Abstract
Triple-negative breast cancer (TNBC) subtype is characterized by aggressive clinical behavior and poor prognosis patient outcomes. Here, we show that ADAR1 is more abundantly expressed in infiltrating breast cancer (BC) tumors than in benign tumors. Further, ADAR1 protein expression is higher in aggressive BC cells (MDA-MB-231). Moreover, we identify a novel interacting partners proteins list with ADAR1 in MDA-MB-231, using immunoprecipitation assay and mass spectrometry. Using iLoop, a protein–protein interaction prediction server based on structural features, five proteins with high iloop scores were discovered: Histone H2A.V, Kynureninase (KYNU), 40S ribosomal protein SA, Complement C4-A, and Nebulin (ranged between 0.6 and 0.8). In silico analysis showed that invasive ductal carcinomas had the highest level of KYNU gene expression than the other classifications (p < 0.0001). Moreover, KYNU mRNA expression was shown to be considerably higher in TNBC patients (p < 0.0001) and associated with poor patient outcomes with a high-risk value. Importantly, we found an interaction between ADAR1 and KYNU in the more aggressive BC cells. Altogether, these results propose a new ADAR-KYNU interaction as potential therapeutic targeted therapy in aggressive BC.
Similar content being viewed by others
Introduction
Breast cancer (BC) presents a major challenge disease despite advancement in early detection and improved treatments due to its heterogeneity among different patients. Well-recognized studies have classified breast cancer tumors into four main intrinsic molecular subtypes with distinct prognostic and therapy implications1. Triple negative breast cancer (TNBC) is the most aggressive subtype of BC and is frequently classified as a subtype of basal-like BC2,3. Basal-like has unfavorable prognosis and aggressive tumor biology with limited therapy4. In contrast, Luminal A-subtype tumors have the most favorable prognosis and tumor biology with sufficient endocrine therapy5. Therefore, understanding the distinctive molecular mechanisms of cancer biology and development will facilitate discovery of new methods/mechanisms and targets for cancer therapy.
RNA editing is a prevalent post-transcriptional modification in the human transcriptome that alters RNA sequence without changing the genomic DNA sequence6,7,8. Despite the discovery of RNA editing more than 30 years ago, it brought a new understanding of genetic complexity that may impact various human diseases, including cancer6,7,8. In mammals, A-to-I RNA editing is the most widespread modification of editing events that involves editing specific nucleotide changes in double-stranded (ds) RNA increasing the diversity of transcriptome. This modification is mediated by a particular enzyme family designated as adenosine deaminase acting on RNAs (ADARs)9,10,11.
Adenosine Deaminase Acting on RNA 1 (ADAR1), a part of the ADARs enzyme family, catalyzes the irreversible conversion of adenosine to inosine by deamination within cellular dsRNA9,10,11. Several studies of ADAR1 expression levels and A-to-I RNA editing events alteration in cancer have been reported in the last few years. Indeed, Prestige’s studies discovered an up-regulation in RNA editing events as well as ADAR1 enzyme expression levels among 17 cancer types from 6,236 patient samples through using ‘The Cancer Genome Atlas’, a large-scale cancer sequencing project13,14. In BC, they found an increase in editing frequency and ADAR expression levels in 68 cancerous breast tissues compared to normal13,14,15. It has been found that higher ADAR1 expression was linked to tumor-infiltrating lymphocytes in patients with TNBC16. Recent finding has shown that ADAR1 promotes BC by regulating the cell cycle and DNA damage response17. ADAR1 was reported to be essential for TNBC transformation and tumorigenesis18. All above studies emphasized the vital role of ADAR1/A-to-I RNA editing process in BC progression and evaluated ADAR1 as the promising therapeutic target in BC. However, the mechanisms and proteins that regulate ADAR1/A-to-I RNA editing process remain mostly unidentified.
The study of protein–protein interaction (PPI) in the cellular milieu provides significant insight into a protein’s functions and mechanism. The usual way to capture PPI is experimental and labor-intensive19. Since, interacting protein pairs share a lot of structural, phylogenetic, and evolutionary features, different bioinformatics algorithms have been developed to exploit these features to predict PPI at a genome-wide scale20. With the surge of genome sequencing technology, the genomes of the organisms have been sequenced at a faster pace and thus gave birth to bioinformatics methods exploiting sequence features to predict PPI21. A wide variety of machine learning techniques have been used to predict PPI22. More recently, deep learning methods are also coming up for the prediction of PPI23,24,25,26.
In this work to gain insights into the interacting ADAR1 patterners, we used immunoprecipitation (IP) and mass spectrometry (MS)-based proteomics approaches to identify potential interacting proteins. We have identified several novel proteins interacting with ADAR1 in BC cells. In aggressive BC cells, five proteins were discovered using iLoop, a protein–protein interaction prediction website based on structural features: Histone H2A.V, Kynureninase (KYNU), 40S ribosomal protein SA, Complement C4-A, and Nebulin. For the first time, we discovered a novel physical interaction between ADAR1 and Kynureninase (KYNU) in aggressive BC cells. These findings could provide valuable resources for understanding cancer progression mechanisms and pave the way for new therapeutic targets in BC.
Materials and methods
Antibodies and reagents
Various antibodies were used, including anti-ADAR1 rabbit polyclonal antibody (Thermo Fisher), anti-Normal rabbit IgG (Sigma Aldrich), anti-GAPDH rabbit monoclonal antibody (Abcam), anti-KYNU rabbit polyclonal antibody (Thermo Fisher), anti-ADAR1 mouse monoclonal antibody (Thermo Fisher). Secondary antibodies were used, including goat anti-mouse (Abcam) and goat anti-rabbit IgG HRP (Thermo Fisher). For western blotting analysis, the primary antibodies dilutions were 1:1000 and GAPDH was used as the loading control. Secondary antibodies for western blotting analysis were diluted to 1:7000. Additional reagents used include pierce protein A/G agarose (Thermo Fisher), Protease inhibitor cocktail, EDTA-Free (100X) (Thermo Fisher), RIPA buffer, Pierce ip lysis buffer (Thermo Fisher), Sterile conical centrifuge tubes 15 ml (Thermo Fisher), cell culture flask 25 cm (Thermo Fisher).
Cell culture
Dr. Alia Al-Amoudi's lab (King Abdulaziz University/King Fahad Medical Research Center, KSA) provided the MDA-MB-231 and MCF7 human BC cell lines. MDA-MB-231 cells were cultured in DMEM media (Thermo Fisher) containing 10% fetal bovine serum (FBS) (Thermo Fisher); while MCF7 cells were maintained in RPMI 1640 media (Thermo Fisher) containing 10% FBS (Thermo Fisher).
Western Blotting
Western blot analysis was carried out by preparing total protein lysates with RIPA lysis buffer (Thermo Fisher) and a cocktail of protease inhibitors (Thermo Fisher). 8% SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to resolve and separate 30 μg of total cellular proteins. Proteins are transferred to a nitrocellulose membrane. Membranes were blocked in 5% skimmed milk. The membranes were then incubated with the appropriate primary and secondary antibodies overnight at 4 °C.
Immunoprecipitation and co-immunoprecipitation
IP lysis buffer (Thermo Fisher) and a protease inhibitor cocktail (Thermo Fisher) were used to generate protein lysates. 1 μg of anti-ADAR1 antibody was bound to 40 μl of protein A/G beads (Thermo Fisher) and incubated with 500 μl of total protein cell lysates. All the mixture incubated overnight at 4 °C. The beads’ washing step was achieved by using IP buffer. The beads were washed four times. Then, western blotting was accomplished by using SDS-PAGE gel and detected using appropriate primary and secondary antibodies.
Immunofluorescence
Cell were seeded on coverslips until they reached between 80 to 90% confluency. The fixation step was accomplished by incubating the cell coated coverslips in 4% paraformaldehyde for 12 min at room temperature (RT). The permeabilization step was performed by incubating the cell coated coverslips in (0.2% Triton X-100) for 20 min at RT. Cells were then blocked for 45 min at RT with 2% goat serum. Cells were incubated overnight at 4 °C with a specific primary antibody (anti-ADAR1 mouse monoclonal antibody (Thermo Fisher). The primary antibody was diluted 1:200. The cells were then incubated for one hour at RT with the secondary antibody. Then, cells were incubated with the mounting media with the DAPI for few minutes. Cells were imaged using confocal microscopy (Zeiss LSM 700) fitted with a × 20 and × 40 objective.
Tissue microarray
Tissue microarrays (TMA)/(BRC1021) were obtained from Pantomics Inc. The TMA includes 102 human BC patient’s samples with different information such as age, grade, stage and TNM. The H&E-stained slides were revised by a pathologist to confirm the BC tumor cases.
Automated immunostaining
Immunohistochemistry (IHC) was performed in King Fahad Medical Research Center (KFMRC), IHC units, KSA following Automated immunostaining process27. The primary rabbit polyclonal antibody anti-ADAR1 (Thermo Fisher) with 1:1000 dilution was used and incubated at RT for one hour. The TMA slides were scanned using Grundium slide scanner.
Scoring and evaluation of ADAR1 expression
ADAR1 protein expression’s evaluation was determine blindly of the clinicopathological features by using regular Nikon light microscope (40X magnification). The intensity of IHC staining was classified into four groups: (0): no/negative expression; (1 +): weak expression; (2 +): moderate, and (3 +): high expression. The scoring and evaluation was done in the KFMRC, IHC units, KSA27. The following formula using equation form was used to calculate both the intensity and the fraction of positively stained cells: I = 0xf0 + 1xf1 + 2xf2 + 3xf3. Following that, the staining was divided into two categories: (1) low (no/weak) expression, and (2) high (moderate/strong) expression27.
Statistical analysis
Statistical analysis was accomplished using the SPSS® (IMB NY, USA) software packages (PASW Statistics for Windows, version 19). The Chi-square test was performed to analyze frequency tables and to calculate the significance of the correlations between the categorical variables. P-values < 0.05 were considered as statistically significant.
Mass spectrometry
Protein was digested and desalted as described elsewhere28. In 0.1% formic acid, the dried peptide mixture was redissolved. The experiment was performed in King Abdullah University of Science and Technology, KSA, proteomics units as described elsewhere29,30.
Results and discussion
ADAR1 is highly expressed in infiltrating BC tumors than the benign tumors irrespective of the clinical-pathological parameters
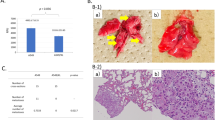
To study the clinical relevance of the role of ADAR1 in human BC, we explored the ADAR1 expression in human BC TMA. The TMA of 102 cases that include 97 BC cases and five normal/benign breast tissues. Importantly, we found that ADAR1 expression was higher (score above the mean value (= 131)) in 62/97 (64%) in BC cases compared to 0/5 (0%) benign cases (p = 0.003) (Fig. 1A,B). Then, we examined whether ADAR1 expression correlated with different clinicopathological parameters such as age and tumor grade and stage, we observed no significant correlation (Supplementary Table 1). Furthermore, we detect no statical correlation between ADAR1 protein expression and recognized biomarkers that define basic BC classification, including estrogen receptor (ER), progesterone receptor (PR) or human epidermal growth factor receptor 2 (HER2) tumor status (Supplementary Table 1). To validate these findings, we used a publicly available database including 5696 BC patients, Gene-Expression Miner v4.8 (bcGenExMiner v4.8), and found no significant association between ADAR1 mRNA levels and ER, PR, or HER2 (Supplementary Fig. 1A–C). Our results show that ADAR1 is highly expressed in infiltrating BC tumors than in benign tumors, regardless of the clinical pathological parameters.
ADAR1 expression in breast cancer. (A) ADAR1 protein expression in malignant versus benign cases; (B) Positive immunohistochemical staining of ADAR1 in normal adjacent tissue, in situ and invasive breast cancer lesions (10X and 40X); (C) Immunoblot analysis of total cell lysates of MDA-MB-231 and MCF7 cells using antibodies against ADAR1and GAPDH; (D) Confocal immunofluorescence images of ADAR1 (green) and nucleus (Dapi) (blue) of breast cancer cells MDA-MB-231 and MCF7. Scale bars, 30 μm; (E) ADAR1 mRNA expression level in non-TNBC in comparison to TNBC tumor tissues using bcGenExMiner v4.8 database; (F) ADAR1 mRNA expression level in with or without lymph nodes involvements in tumor tissues using bcGenExMiner v4.8 database.
To further investigate the role of ADAR1 in BC tumorigenesis, we examined the expression of ADAR1 in human BC cells, including MDA-MB-231 and MCF7 (represent Triple negative and Luminal A, respectively). Interestingly, we found higher ADAR1 protein expression in the more aggressive BC cell (MDA-MB-231) than the less aggressive cell (MCF7) (Fig. 1C). While similar patterns of ADAR1 localization, primarily nuclear and cytoplasmic, were observed in both cells (Fig. 1D). Moreover, ADAR1 mRNA expression was found to be significantly higher in TNBC patients (p < 0.003) and lymph node positive BC patients (LN +) (p < 0.0001) using bcGenExMiner v4.8 database (Fig. 1E,F). Collectively, these findings suggest that ADAR1 is highly expressed in aggressive BC cells and may play a role in aggressive BC behavior.
Analysis of endogenous ADAR1 protein interactors in aggressive BC cells, TNBC (MDA-MB-231)
To discover the ADAR1 protein partner, we first performed an IP assay to pull down the ADAR1 enzyme in the TNBC MDA-MB-231 cells. Normal IgG pull-down was included as negative control and total lysate as the positive control. Western blotting was carried out using primary antibody against ADAR1. Indeed, we found enrichments (bands) of ADAR1 in MDA-MB-231 cells (Fig. 2A), whereas the band was not present in the IgG control. As a result, ADAR1 IP from BC cells was successful.
ADAR1 interaction partners in breast cancer cells. (A) MDA-MB-231 cells were lysed, and immunoprecipitations were performed using a rabbit polyclonal antibody against ADAR1 or control normal rabbit IGg. Western blotting was carried out using an antibody against ADAR1; (B) The interaction network of ADAR1. The ADAR1 interactome in MDA-MB-231 cell line was generated based on structural features using iLoops and visualized in Cytoscape. The thickness of the edge correlates with iLoops score; (C) MCF7 cells were lysed and immunoprecipitations were performed using a rabbit polyclonal antibody against ADAR1 or control normal rabbit IGg. Western blotting was carried out using an antibody against ADAR1; (D) The interaction network of ADAR1. The ADAR1 interactome in MCF-7 cell line was generated based on structural features using iLoops and visualized in Cytoscape. The thickness of the edge correlates with iLoops score.
Next, to identify the ADAR1 proteins interaction we performed MS analysis including two biological replicates and 3 technical replicates. The preparation of samples including digestion/desalted and MS analysis was performed by King Abdullah University of Science and Technology, KSA. The MS data were analyzed using Proteome discoverer (version2.5). The Uniprot human database (released June 25, 2021) was used for the database search. Hundreds of proteins were identified as shown in the table S2 (Supplementary Table 2). The list of identified proteins was then filtered manually by removing contaminates proteins and keeping only these identified on both biological replicates. Furthermore, only significant differentially expressed proteins with p value < 0.05 and fold change > 2 compared to control sample (IgG) were included. These filtration steps generated a total 13 proteins for MDA-MB-231 (Supplementary Table 3).
To further gain insights into the understanding of ADAR1 interact patterners overall, we explore ADAR1 physical interact partner to a publicly available database STRING, a biological database of protein–protein interaction prediction network (STRING DB V 10.5-http://string-db.org/). We found seven proteins (Supplementary Fig. 2): UPF1, DICER1, ILF3, RBMX, ELAVL1, AXIN1 and HDLBP interacted with ADAR based on experimental data such as IP assay in different kind of diseases and posttranscriptional regulatory process31,32,33,34. However, none of these interactions have been studied in BC. Consequently, we found no common proteins between our finding and STRING result. Therefore, we used iLoops, a protein–protein interaction prediction server based on structural features, to investigate the interactome of ADAR1 in the aggressive BC cell line (MDA-MB-231). As a result, five of the 13 proteins were found to interact with the ADAR1 enzyme with a high iloop_score (ranged between 0.6 and 0.8). As shown in Fig. 2B, these proteins are Histone H2A.V, KYNU, 40S ribosomal protein SA, Complement C4-A, and Nebulin.
To further confirm our results, we did the same IP assay and MS experiment followed by the same analysis for the less aggressive BC cell (luminal A subtype), MCF7. As a result, 82 proteins with a high iloop score were found to interact with the ADAR1 enzyme (Fig. 2C,D, and Supplementary Table 4). Interestingly, none of the 82 proteins in MCF7 shared any similarities with the five proteins identified in MDA-MB-231 cells. These results indicate that these five proteins, Histone H2A.V, KYNU, 40S ribosomal protein SA, Complement C4-A and Nebulin, are specific for interacting with ADAR1 enzyme in TNBC, which is define as the most aggressive subtypes of BC.
Implication of Histone H2A.V, KYNU and 40S ribosomal protein SA with poor patient outcome in BC
Next, to validate the role of Histone H2A.V, KYNU, 40S ribosomal protein SA, Complement C4-A and Nebulin in BC progression, we examine the mRNA levels and patient outcomes in relation to relapse free survival (RFS). Kaplan–Meier plotter, an extensive gene profiling database including more than 5 thousand BC cases, was used. Interestingly, high mRNA levels of Histone H2A.V, KYNU, and 40S ribosomal protein SA were found to be significantly associated with decreased RFS and an unfavorable prognosis with high-risk value (1.34, 1.33 and 1.2, respectively) (Fig. 3A–C). On the other hand, Complement C4-A and Nebulin showed less risk factor (0.66 and 0.86, respectively) and were associated with increased RFS and favorable prognosis (Fig. 3D,E).
Following the above results, patients with higher Histone H2A.V, KYNU, and 40S ribosomal protein SA gene expression levels exhibited significantly shorter overall survival (p = 0.018, 0.015, and 1.014, respectively) and distant metastasis free survival (Fig. 4A–F). Overall, these results indicate that the top three predicted ADAR1 interacted proteins (Histone H2A.V, KYNU, and 40S ribosomal protein SA) are linked to an aggressive BC phenotype.
Expression of Histone H2A.V, KYNU, and 40S ribosomal in BC patients
Next, to determine the involvement in BC tumorigenesis, we examined the expression level of the Histone H2A.V, KYNU and 40S ribosomal protein SA using a publicly available database including 5696 BC patients, Gene-Expression Miner v4.8 (bcGenExMiner v4.8). Based on diverse histological classes, Histone H2A.V mRNA expression was significantly higher in invasive ductal carcinomas (IDC) than in invasive lobular carcinomas (p = 0.05). However, there was no significant difference between IDC and other classifications such as mucinous carcinoma (Fig. 5A). IDC, well-known as infiltrating ductal carcinoma, is the most invasive type which can spread to lymph nodes or blood vessels and metastasize throughout the body. We found that Histone H2A.V mRNA expression levels were higher in non-TNBC patients based on TNBC/IHC status (Fig. 5B). Interestingly, in comparison to other classifications, invasive ductal carcinomas had the highest level of KYNU gene expression, followed by invasive lobular carcinoma (p < 0.0001) and mucinous carcinomas (p < 0.0001) (Fig. 5C). Moreover, KYNU mRNA expression was found to be significantly higher in TNBC patients (p < 0.0001) (Fig. 5D).
Next, we performed the same analysis on 40S ribosomal expression and found that IDC had higher mRNA levels but no significant difference from other classifications (Fig. 5E). 40S ribosomal expression was to be higher in TNBC patients (p < 0.0001) (Fig. 5F). Our findings show that upregulation of KYNU expression is the most meaningfully associated with IDC, TNBC, and poor prognosis patient outcomes.
Detection of ADAR1 and KYNU interaction in aggressive BC cell
KYNU is enzymes that play a vital role in tryptophan metabolism through the degradation of intermediate 3'-hydroxy-kynurenine metabolites to produce 3‐hydroxyanthranilic acid. KYNU contributes to the biosynthesis of NAD cofactors from tryptophan via the kynurenine pathway. KYNU has been reported being associated with various diseases such as metabolic, neurological, cardiac and renal disease. The role of KYNU in BC development has not been illustrated and is mostly unclear. Recently, it was found that KYNU promotes tumor cell invasion via CD4435. These finding suggest that the interaction between ADAR1 and KYNU may play a role in ADAR1 oncogenic pathway in aggressive BC. Indeed, we found interaction between ADAR1 and KYNU in MDA-MB-231 cell (Fig. 6A). Next, we evaluate the subcellular localization of KYNU. Interestingly, KYNU was found to be perinuclear, whereas ADAR1 was found in both the nuclear and cytoplasm (Fig. 6B). These findings suggest that the ADAR1-KYNU interaction is important in aggressive BC cells and could be a potential therapeutic target. However, more research is needed to understand the mechanism underlying the ADAR1-KYNU interaction's role in BC.
KYNU and ADAR1 interaction in aggressive breast cancer cell and subcellular localization. (A) MDA-MB-231 cell were lysed and immunoprecipitations were performed using a rabbit polyclonal antibody against ADAR1 or control normal rabbit IGg. Western blotting was carried out using a rabbit polyclonal antibody against KYNU (upper panel). Membrane was reprobed using a rabbit polyclonal against ADAR1 (lower panel); (B) Confocal immunofluorescence images of KYUN (red), ADAR1 (green) (using mouse monoclonal antibody against ADAR1) and nucleus (Dapi) (blue) of breast cancer cell MDA-MB-231. Scale bars, 150 μm.
Conclusion
This study revealed a novel interaction partner ADAR1 enzyme that may play a critical role in mediating the oncogenic process of ADAR1 in aggressive BC. One of the top potential candidates, KYNU, was found to be expressed and is the most meaningfully correlates with invasive ductal carcinomas, TNBC, and poor prognosis patient outcomes. Interestingly, we found an interaction between ADAR1 and KYNU in MDA-MB-231 cells, with perinuclear localization of KYNU, which may play an important role in the aggressiveness of BC. Overall, these findings contribute to a better understanding of the mechanisms underlying cancer progression and the identification of new therapeutic targets in breast cancer.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. The data that support the findings of this study are available from KAUST but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of KAUST.
Abbreviations
- BC:
-
Breast cancer
- TNBC:
-
Triple negative breast cancer
- ADARs:
-
Adenosine deaminases acting on RNA
- KYNU:
-
Kynureninase
- FBS:
-
Fetal bovine serum
- IP:
-
Immunoprecipitation
- RT:
-
Room temperature
- IHC:
-
Immunohistochemistry
- TMA:
-
Tissue microarrays
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- MS:
-
Mass spectrometry
- RFS:
-
Relapse free survival
- IDC:
-
Invasive ductal carcinomas
References
Guiu, S. et al. Molecular subclasses of breast cancer: How do we define them? The IMPAKT 2012 Working Group Statement. Ann. Oncol. 23, 2997–3006 (2012).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Prat, A. et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. The Breast 24, S26–S35 (2015).
Sørlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Gao, J. J. & Swain, S. M. Luminal A breast cancer and molecular assays: A review. Oncologist 23, 556–565 (2018).
Stuart, K. RNA editing: Trypanosomes rewrite the genetic code. Verh. K Acad. Geneeskd. Belg. 60, 63–74 (1998).
Bass, B. L. & Weintraub, H. J. C. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 55, 1089–1098 (1988).
Bass, B. L. & Weintraub, H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 55, 1089–1098 (1988).
Bass, B. L. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 71, 817–846 (2002).
Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 79, 321–349 (2010).
Bass, B. et al. A standardized nomenclature for adenosine deaminases that act on RNA. RNA 3, 947 (1997).
Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 79, 321 (2010).
Fumagalli, D. et al. Principles governing A-to-I RNA editing in the breast cancer transcriptome. Cell Rep. 13, 277–289 (2015).
Paz-Yaacov, N. et al. Elevated RNA editing activity is a major contributor to transcriptomic diversity in tumors. Cell Rep. 13, 267–276 (2015).
Kang, L. et al. Genome-wide identification of RNA editing in hepatocellular carcinoma. Genomics 105, 76–82 (2015).
Song, I. H. et al. ADAR1 expression is associated with tumour-infiltrating lymphocytes in triple-negative breast cancer. Tumor Biol. 39, 1010428317734816 (2017).
Sagredo, E. A. et al. ADAR1 Transcriptome editing promotes breast cancer progression through the regulation of cell cycle and DNA damage response. Biochim. Biophys. Acta (BBA) 1867, 118716 (2020).
Kung, C.-P. et al. Evaluating the therapeutic potential of ADAR1 inhibition for triple-negative breast cancer. Oncogene 40, 189–202 (2021).
Stelzl, U. et al. A human protein-protein interaction network: A resource for annotating the proteome. Cell 122, 957–968 (2005).
Kar, G., Gursoy, A. & Keskin, O. Human cancer protein-protein interaction network: A structural perspective. PLoS Comput. Biol. 5, e1000601 (2009).
Murakami, Y. & Mizuguchi, K. Recent developments of sequence-based prediction of protein–protein interactions. Biophys. Rev. 14, 1393–1411 (2022).
Sarkar, D. & Saha, S. Machine-learning techniques for the prediction of protein–protein interactions. J. Biosci. 44, 104 (2019).
Soleymani, F., Paquet, E., Viktor, H., Michalowski, W. & Spinello, D. Protein–protein interaction prediction with deep learning: A comprehensive review. Comput. Struct. Biotechnol. J. 20, 5316–5341 (2022).
Hu, X., Feng, C., Ling, T. & Chen, M. Deep learning frameworks for protein-protein interaction prediction. Comput. Struct. Biotechnol. J. 20, 3223 (2022).
Lei, Y. et al. A deep-learning framework for multi-level peptide–protein interaction prediction. Nat. Commun. 12, 5465 (2021).
Wang, P., Zhang, G., Yu, Z.-G. & Huang, G. A deep learnING And XGBoost-based method for predicting protein-protein interaction sites. Front. Genet. 12, 752732 (2021).
Hakamy, S. et al. Assessment of prognostic value of tissue inhibitors of metalloproteinase 3 (TIMP3) protein in ovarian cancer. Libyan J. Med. 16, 1937866 (2021).
Hughes, C. S. et al. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 10, 757 (2014).
Zhang, H. & Bensaddek, D. Narrow precursor mass range for DIA-MS enhances protein identification and quantification. Life 11, 982 (2021).
Zhang, H. et al. Arabidopsis proteome and the mass spectral assay library. Sci. Data 6, 1–11 (2019).
Agranat, L., Raitskin, O., Sperling, J. & Sperling, R. The editing enzyme ADAR1 and the mRNA surveillance protein hUpf1 interact in the cell nucleus. Proc. Natl. Acad. Sci. USA 105, 5028–5033 (2008).
Ota, H. et al. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell 153, 575–589 (2013).
Malty, R. H. et al. A map of human mitochondrial protein interactions linked to neurodegeneration reveals new mechanisms of redox homeostasis and NF-κB signaling. Cell Syst. 5, 564–577 (2017).
Wang, Q., Zhang, Z., Blackwell, K. & Carmichael, G. G. Vigilins bind to promiscuously A-to-I-edited RNAs and are involved in the formation of heterochromatin. Curr. Biol. 15, 384–391 (2005).
Al-Mansoob, M., Gupta, I., Stefan Rusyniak, R. & Ouhtit, A. KYNU, a novel potential target that underpins CD44-promoted breast tumour cell invasion. J Cell Mol Med 25, 2309–2314 (2021).
Acknowledgements
We would like to express our gratitude to Dr Huoming Zhang (Proteomics Core Lab, Bioscience Core Lab, Kind Abdullah University of Science and Technology), Dr. Anwar Hashem Lab’s members (King Fahad Medical Research Center, KSA), Dr. Abdelbaset Buhmeida (and GenaTi Tissue & Pathology Research Service (GTPRS) Unit) and Dr. Alia Al-Amoudi's lab (King Fahad Medical Research Center, KSA).
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, grant No# (G: 270–665-1442).
Author information
Authors and Affiliations
Contributions
N.B.: Designed, performed experiments, and drafting the article. M.A.: Contributed to drafting the article. B.A.: Analyzed MS experiments. M.Y.: Analyzed MS experiments. M.R.: Performed bioinformatics data analyses. A.T.: Contributed to drafting the article. N.A.: Performed bioinformatics data analyses. G.A.: Contributed to drafting the article. M.K.: Assisted in performing the experiments. S.S.: contributed to drafting the article. F.A.: Assisted in performing the experiments. S.A.: contributed to drafting and reviewing the article. R.A.: contributed to drafting the article. M.G.: contributed to drafting the article. A.H.: contributed to drafting the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Binothman, N., Aljadani, M., Alghanem, B. et al. Identification of novel interacts partners of ADAR1 enzyme mediating the oncogenic process in aggressive breast cancer. Sci Rep 13, 8341 (2023). https://doi.org/10.1038/s41598-023-35517-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35517-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.