P = 0.010), HR (P = 0.014), ALT (P < 0.001), AST (P < 0.001), creatinine (P < 0.001), RBC (P < 0.001), WBC (P = 0.033), hemoglobin (P < 0.001), hematocrit (P = 0.011), RDW (P < 0.001), PLT (P < 0.001), AG (P = 0.012), chloride (P < 0.001), sodium (P < 0.001), APCHEII score (P = 0.011) and SOFA score (P < 0.001). In Q4 group, the levels of ALT, AST and SOFA score were significantly higher, while the levels of RBC and PLT were significantly lower compared to other three (Q1-Q3) groups. The incidences of septic shock in Q1–Q4 groups were 48.56%, 38.97%, 41.19% and 44.88%, respectively (P = 0.014). The incidences of AKI in Q1-Q4 groups were 70.16%, 74.85%, 78.69% and 79.30%, respectively (P = 0.003).
The median days of LOS in ICU and hospital were 6.72 and 17.28, respectively. The mortalities of in 28-day, 90-day, 180-day and 1-year Q4 groups were the highest compared to Q1-Q3 groups, which were 29.51%, 39.14%, 41.39% and 42.42%, respectively.
Univariate analyses for different outcomes in sepsis
In Table 2, univariate analyses were implemented for different outcomes. Variables were significantly associated with all four different outcomes in sepsis (all P < 0.05) as follow: age, total calcium, PT, TT, urea nitrogen, RBC, hemoglobin, RDW, PLT, AG, chloride, APACHEII score and SOFA score. For ferritin (per 1000 ng/ml increase), the values of OR in mortalities of in 28-day, 90-day, 180-day and 1-year were 1.13 (95% CI 1.08–1.18, P < 0.0001), 1.14 (95% CI 1.09–1.19, P < 0.0001), 1.15 (95% CI 1.10–1.20, P < 0.0001) and 1.15 (95% CI 1.10–1.21, P < 0.0001), respectively.
Association between serum ferritin and outcomes in different models
Table 3 demonstrated the association between serum ferritin and outcomes including mortalities of in 28-day, 90-day, 180-day and 1-year in sepsis. In Model II (adjusted for all potential confounders), for every 1000 ng/ml increment in serum ferritin, the values of OR in mortalities of in 28-day, 90-day, 180-day and 1-year were 1.13 (95% CI 1.07–1.19, P < 0.0001), 1.15 (95% CI 1.09–1.21, P < 0.0001), 1.16 (95% CI 1.10–1.22, P < 0.0001) and 1.17 (95% CI 1.10–1.23, P < 0.0001), respectively. In addition, we changed the serum ferritin from continuous variable to categorial variable (Q1–Q4) and analyzed the associations in three models. Compared Q1 group, the risk of mortalities of 28-day, 90-day, 180-day and 1-year increased significantly in Q4 group in all three models (all values of P for trend < 0.0001). In model II, the values of OR in mortalities of in 28-day, 90-day, 180-day and 1-year were 2.16 (95% CI 1.53–3.07, P < 0.0001), 2.31 (95% CI 1.69–3.17, P < 0.0001), 2.30 (95% CI 1.69–3.14, P < 0.0001) and 2.19 (95% CI 1.61–2.97, P < 0.0001) in Q4 group, respectively.
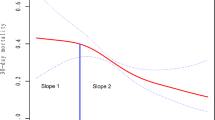
A non-linear relationship between serum ferritin and outcomes
In Table 4, we compared two models including the linear model (model A) and two-segment non-linear model (model B) in all clinical outcomes and found that all the P values for the log-likelihood ratio test were less than 0.05, which indicated the non-linear model was better for expressing the association between serum ferritin and clinical outcomes. In Fig. 1, smooth fitting curves were constructed, which demonstrated the non-linear relationships between serum ferritin level and mortalities of 28-day (A), 90-day (B), 180-day (C) and 1-year (D). The turning points of serum ferritin in the four clinical outcomes were 2340 ng/ml, 2250 ng/ml, 2280 ng/ml and 2300 ng/ml, respectively.
Kaplan–Meier analysis for survival probability
Figure 2 illuminated Kaplan–Meier analysis for survival probability in four groups (Q1–Q4). In Q4 group, the lowest survival probabilities in 28-day (A), 90-day (B), 180-day(C) and 1-year (D) were found (all P < 0.001).
Subgroup analysis
In Supplementary Table 2, subgroup analysis was performed. The results were comparatively stable in most subgroups. Patients who were female with higher levels of WBC (≥ 15.1*109/l) had higher risk of mortality.
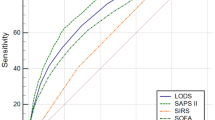
Prognostic value of ferritin in predicting clinical outcomes
In Table 5, predictive performances of serum ferritin, SOFA and APAHCEII in clinical outcomes were compared. The areas under the ROC curve (AUC) of ferritin for predicting mortalities of 28-day, 90-day, 180-day and 1-year were 0.597 (95% CI 0.563–0.629), 0.593 (95% CI 0.564–0.621), 0.595 (95% CI 0.567–0.623) and 0.592 (95% CI 0.564–0.620), respectively. The cut-off values of serum ferritin were 800.00 ng/ml, 599.50 ng/ml, 599.50 ng/ml and 597.5 ng/ml, respectively. In Fig. 3, ROCs of ferritin, SOFA and APACHEII for predicting mortalities of 28-day (A), 90-day (B), 180-day (C) and 1-year (D) in sepsis were demonstrated.
Discussion
In the present study, the non-linear relationships between serum ferritin and clinical outcomes in sepsis were found. For every 1000 ng/ml increment in serum ferritin, the risks in mortalities of in 28-day, 90-day, 180-day and 1-year increased by 13%, 15%, 16% and 17%, respectively. In addition, serum ferritin had a predictive value for outcomes in sepsis.
Ferritin, as a significant protein in iron metabolism, was involved both in the iron homeostasis and inflammatory process23,24,25. The potential prognostic and diagnostic values of serum ferritin level have been proved in various disorders25,26,27. In hemophgocytosis, a serum ferritin > 2000 ug/l for predicting mortality had a specificity of 76% and a sensitivity of 71%28. In hospitalized patients, ferritin levels greater than 2000 ng/ml were identified to be significantly associated with severe diseases29. In metabolic syndrome, the serum ferritin levels were found to be positively related with the levels of insulin resistance, cholesterol, and triglyceride30. One recent study with hemorrhagic fever with renal syndrome in China revealed that the value of serum ferritin for predicting mortality was comparatively good compared with procalcitonin and C-reactive protein31. For all-cause mortality risk within five-year in hemodialysis patients, serum ferritin > 1500 ng/ml was an early indicator32.
In critical ill and sepsis, accumulating evidences also clarified the close relationship between serum ferritin and clinical outcomes. In children with sepsis and septic shock, a ferritin > 500 ng/ml increased the relative risk of mortality with a 2.2 folds33. For predicting death in multiple organ dysfunction due to sepsis, 1994.3 ng/ml might be a cut-off value in serum ferritin34. Ferritin > 4420 ng/ml was described to be diagnostic of macrophage activation-like syndrome and predictive of short (10-day) and 28-day mortality in sepsis35. Current evidence suggested the biomarker of serum ferritin was good for immunotyping and providing immunomodulatory treatment in sepsis with encouraging results36. Based on the results from one large research in critical ill patients, the AUCs for ferritin in predicting in-hospital mortality and organ failure were 0.655 and 0.646, respectively. In sepsis, the AUCs for ferritin in predicting in-hospital mortality and organ failure were 0.628 and 0.608, respectively, which the cut-off values were 411 ng/ml and 581 ng/ml, respectively37, which were partly similar with our results.
The potential mechanisms why elevated serum ferritin levels were correlated with poorer outcomes in sepsis could be explained as follow: (1) The inflammation due to sepsis usually produces the endotoxin, which upregulates the ferritin coding gene and leads to increased levels of serum ferritin38; (2) Ferroptosis, as a way of cell death, might be mediated by different levels of serum ferritin, resulting in cellular injury and organ dysfunction39; (3) Inflammatory cytokines are able to upregulate the ferritin production, which in turn strengthens the release of proinflammatory and anti-inflammatory factors, leading to more severe inflammatory response and poor prognosis40,41.
The strength of our study was that we investigated the relationships between serum ferritin and clinical outcomes with short-term and long-term in sepsis and also explored the predictive values of serum ferritin. We found that serum ferritin was closely associated not only with short-term outcomes but also with 1-year mortality in sepsis patients. Moreover, serum ferritin also had a comparatively good value for predicting the outcomes in sepsis patients. It might help physicians to early differentiating the patients with higher risk of adverse outcomes.
Anyway, some limitations in the research were not avoided. First, due to the lack of some data, some other factors of iron metabolism including transferrin, free iron level, and ferritin saturation were not included. Second, due to our exclusion criteria, the proportion of excluded subjects with missing variables might cause bias in the relationships. Due to the lack of serum ferritin, a number of sepsis patients were excluded. Third, it was a retrospective study based on public database and the limitations of applicability for our results should be considered. In the study, we only used the U.S public database and didn’t validate our results in other cohorts. Further study with large samples in multiple centers and different regions should be done for validating our results.
Conclusion
In the present study, the non-linear relationships between serum ferritin and clinical outcomes in sepsis were found. In addition, serum ferritin had a predictive value for outcomes in sepsis.
Data availability
The data that support the findings of this study are available from the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC).
References
Williams, V. et al. Serum ferritin predicts neither organ dysfunction nor mortality in pediatric sepsis due to tropical infections. Front. Pediatr. 8, 607673 (2020).
Wang, D. et al. Caspse-11-GSDMD pathway is required for serum ferritin secretion in sepsis. Clin. Immunol. 205, 148–152 (2019).
Branco, R. G. & Garcia, P. C. Ferritin and C-reactive protein as markers of systemic inflammation in sepsis. Pediatr. Crit. Care Med. 18(2), 194–196 (2017).
Takasawa, K., Takaeda, C., Wada, T. & Ueda, N. Optimal serum ferritin levels for iron deficiency anemia during oral iron therapy (OIT) in Japanese hemodialysis patients with minor inflammation and benefit of intravenous iron therapy for OIT-nonresponders. Nutrients 10(4), 428 (2018).
Park, J. M. et al. A case-control study in Taiwanese cohort and meta-analysis of serum ferritin in pancreatic cancer. Sci. Rep. 11(1), 21242 (2021).
Tran, T. N., Tran, H. D., Tran-Huu, T. T., Tran, D. M. & Tran, Q. N. A Cross-sectional study of serum ferritin levels in vietnamese adults with metabolic syndrome. Diabetes Metab. Syndr. Obes. 15, 1517–1523 (2022).
Wang, J. et al. Elevated serum ferritin levels are associated with severity and prognosis of severe acute pancreatitis: A preliminary cohort study. BMC Gastroenterol. 22(1), 408 (2022).
Kurian, S. et al. Association of serum ferritin with severity and clinical outcome in COVID-19 patients: An observational study in a tertiary healthcare facility. Clin. Epidemiol. Global Health 21, 101295 (2023).
Chen, Q. et al. Serum ferritin levels are associated with advanced liver fibrosis in treatment-naive autoimmune hepatitis. BMC Gastroenterol. 22(1), 23 (2022).
Lalueza, A. et al. Elevation of serum ferritin levels for predicting a poor outcome in hospitalized patients with influenza infection. Clin. Microbiol. Infect. 26(11), 1557.e1559-1557.e1515 (2020).
Kanda, J. et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 46(2), 208–216 (2011).
Brands, X. et al. Plasma ferritin as marker of macrophage activation-like syndrome in critically ill patients with community-acquired pneumonia. Crit. Care Med. 49(11), 1901–1911 (2021).
von Bahr, G. T. et al. Elevated ferritin and soluble CD25 in critically ill patients are associated with parameters of (hyper) inflammation and lymphocyte cytotoxicity. Minerva Anestesiol. 85(12), 1289–1298 (2019).
McCullough, K. & Bolisetty, S. Iron homeostasis and ferritin in sepsis-associated kidney injury. Nephron 144(12), 616–620 (2020).
Ding, Q., Su, Y., Li, C. & Ding, N. Red cell distribution width and in-hospital mortality in septic shock: A public database research. Int. J. Lab. Hematol. 44(5), 861–867 (2022).
Giesa, N. et al. MIMIC-IV as a clinical data schema. Stud. Health Technol. Inf. 294, 559–560 (2022).
Cao, Y., Su, Y., Guo, C., He, L. & Ding, N. albumin level is associated with short-term and long-term outcomes in sepsis patients admitted in the ICU: A large public database retrospective research. Clin. Epidemiol. 15, 263–273 (2023).
Gotts, J. E. & Matthay, M. A. Sepsis: Pathophysiology and clinical management. BMJ 353, i1585 (2016).
Bjerregaard, L. G., Pedersen, D. C., Mortensen, E. L., Sorensen, T. I. A. & Baker, J. L. Breastfeeding duration in infancy and adult risks of type 2 diabetes in a high-income country. Matern. Child Nutr. 15(4), e12869 (2019).
Jaddoe, V. W. et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ 348, g14 (2014).
Pan, X. et al. Admission lysophosphatidic acid is related to impaired kidney function in acute aortic dissection: 2-year retrospective follow-up study. Front. Cardiovasc. Med. 9, 905406 (2022).
Yang, G., Chai, X., Ding, N., Yang, D. & Ding, Q. A retrospective observational study of serum uric acid and in-hospital mortality in acute type A aortic dissection. Sci. Rep. 12(1), 12289 (2022).
Behairy, B. E. et al. Serum ferritin in neonatal cholestasis: A specific and active molecule or a non-specific bystander marker?. Hepatobiliary Pancreat Dis. Int. 18(2), 173–180 (2019).
Hua, Z. et al. Serum ferritin is a good indicator for predicting the efficacy of adult HLH induction therapy. Ann. Med. 54(1), 283–292 (2022).
Suarez-Ortegon, M. F. et al. Serum ferritin and incident cardiometabolic diseases in Scottish adults. Cardiovasc. Diabetol. 21(1), 26 (2022).
Zhang, R. et al. Serum ferritin as a risk factor for type 2 diabetes mellitus, regulated by liver transferrin receptor 2. Endocr. Connect 10(12), 1513–1521 (2021).
Naymagon, L., Tremblay, D. & Mascarenhas, J. Reevaluating the role of ferritin in the diagnosis of adult secondary hemophagocytic lymphohistiocytosis. Eur. J. Haematol. 104(4), 344–351 (2020).
Grange, S. et al. The use of ferritin to identify critically ill patients with secondary hemophagocytic lymphohistiocytosis. Crit .Care Med. 44(11), e1045–e1053 (2016).
Israel, A. et al. Clinical and prognostic significance of elevated ferritin levels in hospitalised adults. Postgrad. Med. J. 98(1162), 622–625 (2021).
Srivastav, S. K. et al. Serum ferritin in metabolic syndrome-mechanisms and clinical applications. Pathophysiology 29(2), 319–325 (2022).
Che, L. et al. Evaluation of serum ferritin, procalcitonin, and C-reactive protein for the prediction of severity and mortality in hemorrhagic fever with renal syndrome. Front. Microbiol. 13, 865233 (2022).
Erdem, E., Karatas, A. & Ecder, T. The relationship between serum ferritin levels and 5-year all-cause mortality in hemodialysis patients. Blood Purif. 51(1), 55–61 (2022).
Garcia, P. C. et al. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 96(12), 1829–1831 (2007).
Nandy, A. et al. Serum ferritin as a diagnostic biomarker for severity of childhood sepsis. Indian Pediatr. 58(12), 1143–1146 (2021).
Kyriazopoulou, E. et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 15(1), 172 (2017).
Leventogiannis, K. et al. Toward personalized immunotherapy in sepsis: The PROVIDE randomized clinical trial. Cell Rep. Med. 3(11), 100817 (2022).
Sang, L. et al. Association between serum ferritin and outcomes in critically ill patients: A retrospective analysis of a large intensive care unit database. Chin. Med. J. 135(21), 2634 (2022).
Ochiai, M. et al. An elevation of serum ferritin level might increase clinical risk for the persistence of patent ductus arteriosus, sepsis and bronchopulmonary dysplasia in erythropoietin-treated very-low-birth-weight infants. Neonatology 111(1), 68–75 (2017).
Jiao, Y., Yong, C., Zhang, R., Qi, D. & Wang, D. Hepcidin alleviates LPS-induced ARDS by regulating the ferritin-mediated suppression of ferroptosis. Shock (Augusta, Ga) 57(6), 274–281 (2022).
Zarjou, A. et al. Ferritin light chain confers protection against sepsis-induced inflammation and organ injury. Front. Immunol. 10, 131 (2019).
Brinza, C., Floria, M., Popa, I. V. & Burlacu, A. The prognostic performance of ferritin in patients with acute myocardial infarction: A systematic review. Diagnostics (Basel) 12(2), 476 (2022).
Funding
Changsha Natural Science Foundation (kq2208445), Changsha Central Hospital (YNKY202306), Changsha Science and Technology Bureau (kzd21084).
Author information
Authors and Affiliations
Contributions
Conception and design: N.D., L.H. Administrative support: N.D. Provision of study materials or patients: N.D., Y.S. Collection and assembly of data: N.D., C.G. Data analysis and interpretation: C.G., L.H., Y.S. Manuscript writing: N.D.. Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, L., Guo, C., Su, Y. et al. The relationship between serum ferritin level and clinical outcomes in sepsis based on a large public database. Sci Rep 13, 8677 (2023). https://doi.org/10.1038/s41598-023-35874-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35874-2
This article is cited by
-
Ferritin-mediated neutrophil extracellular traps formation and cytokine storm via macrophage scavenger receptor in sepsis-associated lung injury
Cell Communication and Signaling (2024)
-
Association between ferritin to albumin ratio and 28-day mortality in patients with sepsis: a retrospective cohort study
European Journal of Medical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.