Abstract
Plants have evolved mechanisms of adaptation to fluctuations in their environmental conditions that have been given the term “stress memory”. Synthetic wheat offers new hope for breeders to restore useful genes lost during the genetic bottleneck. We aimed to test whether drought priming and seed priming could improve drought tolerance in a diverse germplasm of synthetic and common wheat under field conditions. In this research, 27 wheat genotypes (including 20 synthetics, 4 common local and 3 common exotic bread wheat) were field evaluated under four water environments. These treatments included: 1) normal condition (N), plants were irrigated when 40% of the total available soil water was depleted from the root-zone, 2) seed priming-secondary stress (SD2), only water stress was applied at anthesis when 90% of the total available soil water was depleted and seeds were planted for evaluating, 3) primary stress- secondary stress (D1D2), primary water stress was applied at jointing stage when 70% of the total available soil water was depleted then secondary water stress was applied at the anthesis stage when 90% of the total available soil water was depleted, and 4) secondary stress (D2) only water stress was applied at the anthesis when 90% of the total available soil water was depleted. Our results indicated that improved efficient enzymatic antioxidant system leads to less yield reduction in D1D2 treatment. However, the positive effects of drought priming were more pronounced in drought primed (D1D2) than seed primed treatment (SD2). Synthetic wheat genotypes had a significant superiority in terms of yield, yield components and drought tolerance compared to common wheat genotypes. Nevertheless, the response of genotypes to stress memory was very different. Drought sensitive genotypes had better response to stress memory. Superior genotypes were identified as high yield and drought tolerant genotypes which can be used for future studies.
Similar content being viewed by others
Introduction
Droughts are often regarded as major threats to ecosystems under global climate change1. More or less 45% of the world cultivated area is faced with frequent and continuous drought2. However, plants may undergo different physiological and morphological adaptations for acquiring tolerance to drought stress. Stress memory is a phenomenon evolved in plants to adapt to fluctuations in their environmental conditions3. The mechanisms behind this phenomenon involve complex, multi-level responses which manifest themselves in the form of altered physiological signaling and protective metabolites, as well as epigenetic modifications3. Ultimately, stress memory may provide a mechanism for acclimation and adaptation4.
Bread wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD) is the most important cereal crop with significant increase in contribution of human food5. The major limiting factor for wheat improvement is the narrow genetic variation in common cultivars, especially in the D genome. To enhance the genetic variation in this genome, synthetic hexaploid wheat (SHW) was artificially created by simulating the evolution of bread wheat by crossing different durum or emmer wheat cultivars (AABB) with Aegilops tauschii (the wild diploid progenitor of the D genome)6. Genetic diversity in the Ae. tauschii populations is considerably higher than that in the D genome of bread wheat, with many useful genes for biotic and abiotic stresses and seed storage protein. Studies showed that synthetic derivative lines (SDLs) provide up to 46% increase in grain yield compared to their common wheat parents under water deficit conditions7,8. In Australia, increases of 9–31% in grain productivity, in comparison with parental genotypes and local control varieties, were achieved under rain-fed conditions in synthetic lines9. Similar grain yield increase was also achieved in Argentina, Ecuador, India and, Pakistan. Additional genetic variation for salt tolerance and tolerance to higher temperatures has been reported in SHW10,11,12, which is limited in cultivars of common wheat lines.
Recent reports in some crops have showed that the plants pre-exposed to environmental stress can achieve the potential to display a stronger and faster activation of their defense system in response to the subsequent stress challenges13,14. An early drought episode showed an improved photo-protection and higher production under a second drought event than non pre-exposed plants in Arrhenatherum elatius1. Similarly, pre-exposure of vegetative stage plants to heat stress improved the thermo-tolerance during grain filling in rice15. Likewise, stress memory benefits have also been reported in radish14, tobacco16, and some grass species17. A few studies addressing the inheritance effects of pre-stress priming in wheat. Primed seeds exhibited greater stress-tolerance and an enhanced germination ability over non-primed seeds under multiple adverse environmental conditions in wheat18,19. In another wheat study, plants which were exposed to early stage heat stress, displayed an improved antioxidative activity and higher seed yield under heat stress20. Li et al21 investigated that pre-treatment for water logging during vegetative growth stage enhanced dry matter accumulation and its distribution to grain formation in wheat. Positive effects of drought priming by alleviating drought stress and heat stress during the grain-filling stage has been recently reported in two cultivars of wheat22. Improvements in photosynthetic capacity, seed yield, oxidative stress reduction20, alleviation of photo-inhibition23 and enhancements in regulation of growth hormones24 at grain-filling stage are also attributed to drought priming.
Up to now, experiments investigating these stress imprint have been mostly restricted to small time spans of less than one week and on limited common wheat cultivars. To the best of our knowledge, no information is available about stress memory in synthetic hexaploid wheat. In addition, there is no study in terms of stress memory with this number of genotypes under field with the emphasis of agronomic and physiological traits. Therefore, we aimed to investigate whether the effect of seed priming and mild drought priming could be responsible for improving performance, physiological and antioxidant system under drought in different types of synthetic and commercial wheat cultivars under farm conditions.
Results
Analysis of variance
Analysis of variance (ANOVA) indicated that year effect was not significant for most of traits. Therefore, data of two years were pooled and ANOVA were performed based on two-way analysis (Combined analysis of treatments and genotypes). There were significant differences (P < 0.01) between treatments for most of the measured traits with the exception of spike length, plant height, catalase and relative water content. The effect of genotype was also significant for all of the functional and phenological traits and some physiological traits including proline, carbohydrate, catalase, ascorbate, peroxidase activities and relative water content indicating significant variation among the selected genotypes (Tables S2 and S4). Genotype × treatment effects were significant for the traits including days to pollination, proline, catalase, ascorbate peroxidase and peroxidase activities (Tables S2 and S4).
Means of moisture treatments for functional and physiological traits
The results showed that secondary drought stress significantly reduced grain yield and yield components under three stress conditions (SD2, D1D2, D2) compared to normal condition (N) (Table 1). This reduction was 43% in (SD2), 35% in (D1D2) and 47% in (D2) for grain yield. The lowest yield reduction was related to the primary stress-secondary stress (D1D2) (Table 1). The main difference between this moisture treatment (D1D2) with other moisture treatments (SD2 and D2) is primary stress (D1) which was applied at joining stage. It seems that stress memory had been an important factor in preventing yield reduction. In addition, D1D2 treatment had a higher number of spikes and harvest index compared to other stress conditions (SD2, D2) and the effect of primary stress was quite evident for days to heading (Table 1).
There were two points sampling for physiological traits in this study as mentioned before. Firstly, at recovery period from normal (N) and primary stress—secondary stress (D1D2) treatments. Secondly, at end of second stress (D2) from all of treatments (N, SD2, D1D2, D2). The results of recovery sampling showed that the effect of treatments was significant for chlorophyll a, total chlorophyll (a + b), proline, carbohydrate and ascorbate peroxidase enzyme (Table S3). The level of chlorophyll a, chlorophyll (a + b), proline, carbohydrate and ascorbate peroxidase enzyme (APX) even after four weeks of the primary stress (D1) were significantly higher in (D1D2) treatment camper to normal treatment (N) (Table 2). Furthermore, the results of second sampling showed that the level of ascorbate peroxidase (APX) and peroxidase activity (POX) were significantly higher in (D1D2) treatment compare to others treatments (N, SD2, D2). The level of proline and carbohydrates in stress treatments (SD2, D1D2, D2) were significantly higher than the normal treatment (N) (Table 2).
Means of genotypes or functional and physiological traits
The results showed that there were significant differences among the studied genotypes for the most measured traits (Tables S2, S3 and S4). Synthetic wheat genotypes had a significant superiority in terms of yield, yield components and drought tolerance compared to common wheat genotypes (Figs. 1, 2 and 3). While synthetic wheat genotypes had higher grain yield in normal condition (N), seed priming-second stress (SD2), and secondary stress (D2), the difference between the three genotypic groups (synthetic genotypes, common local genotypes and common Canadian genotypes) was not significant under primary stress-secondary stress (D1D2) (Fig. 2). In this respect, genotypes 200, 80, 196, 159 in normal environment and genotypes 54, 200, 7, 80 in SD2 environment and genotypes 54, 80, 196, 200 in D1D2 environment and genotypes 58, 80, 82,196 in D2 environment were identified as the superior ones in terms of grain yield (Table 3). The highest values of drought tolerance index (STI) were observed for 200, 80 as synthetic genotypes and the lowest values were detected for 4, 1 as common Canadian genotypes (Table 3).
General steps and designing of this study: Seed of wheat lines were collected and reproduced during 2017. Then the lines were evaluated in the field under two treatments (normal condition (N) and intensive drought stress at anthesis stage (D2)) in 2018. Then the seed of both treatments were collected and evaluated in the field under four treatments of normal condition (N), seed priming-secondary stress (SD2), primary stress-secondary stress (D1D2) and secondary stress (D2) in 2019 and 2020.
Although three genotypic groups had specific behaviors for functional traits, there were not specific behaviors for physiological traits among three genotypic groups. In this respect, among 10 wheat genotypes (6 synthetic wheat genotypes, 2 common local and 2 common Canadian wheat genotypes) in normal treatment (N) genotypes 14, 2, 4, 58 and in seed priming-secondary stress (SD2) treatment genotypes 4, 58, 14, 2000 and in primary stress- secondary stress treatment (D1D2) genotypes 4000, 58, 2, 4 and in secondary stress treatment (D2) genotypes 159, 85, 2000, 58, 4 had the highest peroxidase enzyme activity (POX) (Tables 4). It seems that genotypes 4 and 4000 which were detected as lowest values of STI had evident enzyme activities. On the other hand, genotype 58, which was one of the genotypes with highest values of STI, had an active antioxidant system.
The response of genotypes to stress memory (D1D2 treatment) was also very different among genotypes. In fact, yield reduction was 34% in synthetic wheat, 44% in common local wheat, 26% in common Canadian wheat (Fig. 4). Thus, Canadian wheat, which often had the lowest yield in four moisture environments (N, SD2, D1D2, D2) (Fig. 2), had the lowest yield loss in drought primed plants (D1D2) (Fig. 4). Moreover, by comparing rank of each genotype based on grain yield (Table 3), yield components (Table S5), drought tolerance index (STI) (Table 3) and physiological traits (Table 4), three synthetic genotypes (200, 80, 58) were identified as high yield and drought tolerant genotypes.
Principal component analysis
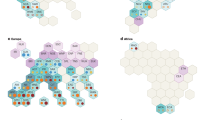
Principal component analyses (PCA) based on functional, phenological, physiological, and drought tolerance index (STI) were performed (Figs. 5 and 6). The principal component graph revealed that the first two components explained 79.20% and 81.54% of trait variation at normal condition (N) and seed priming-second stress (SD2), respectively (Fig. 5a, b). Under normal irrigation, the first principal component (PC1) had higher correlation with chlorophyll a, chlorophyll b, chlorophyll a + b, and carotenoids concentrations; the second principal component (PC2) had higher correlation with grain yield, biological yield, harvest index, number of spike (Fig. 5a).
Principal component analysis of traits measured in the 10 synthetic and common wheat genotypes (a) under normal (N) and (b) seed priming- secondary stress (SD2) conditions. Horizontal and vertical axes are the first and second principal components, respectively. DH, days to heading; DP, days to pollination; PH (cm), plant height; SL (cm), spike length; NS, spike per m2; GY (g/m2), grain yield; SW (g), thousand-grain weight; BY (g/m2), biological yield; HI (%), harvest index; STI, stress tolerance index; RWC (%), relative water content; POX (µmol/mg/protein), peroxidase; APX (µmol/mg/protein), ascorbate; CAT (µmol/mg/protein), catalase; Pro (µmol/g leaf) Proline; TSC (mg/ml), total soluble carbohydrates; Chl-a (mg/g leaf), chlorophyll a; Chl-b (mg/g leaf), chlorophyll b; TChl (mg/g leaf), total chlorophyll a and b; Cars (mg/g leaf), carotenoids concentrations. The Number of genotypes is according to the first column (Genotype code) in table S1.
Principal component analysis of traits measured in the 10 synthetic and common wheat genotypes (a) under primary stress—secondary stress (D1D2) and (b) secondary stress (D2) conditions. Horizontal and vertical axes are the first and second principal components, respectively. DH, days to heading; DP, days to pollination; PH (cm), plant height; SL (cm), spike length; NS, spike per m2; GY (g/m2), grain yield; SW (g), thousand-grain weight; BY (g/m2), biological yield; HI (%), harvest index; STI, stress tolerance index; RWC (%), relative water content; POX (µmol/mg/protein), peroxidase; APX (µmol/mg/protein) ascorbate; CAT (µmol/mg/protein), catalase; Pro (µmol/g leaf) Proline; TSC (mg/ml) , total soluble carbohydrates; Chl-a (mg/g leaf), chlorophyll a; Chl-b (mg/g leaf), chlorophyll b; TChl (mg/g leaf), total chlorophyll a and b; Cars (mg/g leaf), carotenoids concentrations. The Number of genotypes is according to the first column (Genotype code) in table S1.
Under seed priming-second stress (SD2), PC1 had positive correlations with total soluble carbohydrates and biological yield. PC2 positively correlated with harvest index, grain yield, number of spike and stress tolerance index (Fig. 5b) As a result, genotypes 196, 159, and 58 had high values for both PC1 and PC2 under seed priming-second stress (SD2) condition (Fig. 5b).
The first two components justified 81.01 and 83.43% of total variance at primary stress-secondary stress (D1D2) and secondary stress (D2) conditions, respectively (Fig. 6a, b). Under primary stress-secondary stress (D1D2), the first principal component (PC1) had higher correlation with stress tolerance index, harvest index, grain yield, number of spike, proline and total soluble carbohydrates (Fig. 6a). The second principal component (PC2) had high correlation with chlorophyll a, chlorophyll b, chlorophyll a + b, and carotenoids concentrations (Fig. 6a). Under secondary stress (D2) condition, PC1 had positive correlations with chlorophyll a, chlorophyll a + b, carotenoids concentrations (Fig. 6b). The second principle component (PC2) had had positive correlations with biological yield, grain yield, stress tolerance index and number of spike (Fig. 6b). As a result, genotypes 196, 58 and 199 under secondary stress condition (D2) and genotypes 159, 85 and 198 under primary stress-secondary stress (D1D2) were found to have high yield potential.
Discussion
Plants pre-exposed to environmental stress can achieve the potential to display a stronger and faster activation of their defense system in response to the subsequent stress challenges, here defined as priming25. We tested whether seed priming and drought priming during the joining stage could alleviate yield reduction of intense stress imposed during anthesis in a diverse germplasm of synthetic and common wheat under field conditions.
The results of present study indicated that drought stress during the reproductive stage had considerable negative effects on yield and yield components but it was observed that drought primed plants (D1D2) reduced this negative effects compared to the non-primed plants (D2) (Table 1). Pre-exposed drought during vegetative growth proved to be a valuable strategy to facilitate wheat plants to initialize an efficient tolerance mechanism. The results confirm that drought primed plants were able to acclimate to frequent drought episodes by altering physiological factors. Indeed, plants are altering their physiology and metabolism in response to prior experience which is in agreement with other researchers in different wheat studies1,26,27. However, their research was restricted to a small number of cultivars and under pot conditions. The positive effects of drought priming to tolerate drought stress were more pronounced in drought primed plants (D1D2) than seed primed plant (SD2). However, seed primed genotypes (SD2) were also superior to non-primed plant (D2) in terms of biological yield and 100-seed weight. In other pervious research seed composition during terminal drought and seed priming improved the salt tolerance in wheat by modulating the water relations, osmolytes accumulation and lipid peroxidation28.
During water stress, physiological changes active important adaptation mechanisms for plants to resist drought16. Final sampling after applying reproductive stress (D2) showed that D1D2 treatment had more activated antioxidant system than the moisture treatment (D2). The development of the efficient reactive oxygen species (ROS) detoxifying system in the pre-drought stressed wheat lines indicated the existing of stress memory. The higher POX and APX activities identified in primed plants (D1D2) of this study indicated their improved redox defense status to scavenge ROS damage by down-regulating peroxidation of cell membrane lipids under water deficit stress. The effect of primary stress (D1) on physiological measured traits during recovery period was also quite evident. For example, there was a significant difference at recovery period (four weeks normal irrigation after primary stress (D1)) for some traits such as the activity of antioxidant enzymes (APX and GPX) and proline in D1D2 treatment compared to the control treatment (N). It seems that mild primary drought stress (D1) has activated the plant's physiological defense system, creating a kind of cellular readiness that will help the plant perform better than a plant which has only seen reproductive stress. We demonstrate that drought priming boosts the activity of anti-oxidative enzymes, which is vital for depressing oxidative damage and for enhancing tolerance to repeated drought stress in T. aestivum. These findings suggest that primary stress has led to the induction of drought stress memory mechanism and subsequently yielded improvement of genotypes under the secondary drought stress which is in agreement with Abide et al.26 However, our results indicated that the response of genotypes under different moisture treatments was different causing to significant effect of treatment by genotype interaction for some traits such as APX, POX and CAT. For example, the genotype number 4000 had the maximum value of POX under D1D2 treatment, while this genotype has an average value for the APX. Such responses show that stress memory is dependent on the type of genotype and this point should be considered in the breeding selection to improve this trait. The results of principal component analysis indicate a high correlation between APX and POX (The correlation coefficient between two variables is defined as the cosine of the angle between their respective vectors), which shows that the changing trend of these two variables is in the same direction and one of them can be used as a marker of the other. For developing drought tolerant varieties, selection genotypes with high yield under normal conditions and less reduction of yield under moisture stress is ideal. Some selection indices such as drought tolerance index (STI) have been developed to distinguish tolerant and non- tolerant genotypes29. In the present study, significant differences were observed among the 27 genotypes not only for measured traits but also for STI indicating considerable genotypic variation in the studied germplasm. Synthetic wheat genotypes had a significant superiority in terms of yield and yield components and drought tolerance (STI) compared to common wheat genotypes (local and foreign), in both normal and drought conditions. Synthetic genotypes have been obtained from the cross between tetraploid wheat (Emmer and Durum) with Aegilops tauschii (the wild diploid progenitor of the D genome). Genome D contains resistant genes to various biotic and abiotic stresses making it as great potential for wheat improvement. Despite little difference in antioxidant activity between synthetic and common wheat (the results of present study), large potential of synthetic wheats in terms of drought tolerance and grain production indicates that root characteristic system may play more contribution in synthetic wheat which need further studies. In a study by Song et al.30 synthetic wheat displayed increased plant height, larger flag leaf area, longer spikes and more biomass plant than the control cultivar. Synthetic wheat genotypes can provide new sources for grain yield potential, disease resistance, abiotic tolerance, and nutrient-use efficiency and it is becoming more and more important for modern wheat improvment31 to enhance yield traits to face global climate changes and feed the world’s increasing population, especially in arid environments.
Although synthetic wheat genotypes had higher grain yield in normal condition (N), seed priming-second stress (SD2), and secondary stress (D2), the difference between the three genotypic groups was not significant under primary stress-secondary stress (D1D2) (Fig. 2). Indeed, three genotypic groups had similar grain yield under primary stress-secondary stress (D1D2). It seems that drought stress memory helps common wheat genotypes to boost their performance under drought stress. The response of genotypes to stress memory was very different. Canadian wheat, which often had the lowest yield in four water conditions, had the lowest yield loss in drought primed plants (D1D2). It seems that sensitive genotypes had better response to stress memory which was in consensus with previous reports by Mendanha et al.32 but they had similar behavior at physiological level compare to other cultivars27. Therefore, the type of response to priming appears to be cultivar dependable, and thus phenotypical variation should be expected when studying the effects of abiotic priming. While three genotypic groups had specific behaviors for functional traits and secondary drought stress induced a strong synthesis of proline, carbohydrate, catalase and ascorbate peroxidase activities, there were not specific behaviors for physiological traits between three genotypic groups. In fact, among the ten genotypes that were selected in order to measure physiological traits there were not different physiological behaviors. For example, both sensitive and tolerant genotypes had higher amount of proline, catalase and peroxidase. Thus, it is very likely that differences between synthetic and common wheat genotypes, and factors that make synthetic genotypes superior to common genotypes, are more related to the genetic and cellular level than the physiological level but more studies are needed to reveal the reasons for superiority of synthetic wheats to common wheats at the physiological, biochemical and root characteristic system.
Conclusions
It was concluded that wheat plants pre-exposed to primary moderate drought stress in a field trail retained a drought stress memory that triggered more efficient and faster stress scavenging mechanism towards severe later drought stress. Wheat lines subjected to drought priming (D1D2) showed efficient enzymatic antioxidant system leading to less reduction in grain yield. However, high genetic variation observed in term of stress memory within and between two wheat groups (synthetic and common wheat lines). Our results also indicated that the response of genotypes under different treatments was different causing to significant effect of treatment by genotype interaction for some traits such as APX, POX and CAT. Such responses show that stress memory is dependent on the type of genotype and this point should be considered in the breeding selection to improve this trait. Based on the results of PCA, genotypes number 58, 159 and 196 were recognized as the superior genotypes in term of drought tolerance. Furthermore, synthetic wheat genotypes had a significant superiority in terms of yield and yield components and drought tolerance index (STI) compared to common wheat genotypes. However, exotic cultivars, which were more sensitive to drought, exhibited better effects of stress memory in response to water deficit stress. The jointing stage primed plants (D1D2) showed higher efficient enzymatic antioxidant system and effectively alleviated grain yield inhibition than those seed primed plants (SD2). In addition to physiological studies, root characteristic system, epigenetic and molecular elucidations suggest to better understand the mechanisms of drought tolerance resulting from stress memory.
Materials and methods
Plant material and experimental site
Twenty-seven wheat genotypes including 20 synthetic hexaploid wheat, 4 local (Iranain) hexaploid bread wheat and 3 common Canadian hexaploid bread wheat genotypes were evaluated during 2019 and 2020. Canadian and Iranian cultivars (common wheat) were chosen among the famous commercial varieties. Synthetic lines were chosen from the collection available at the gene bank at CIMMYT (Table S1). Our plant material is a public panel and comply with relevant institutional, national, and international guidelines and legislation.
The research farm of the Isfahan University of Technology, 32–30°N, 51–20° E, Isfahan, Iran was used to conduct this study. The annual mean of temperature and precipitation were 14.4 °C and 141 mm, respectively. The soil was non-sodic and non-saline categorizing as Typic Haplargid, silty clay loam containing 389 g/kg Ca-carbonate equivalent and 49.0 g/kg organic C and 0.76 g/kg total N, with the pH of 8.2. The electrical conductivity (EC) of the farm soil-saturated extract was 1.59 dS/m and the sodium adsorption (SA) ratio was 1.39 (mmol/l).
Treatments
The study was comprised of two separate phases. In the first phase, genotypes were evaluated at two moisture environments of well-watered and intensive drought stress (as follow) in 2018. In the second phase, seeds collected from well-watered were used to plan three priming treatments and seed collected from drought stress was used as fourth treatment. All four treatments were evaluated in 2019 and 2020 in field according to the following explanation (Fig. 1). Genotypes was sown on first November of each year.
The experiment was conducted with four treatments: 1) normal treatment (N), plants were irrigated when 40% of the total available soil water was depleted from the root-zone. 2) Primary stress—secondary stress (D1D2) treatment, primary water stress was applied at jointing stage when 70% of the total available soil water was depleted from the root-zone for two weeks and then after applying four weeks recovery (normal irrigation), secondary water stress was applied at the anthesis stage when 90% of the total available soil water was depleted from the root-zone. 3) Secondary stress (D2), only water stress was applied at the anthesis when 90% of the total available soil water was depleted from the root-zone. 4) Seed stress—secondary stress (SD2) treatment, only water stress was applied at anthesis when 90% of the total available soil water was depleted from the root-zone and continued until physiological ripening. The seeds of fourth treatment were stressed seeds from the previous year (2018) while the seeds of other treatments were the result of normal condition of the previous year.
There were also two sampling points: firstly, at the end of the recovery period, four weeks after applying D1, samples from N and D1D2 treatments were taken. Secondly, samples were taken at the end of second stress from all treatments (N, SD2, D1D2 and D2) (Fig. 1).
Soil samples were collected every second day at depths of 0–20, 20–40 and 40–60 cm between two irrigations and exactly one day before irrigation to measure soil moisture. The irrigation depth was determined according to the following formula:
where Id is irrigation depth (cm), θirrig is soil gravimetric moisture percentage at irrigating time, θFC is soil gravimetric moisture percentage at field capacity, ρb is the soil bulk density at root-zone (1.4 gcm3), D is the root-zone depth (60 cm) and ρw is water density. Water was delivered from a pumping station via polyethylene pipe and the water volumes for irrigation were measured with a volumetric counter.
Measurements
A set of traits including days to heading (DH); days to pollination (DP); number of spike (NS), plant height (PH), grain yield (GY), biological yield (BY), 1000-seed weight (SW), harvest index (HI) were measured. In addition, Relative water content (RWC) was estimated according to Ritchie et al.33. Carotenoid, Chlorophyll a (Chla) and chlorophyll b (Chlb) were measured using Lichtenthaler and Buschmann34 method at the wave lengths of 661.5, 644.8 and 470 nm, respectively. The sum of Chla + Chlb was consider as total chlorophyll content (TChl). The Bates’s method35 was used to measure proline content. Water-soluble carbohydrate content was recorded according to Dubois et al.36. The activity of catalase (CAT) was determined by measuring the oxygen and water molecules generated from conversion of H2O237. The activity of Ascorbate peroxidase (APX) was determined based on the oxidation of ascorbate to dehydroascorbate38 identified via monitoring the decrease in absorbance at 290 nm for 2 min by spectrophotometer. The method of Herzog and Fahimi39 was used for measuring peroxidase activity (POX) which is based on the increase in absorbance at 470 nm over the course of 2 min.
Drought tolerance index was calculated based on the seed yield under normal and drought stress conditions according to the following equation:
where Ymp refers to grain yield mean over all genotypes grown under normal conditions. Ypi and Ysi are seed yield of the ith genotype under normal and stress irrigation conditions, respectively.
Statistical analysis
After normality test, three-way ANOVA indicated that year effect is non-significant for most of the traits. Therefore, the data of two years were pooled and the two-way analysis of variance (combined analysis) was performed to examine differences between the moisture environments, genotypes, and their interactions by using the general linear model (GLM) in the SAS Software32. Least significant differences (LSD) method was used for mean comparisons. Principal component analyses (PCA) were also performed based on a correlation matrix on all functional, phenological and physiological traits.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Walter, J. et al. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 71, 34–40. https://doi.org/10.1016/j.envexpbot.2010.10.020 (2011).
Majidi, M. M., Shafiei-Koij, F., Pirnajmedin, F., Jami, M. & Radan, Z. Fatty acid profile, silymarin content and production properties of milk thistle (Silybum marianum) germplasm under different water environments. Crop Pasture Sci. 72, 302–310. https://doi.org/10.1071/CP20489 (2021).
Nosalewicz, A., Siecińska, J., Kondracka, K. & Nosalewicz, M. The functioning of Festuca arundinacea and Lolium perenne under drought is improved to a different extend by the previous exposure to water deficit. Environ. Exp. Bot. 156, 271–278. https://doi.org/10.1016/j.envexpbot.2018.09.016 (2018).
Avramova, Z. Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 83, 149–159. https://doi.org/10.1111/tpj.12832 (2015).
Braun, H. J., Atlin, G. & Payne, T. Multi-location testing as a tool to identify plant response to global climate change. Clim. Change Crop Prod. https://doi.org/10.1079/9781845936334.0115 (2010).
McFadden, E. & Sears, E. The artificial synthesis of Triticum spelta. Records Genet. Soc. Am. 13, 26–27 (1944).
Rafique, K. et al. Evaluation of d-genome synthetic hexaploid wheats and advanced derivatives for powdery mildew resistance. Pak. J. Bot. 49, 735–743 (2017).
Trethowan, R. M. & Mujeeb-Kazi, A. Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Sci. 48, 1255–1265. https://doi.org/10.2135/cropsci2007.08.0477 (2008).
Dreccer, M. F., Borgognone, M. G., Ogbonnaya, F. C., Trethowan, R. M. & Winter, B. CIMMYT-selected derived synthetic bread wheats for rainfed environments: Yield evaluation in Mexico and Australia. Field Crops Res. 100, 218–228. https://doi.org/10.1016/j.fcr.2006.07.005 (2007).
Ginkel, M. V. & Ogbonnaya, F. Novel genetic diversity from synthetic wheats in breeding cultivars for changing production conditions. Field Crops Res. 104, 86–94. https://doi.org/10.1016/j.fcr.2007.02.005 (2007).
Jamil, M. et al. Relationship among water use efficiency, canopy temperature, chlorophyll content and spot blotch (Cochliobolus sativus) resistance in diverse wheat (Triticum aestivum L.) Germplasm. Pak. J. Bot. 48, 993–998 (2016).
Munns, R., Schachtman, D. P. & Condon, A. G. The significance of a two-phase growth response to salinity in wheat and barley. Funct. Plant Biol. 22, 561–569. https://doi.org/10.1071/PP9950561 (1995).
Backhaus, S. et al. Recurrent mild drought events increase resistance toward extreme drought stress. Ecosystems 17, 1068–1081. https://doi.org/10.1007/s10021-014-9781-5 (2014).
Vu, W. T., Chang, P. L., Moriuchi, K. S. & Friesen, M. L. Genetic variation of transgenerational plasticity of offspring germination in response to salinity stress and the seed transcriptome of Medicago truncatula. BMC Evol. Biol. 15, 1–14. https://doi.org/10.1186/s12862-015-0322-4 (2015).
Shi, W., Lawas, L. M. F., Raju, B. R. & Jagadish, S. V. K. Acquired thermo-tolerance and trans-generational heat stress response at flowering in rice. J. Agron. Crop. Sci. 202, 309–319. https://doi.org/10.1111/jac.12157 (2016).
Choi, C. S. & Sano, H. Abiotic-stress induces demethylation and transcriptional activation of a gene encoding a glycerophosphodiesterase-like protein in tobacco plants. Mol. Genet. Genom. 277, 589–600. https://doi.org/10.1007/s00438-007-0209-1 (2007).
Meisner, A., De Deyn, G. B., de Boer, W. & van der Putten, W. H. Soil biotic legacy effects of extreme weather events influence plant invasiveness. Proc. Natl. Acad. Sci. 110, 9835–9838. https://doi.org/10.1073/pnas.1300922110 (2013).
Farooq, M., Irfan, M., Aziz, T., Ahmad, I. & Cheema, S. A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop. Sci. 199, 12–22. https://doi.org/10.1111/j.1439-037X.2012.00521.x (2013).
Korkmaz, A. & Korkmaz, Y. Promotion by 5-aminolevulenic acid of pepper seed germination and seedling emergence under low-temperature stress. Sci. Hortic. 119, 98–102. https://doi.org/10.1016/j.scienta.2008.07.016 (2009).
Wang, X., Vignjevic, M., Jiang, D., Jacobsen, S. & Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 65, 6441–6456. https://doi.org/10.1093/jxb/eru362 (2014).
Li, C. et al. Waterlogging pretreatment during vegetative growth improves tolerance to waterlogging after anthesis in wheat. Plant Sci. 180, 672–678. https://doi.org/10.1016/j.plantsci.2011.01.009 (2011).
Mendanha, T., Rosenqvist, E., Nordentoft Hyldgaard, B., Doonan, J. H. & Ottosen, C. O. Drought priming effects on alleviating the photosynthetic limitations of wheat cultivars (Triticum aestivum L.) with contrasting tolerance to abiotic stresses. J. Agron. Crop. Sci. 206, 651–664. https://doi.org/10.1111/jac.12404 (2020).
Wang, X. et al. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 75, 677–687. https://doi.org/10.1007/s10725-014-9969-x (2015).
Abid, M. et al. Pre-drought priming sustains grain development under post-anthesis drought stress by regulating the growth hormones in winter wheat (Triticum aestivum L.). Planta. 246, 509–524. https://doi.org/10.1007/s00425-017-26984 (2017).
Martinez-Medina, A. et al. Recognizing plant defense priming. Trends Plant Sci. 21, 818–822. https://doi.org/10.1016/j.tplants.2016.07.009 (2016).
Abid, M. et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 145, 12–20. https://doi.org/10.1016/j.envexpbot.2017.10.002 (2018).
Auler, P. A. et al. Stress memory of physiological, biochemical and metabolomic responses in two different rice genotypes under drought stress: The scale matters. Plant Sci. 311, 110994–111003. https://doi.org/10.1016/j.plantsci.2021.110994 (2021).
Tabassum, T., Farooq, M., Ahmad, R., Zohaib, A. & Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 118, 362–369. https://doi.org/10.1016/j.plaphy.2017.07.007 (2017).
Saeidnia, F., Majidi, M. M., Mirlohi, A. & Soltan, S. Physiological and tolerance indices useful for drought tolerance selection in smooth bromegrass. Crop Sci. 57, 282–289. https://doi.org/10.2135/cropsci2016.07.0636 (2017).
Song, Q., Liu, C., Bachir, D. G., Chen, L. & Hu, Y. G. Drought resistance of new synthetic hexaploid wheat accessions evaluated by multiple traits and antioxidant enzyme activity. Field Crops Res. 210, 91–103. https://doi.org/10.1016/j.fcr.2017.05.028 (2017).
Li, A., Liu, D., Yang, W., Kishii, M. & Mao, L. Synthetic hexaploid wheat: yesterday, today, and tomorrow. Crop Genet. Breed. 4, 552–558. https://doi.org/10.1016/j.eng.2018.07.001 (2018).
Mendanha, T., Rosenqvist, E., Hyldgaard, B. & Ottosen, C. O. Heat priming effects on anthesis heat stress in wheat cultivars (Triticum aestivum L.) with contrasting tolerance to heat stress. Plant Physiol. Biochem. 132(213–221), 2018. https://doi.org/10.1016/j.plaphy.2018.09.002 (2018).
Ritchie, S. W., Nguyen, H. T. & Holaday, A. S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci. 30, 105–111. https://doi.org/10.2135/cropsci1990.0011183X003000010025x (1990).
Lichtenthaler, H. K. & Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 1, 4–3. https://doi.org/10.1002/0471142913.faf0403s01 (2001).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil. 39, 205–207. https://doi.org/10.1007/BF00018060 (1973).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. https://doi.org/10.1021/ac60111a017 (1956).
Khamesi, F., Amini, A. & Ehsanzadeh, P. Chickpea response to saline water: Concurrence of ion homeostasis sustainment and antioxidative defense measures. S. Afr. J. Bot 133, 245–252. https://doi.org/10.1016/j.sajb.2020.08.001 (2020).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232 (1981).
Herzog, V. & Fahimi, H. D. A new sensitive colorimetric assay for peroxidase using 3, 3′-diaminobenzidine as hydrogen donor. Anal. Biochem. 55, 554–562. https://doi.org/10.1016/0003-2697(73)90144-9 (1973).
SAS Institute. SAS user’s guide. Release 9.2. SAS Inst. Inc., Cary, NC (2008).
Sun, L. et al. Cold priming induced tolerance to subsequent low temperature stress is enhanced by melatonin application during recovery in wheat. Molecules 23, 1091–1102. https://doi.org/10.3390/molecules23051091 (2018).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. AA conducted the study, collected and analyzed the data. MMM supervised and administered the project. NM and MG helped in data collection. The first draft of the manuscript was written by AA and all authors commented on this versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amini, A., Majidi, M.M., Mokhtari, N. et al. Drought stress memory in a germplasm of synthetic and common wheat: antioxidant system, physiological and morphological consequences. Sci Rep 13, 8569 (2023). https://doi.org/10.1038/s41598-023-35642-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35642-2
This article is cited by
-
Drought Priming May Enhance the Tolerance of Cotton Seedlings to Subsequent Drought Stress
Plant Molecular Biology Reporter (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.