Abstract
Traumatic brain injury (TBI) is a silent epidemic that has been easily ignored. The safety and efficacy of restarting antiplatelet therapy after encountering traumatic brain injury (TBI) events remain a challenge. We explored the outcomes of restarting aspirin use on secondary stroke and mortality in patients with chronic stroke 4 weeks after suffering from a TBI episode in Taiwan. This study analyzed data from the National Health Insurance Research Database from January 2000 to December 2015. Overall, 136,211 individuals diagnosed with chronic stroke who suffered from acute TBI and received inpatient service were enrolled. The study outcomes were a competing risk of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality. We identified a case group of 15,035 patients with chronic stroke (mean [SD] age of 53.25 [19.74] years; 55.63% male) who restarted aspirin use 4 weeks after suffering from TBI and a control group of 60,140 patients with chronic stroke (mean [SD] age of 53.12 [19.22] years; 55.63% male) who discontinued aspirin use after suffering from TBI. The risk of hospitalization of secondary ischemic stroke [adjusted hazard ratio (aHR) 0.694; 95% confidence interval (CI) 0.621–0.756; P < 0.001] and hemorrhagic stroke (aHR 0.642; 95% CI 0.549–0.723; P < 0.001) and all-cause mortality (aHR 0.840; 95% CI 0.720–0.946; P < 0.001) significantly decreased in patients with chronic stroke restarting aspirin use 1 month after suffering from TBI events (including intracranial hemorrhage) in comparison with the control subjects, regardless of those with or without diabetes mellitus, chronic kidney disease, myocardial infarction, atrial fibrillation, clopidogrel use, and dipyridamole use. Restarting aspirin use could lower the risks of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality in patients with chronic stroke 1 month after suffering from TBI episodes.
Similar content being viewed by others
Introduction
Acute stroke, the most severe and disabling neurological disorder worldwide, is a crucial public health concern, and over the past decades, there have been progress in prevention and treatment. The benefits of aspirin in preventing early recurrent ischemic changes are more significant than earlier recognized1. Other strategies to prevent acute stroke comprise alternative anticoagulants warfarin for atrial fibrillation, intravenous alteplase, and carotid stenting with or without endovascular thrombectomy for treating acute ischemic stage2. Pharmacological and interventional therapies promise to facilitate brain rehabilitation and functional recovery3,4. Despite decreasing stroke mortality through these treatments, the global burden of acute stroke is on the rise. A comprehensive approach targeting people at risk for other diseases who share common risk factors to prevent secondary stroke is obligatory.
Traumatic brain injury (TBI) is a silent epidemic that has been easily ignored5; it is associated with epilepsy6, cognitive decline7,8, and psychiatric conditions9. TBI has also been reported to be associated with a tenfold risk of stroke within 3 months, such as risks of increasing ischemic stroke and intracerebral hemorrhage, according to a study with a total of 23,199 patients10. It has been postulated that a TBI causes brain damage, further involving the loss of brain functions by triggering disturbances in the blood supply to the brain and leading to a stroke11. Patients with TBI often experience traumatic cerebral microbleeds (also known as hemorrhagic diffuse axonal injury) associated with the severity of the injury, especially in moderate-to-severe traumatic brain injury patients. Brain microbeads are useful markers for determining the nature and severity of the underlying chronic condition of small vessels12. Moreover, Microbleeds International Collaborative Network, new risk scores incorporating clinical variables and cerebral microbleeds, provide predictive value with the long-term risks of intracranial hemorrhage and ischaemic stroke in patients with chronic stroke for secondary stroke prevention13. Thus, we need initiatives to raise patient and public awareness about the risk of stroke following a TBI, especially in the first few months.
However, up to date, there is little evidence of the outcome of restarting aspirin use on secondary stroke in patients with chronic stroke following a TBI episode. In this study, using the National Health Insurance Research Database (NHIRD) in Taiwan, we explored the outcomes of secondary stroke (ischemic and hemorrhagic) and all-cause mortality in patients with chronic stroke with and without starting aspirin use 4 weeks after suffering from a TBI episode in Taiwan.
Methods
Data source
The Taiwan National Health Insurance system is a universal single-payer insurance system and enrolled all these insurance schemes into a single national insurance system. The NHIRD provides longitudinal databases which randomly sampled two million beneficiaries from the original NHIRD14. The representativeness of NHIRD has been validated by Taiwan’s National Health Research Institutes14. Informed consent is waived due to personal information that had been de-identified in the NHIRD and informed consent waiver was approved by the Institutional Review Board of the Tri-Service General Hospital (IRB Number: B-109-36). All information allowing an enrolled patient to be identified was encrypted. This study was carried out in accordance with the approved protocol and the Declaration of Helsinki. All experimental protocols were approved by the Institutional Review Board of the Tri-Service General Hospital, Taipei, Taiwan. A request for the analytic methods should be sent to the corresponding author.
This retrospective study investigated all patients diagnosed with chronic stroke who suffered from a TBI episode and received inpatient service linked with the NHIRD. The time interval between chronic stroke and TBI was within half one year. All patients were identified based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes in the Registry of Catastrophic Illness in 2000. Information on the study's identification was collected within 15 years—specifically, data were recorded from January 2000 to December 2015.
Study design and outcomes
Using the Taiwan NHIRD, patients with chronic stroke following a TBI episode and who received inpatient service were collected in the following two groups: aspirin users and nonusers. The definition of TBI included intracranial hemorrhage at the same time (Supplemental Table S1). This study was conducted using the inpatient and outpatient claims data recorded from January 2000 to December 2015. The aspirin group was made up of patients with chronic stroke in whom aspirin use was restarted 4 weeks after suffering from a TBI episode. The control group was made up of patients with chronic stroke in whom aspirin use was still discontinued after they suffered from a TBI episode.
We excluded patients whose ages and genders were not recorded, who were younger than 40 years, who had a minimum of 2 years of unavailable data following TBI initiation, who were chronic stroke and in whom aspirin use was not prescribed within 6 months before suffering from TBI, who had a history of cancer and human immunodeficiency virus, and anticoagulation (warfarin and rivaroxaban) user.
The index date was defined as the start of aspirin use at 4 weeks after suffering from a TBI episode in patients with chronic stroke. The study follow-up period started from the index date to the onset date of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality. We assigned a date for the control patients who did not continue aspirin use in chronic stroke after suffering from a TBI episode, which matched their corresponding case patients (referred to as the index date). Because controls did not have aspirin use, they were assigned a date for a pseudo-aspirin event, which corresponded to the index date of their matched patients (referred to as the index date hereafter).
Finally, cases and controls were all matched for age, gender, and covariates, including comorbidities and medications that had continued use for 6 months before suffering from a TBI episode (Supplemental Table S1). We used the incidence density sampling approach to match controls with each case according to age (± 1 year), sex, and the follow-up period of aspirin use in patients with chronic stroke after suffering from TBI. This approach allowed us to observe both patient groups for similar periods, thus eliminating the bias caused by time frame differences.
The study outcomes were the competing risk of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality. The outcome of interest was the first hospitalization for stroke causes from the NHI claims database after study initiation. All-cause mortality within the 15 years follow-up period was considered an event of interest. The causes of mortality after study initiation, such as ischemia stroke, hemorrhagic stroke, myocardial infarction, arrhythmia, out-of-hospital cardiac arrest, and infectious disease, were also assessed according to the primary diagnostic codes entered in the inpatient claims and emergency claims 30 days before death. Patients were followed up from the index date to the earliest of the following: outcome occurrence, death, disenrollment of the NHI program, or December 31, 2015.
Covariates
Baseline demographic and clinical characteristics recorded before the index date were obtained. The comorbidities included diabetes mellitus (DM), chronic kidney disease (CKD), myocardial infarction, atrial fibrillation, hypertension, hyperlipidemia, heart failure, peripheral vascular disease, chronic pulmonary disease, chronic liver disease, and dementia. We measured the risk of outcomes in patients who had received medications within 6 months before the index date. The prescribed medications included DM and non-DM medications. The major classes of DM medications included metformin, sulfonylureas, thiazolidinediones, a-glucosidase inhibitor, dipeptidyl peptidase 4 inhibitors, and insulin. The non-DM medications included clopidogrel, dipyridamole, antiplatelet drug, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, calcium channel blockers, statins, and nonsteroidal anti-inflammatory drugs. The ICD-9-CM disease diagnostic codes used for previous or coexisting diseases and the Anatomical Therapeutic Chemical codes used for medications are listed in Supplemental Table S1.
Statistical analysis
We applied propensity score matching to balance the two groups with respect to known confounders and to ensure comparability during analysis15. Propensity score methods for reducing the effects of confounding in observational studies mimic some of the particular characteristics of a randomized controlled trial16. We adjusted for potential confounding variables of the comorbidities, DM medications, and non-DM medication on the basis of clinical relevance. Continuous variables are reported as mean (SD) depending on the distribution of the data. Categorical variables are reported as numbers and percentages. The patients’ baseline characteristics were compared using standardized differences that reflect the mean difference as a percentage of the standard deviation, as indicated by Mamdani et al.17. We compared baseline characteristics between the group that restarted aspirin for chronic stroke patients who suffered from TBI episodes in the matched control group using standardized differences, with a standardized difference greater than 10% considered meaningful. All variables in the analysis were complete, which were missing for less than 0.5% of the cohort. The SAS statistical software (SAS System for Windows, version 9.1.3; SAS Institute, Cary, NC, USA) was used for statistical analyses. The χ2 test and t-test were used to analyze and evaluate the differences in age or comorbidities and medications between the aspirin and non-aspirin groups. The Fisher's exact test was applied for categorical variables to statistically compare the differences between the two cohorts. The Cox proportional regression hazards model was used to compare the incidence rates of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality between the aspirin and non-aspirin groups after the modification of comorbidities. We tested the proportionality assumption by adding a time-dependent exposure covariate to the model. The Kaplan–Meier method and log-rank test were used to estimate the outcomes of the two groups. A two-tailed P value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
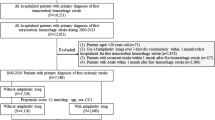
A total of 136,211 individuals diagnosed with chronic stroke who suffered from TBI for the first time and received inpatient service were enrolled in this study, as shown in Fig. 1. This study had been conducted from January 2000 to December 2015. Of these individuals, 17,910 patients with chronic stroke suffering from TBI were excluded [including patients whose ages and genders were not recorded, those younger than 40 years, without at least 2 years of data available following TBI initiation, without aspirin use in chronic stroke patients within 6 months before suffering from head TBI, a history of cancer and human immunodeficiency virus, and anticoagulation (warfarin and rivaroxaban) user]. The baseline characteristics of those subjects before using the propensity score matching was showed in Supplemental Table S2. After using the propensity score matching according to the index date, gender, age, comorbidities, and medications, we identified a case group of 15,035 patients with chronic stroke (mean [SD] age of 53.25 [19.74] years; 55.63% male) who restarted aspirin use 1 month after suffering from TBI and a control group of 60,140 patients with chronic stroke (mean [SD] age of 53.12 [19.22] years; 55.63% male) who discontinued aspirin use after suffering from TBI. Table 1 shows the baseline characteristics of patients with chronic stroke who did or did not receive aspirin following a TBI episode after using the propensity score matching. There were no significant differences in gender, age, and all covariates, including comorbidities and medications between the groups. The standardized differences before and after using the propensity score matching was showed in Supplemental Table S3. In this study, the mean ± standard deviation of follow-up was 9.24 ± 5.17 years, with a minimum of 0.01 years and a maximum of 15.87 years, and the median was 7.22 years.
Effects of restarting aspirin use on secondary stroke 4 weeks after suffering from TBI episodes in chronic stroke patients
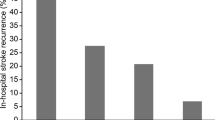
The cumulative incidence of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality in the Cox model with competing risks are shown in Table 2 and Fig. 2. The competing risk of hospitalization of secondary ischemic stroke [adjusted hazard ratio (aHR), 0.694; 95% confidence interval (CI) 0.621–0.756; P < 0.001] and hemorrhagic stroke (aHR 0.642; 95% CI 0.549–0.723; P < 0.001) and all-cause mortality (aHR 0.840; 95% CI 0.720–0.946; P < 0.001) in patients with chronic stroke restarting aspirin use 4 weeks after suffering from TBI episodes significantly decreased in comparison with the control subjects during the follow-up period after adjusting for gender, age, comorbidities, and medications in Taiwan.
Furthermore, we performed a subgroup analysis of the association between aspirin use and risks of secondary stroke (ischemic and hemorrhagic) hospitalization and all-cause mortality (Supplemental Tables S4 and S5). Regardless of their underlying diseases, including DM, CKD, myocardial infarction, and atrial fibrillation, and medications, including clopidogrel and dipyridamole, restarting aspirin use still had beneficial effects on lowering the risks of secondary stroke hospitalization (ischemia and hemorrhage) in patients with chronic stroke 1 month after TBI, as compared with that in the matched control group that discontinued aspirin use after TBI (Supplemental Table S4). For all-cause mortality, moreover, restarting aspirin was associated with decreased death risk in patients with and without DM, myocardial infarction, and medications, including clopidogrel and dipyridamole use (Supplemental Table S5).
Cumulative dosages of aspirin were also associated with reducing the risk of hospitalization (ischemic and hemorrhagic stroke) and all-cause mortality in those patients with TBI episodes, as shown in Table 3, respectively. Ischemic stroke, hemorrhagic stroke, and myocardial infarction were significantly associated with the causes of all-cause mortality after restarting aspirin use in patients with chronic stroke 1 month after TBI episodes (Supplemental Table S6).
Furthermore, we defined severe TBI as TBI cases with long-stay hospitalization or surgical treatment. As shown in Supplemental Table S7 for severe TBI, we found that besides all-cause mortality, there is a lower competing risk of hospitalization of secondary ischemic stroke and hemorrhagic stroke after restarting aspirin use 1 month after TBI episodes in patients with chronic stroke.
Discussion
The purpose of aspirin use in patients with chronic stroke is to ameliorate stroke progression and secondary attack, but restarting aspirin use following TBI episodes remains a significant challenge, especially for hemorrhage tendency. Our study explored the outcomes of secondary stroke (ischemic and hemorrhagic) hospitalization and mortality in patients with chronic stroke with and without restarting aspirin use 4 weeks after suffering from a TBI episode in Taiwan. After multivariate adjustment and subgroup analysis, this study presented that restarting aspirin use is associated with reducing secondary stroke, including ischemic and hemorrhagic, and all-cause mortality in patients with chronic stroke with TBI episodes (including intracranial hemorrhage episodes) 4 weeks later. Regardless of those with or without DM, CKD, myocardial infarction, and atrial fibrillation and clopidogrel, and dipyridamole, restarting aspirin use remains safe and beneficial in lowering secondary stroke hospitalization and all-cause mortality in patients with chronic stroke 4 weeks after TBI episodes.
The safety and efficacy of restarting antiplatelet therapy after encountering TBI episodes remain a challenge. Despite expectations that restarting aspirin following episodes of hemorrhage-associated stroke might increase the risk for a recurrent brain hemorrhage, researchers have found the opposite18,19,20,21,22,23. For example, in the RESTART trial that recruited 573 subjects with chronic stroke following a brain hemorrhage, the researchers found that restarting antiplatelet agents did not increase the rate of a recurrent brain hemorrhage, which indicated that hemorrhagic episodes were found not to differ between the restart and avoid-antiplatelet therapy groups significantly18. Also, the RESTART study showed that restarting antiplatelet agents tended to reduce brain hemorrhage (adjusted HR, 0.51; 95% CI 0.25–1.03; P = 0.060); this effect seems to be primarily derived from the prevention of thrombotic events19,21. Similarly, our study observed that restarting aspirin therapy is associated with a low risk of hemorrhagic stroke in patients with chronic stroke suffering TBI episodes.
No expert consensus or strategies elucidated the benefits and risks of restarting aspirin in patients with chronic stroke with TBI episodes. Emerging report of a meta-analysis that combined observational cohort for 1916 patients showed that aspirin resumption had a lower risk for thromboembolic events (relative risk, 0.61; 95% CI 0.48–0.79; P < 0.01) without increasing the risk of brain hemorrhage recurrence in patients with primary intracranial hemorrhage19. Our study also indicated that restarting aspirin decreased the risk of ischemic stroke in patients with chronic stroke with TBI episodes, similar to those previously reported19,24. Furthermore, we observed that restarting aspirin benefited from survival in chronic stroke with TBI episodes, regardless of those with or without DM, myocardial infarction, and atrial fibrillation and clopidogrel, and dipyridamole. An unnecessary closing of aspirin therapy in those patients might increase the ischemic or thromboembolic risk. Thus, whether to restart aspirin uses or not requires physicians to balance ischemic and hemorrhagic risk.
The optimal timing of restarting antiplatelet therapy in patients with TBI or brain hemorrhage remains unclear. This study indicated that restarting aspirin had lower all-cause mortality in those patients 1 month after TBI episodes. In a nationwide cohort study of three Danish nationwide registries between 1997 and 2013, restarting anticoagulant treatment had lower all-cause mortality (HR, 0.55; 95% CI 0.37–0.82) 6 weeks after intracranial hemorrhage in patients with atrial fibrillation24. Moreover, Gonzalez-Perez et al.25 showed that significantly improved survival was associated with the current uses of starting low-dosage aspirin 30 days after a hemorrhagic stroke episode during a median of 6.4 years. On the contrary, aspirin discontinuation was associated with increased mortality (aHR 1.54; 95% CI 1.21–1.97)25. For those survivors of cerebral hemorrhage, recovery is slow. Minor patients could recover near-complete functioning within 30 days after the stroke; however, most recovery from intracranial hemorrhage occurs in the first few months after the stroke26. Therefore, the optimal timing for restarting antiplatelet agents 4 weeks after an episode of TBI or brain hemorrhage to decrease vascular risks is relatively safe, particularly in patients on antiplatelet therapy before the episode.
Our study’s strengths are the large sample size, the analysis of a national database, the consideration of the cumulative dose effect of drug use, and the subgroup analysis. This study minimized the selection bias and confounding factors by all matching the control group for age, sex, comorbidities, and medications. Despite its strengths and novelty, this study has some limitations that require clarifications. First, NHIRD does not provide laboratory data for further investigation. Second, lifestyle and personal habits cannot be obtained from the NHIRD. Third, previous stroke etiology, TBI severity, and skull fracture may have a bias in the use of aspirin. Forth, given that this study was an observational study of drug epidemiology and not a randomized controlled trial, there may have been allocation bias and prescription bias. Although a randomized controlled trial is the best way to demonstrate pharmaceuticals’ effects, most medical research is still conducted using drug epidemiology27,28,29. There are many situations where a randomized controlled trial is unsuitable. For example, there may be unethical occurrences, like adverse effects or other outcomes, studying drug interactions, a genetic disposition to diseases, and examining drug overdose.
Conclusions
This study revealed that restarting aspirin use is associated with reducing the occurrence of secondary stroke (ischemic and hemorrhagic) and all-cause mortality 4 weeks after a TBI episode (including intracranial hemorrhage epsiodes) in patients with chronic stroke. Prospective studies are required before providing any definitive recommendations regarding the resumption's optimal timing and method.
Data availability
The datasets collected and analyzed in our study are available from the corresponding author (Wu-Chien Chien, email: chienwu@ndmctsgh.edu.tw) on reasonable request.
References
Chen, Z. M. et al. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40,000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke 31, 1240–1249 (2000).
Campbell, B. C. V. & Khatri, P. Stroke. Lancet 396, 129–142 (2020).
Szelenberger, R., Kostka, J., Saluk-Bijak, J. & Miller, E. Pharmacological interventions and rehabilitation approach for enhancing brain self-repair and stroke recovery. Curr. Neuropharmacol. 18, 51–64 (2020).
Chavez, J. C., Hurko, O., Barone, F. C. & Feuerstein, G. Z. Pharmacologic interventions for stroke: Looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke 40, e558-563 (2009).
Langlois, J. A., Marr, A., Mitchko, J. & Johnson, R. L. Tracking the silent epidemic and educating the public: CDC’s traumatic brain injury-associated activities under the TBI Act of 1996 and the Children’s Health Act of 2000. J. Head Trauma Rehabil. 20, 196–204 (2005).
Lowenstein, D. H. Epilepsy after head injury: An overview. Epilepsia 50(Suppl 2), 4–9 (2009).
Shively, S., Scher, A. I., Perl, D. P. & Diaz-Arrastia, R. Dementia resulting from traumatic brain injury: what is the pathology?. Arch Neurol 69, 1245–1251 (2012).
Ramos-Cejudo, J. et al. Traumatic brain injury and alzheimer’s disease: The cerebrovascular link. EBioMedicine 28, 21–30 (2018).
Fleminger, S. Long-term psychiatric disorders after traumatic brain injury. Eur. J. Anaesthesiol. Suppl. 42, 123–130 (2008).
Chen, Y. H., Kang, J. H. & Lin, H. C. Patients with traumatic brain injury: Population-based study suggests increased risk of stroke. Stroke 42, 2733–2739 (2011).
Hekmatpanah, J. & Hekmatpanah, C. R. Microvascular alterations following cerebral contusion in rats. Light, scanning, and electron microscope study. J. Neurosurg. 62, 888–897 (1985).
Puy, L. et al. Cerebral microbleeds: from depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 92, 598–607 (2021).
Best, J. G. et al. Development of imaging-based risk scores for prediction of intracranial haemorrhage and ischaemic stroke in patients taking antithrombotic therapy after ischaemic stroke or transient ischaemic attack: A pooled analysis of individual patient data from cohort studies. Lancet Neurol. 20, 294–303 (2021).
Hsieh, C. Y. et al. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 11, 349–358 (2019).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383 (1987).
Austin, P. C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 46, 399–424 (2011).
Mamdani, M. et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 330, 960–962 (2005).
Collaboration, R. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet 393, 2613–2623 (2019).
Ding, X. et al. Resumption of antiplatelet therapy in patients with primary intracranial hemorrhage-benefits and risks: A meta-analysis of cohort studies. J. Neurol. Sci. 384, 133–138 (2018).
Biffi, A. et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 75, 693–698 (2010).
Flynn, R. W., MacDonald, T. M., Murray, G. D., MacWalter, R. S. & Doney, A. S. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke 41, 2606–2611 (2010).
Chong, B. H. et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thromb. Haemost. 107, 241–247 (2012).
Guha, D., Coyne, S. & Macdonald, R. L. Timing of the resumption of antithrombotic agents following surgical evacuation of chronic subdural hematomas: A retrospective cohort study. J. Neurosurg. 124, 750–759 (2016).
Nielsen, P. B. et al. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: A nationwide cohort study. Circulation 132, 517–525 (2015).
Gonzalez-Perez, A., Gaist, D., de Abajo, F. J., Saez, M. E. & Garcia Rodriguez, L. A. Low-dose aspirin after an episode of haemorrhagic stroke is associated with improved survival. Thromb. Haemost. 117, 2396–2405 (2017).
Winstein, C. J. et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke 47, e98–e169 (2016).
McMahon, A. D. & MacDonald, T. M. Design issues for drug epidemiology. Br. J. Clin. Pharmacol. 50, 419–425 (2000).
Herman, J. Experiment and observation. Lancet 344, 1209–1211 (1994).
Vandenbroucke, J. P. Is the randomized controlled trial the real paradigm in epidemiology?. J Chronic Dis 39, 572–574 (1986).
Acknowledgements
We appreciate Taiwan’s Health and Welfare Data Science Center and Ministry of Health and Welfare (HWDC, MOHW) for providing the National Health Research Database. Data are available from the National Health Insurance Research Database (NHIRD) published by Taiwan’s National Health Insurance (NHI) Administration. Due to the legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw, accessed on 30th Dec, 2020).
Funding
This study was supported by the Tri-Service General Hospital Research Foundation (TSGH-B-112020) and was financially sponsored by the Taipei Medical University (TMU108-AE1-B23). The sponsor has no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.L.C., C.H.C, W.C.C., S.E.T., L.Y.F. had full access to all the data in the study and taken responsibility for the integrity and the accuracy of the data analysis. Concept and design: C.L.C., C.H.C, W.C.C., S.E.T., L.Y.F. Acquisition, analysis, or interpretation of data: C.L.C., C.H.C, Y.H.H., C.C.W., C.A.S., W.C.C., S.E.T., L.Y.F. Drafting of the manuscript: C.L.C., C.H.C, W.C.C. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: C.H.C, W.C.C. Administrative, technical, or material support: all authors. Supervision: W.C.C., S.E.T., L.Y.F.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chou, CL., Chung, CH., Hsu, YH. et al. Risk of secondary stroke subsequent to restarting aspirin in chronic stroke patients suffering from traumatic brain injury in Taiwan. Sci Rep 13, 8001 (2023). https://doi.org/10.1038/s41598-023-34986-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34986-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.