Abstract
Bilinguals with a high proficiency in their first (L1) and second language (L2) often show comparable reaction times when switching from their L1 to L2 and vice-versa (“symmetrical switch costs”). However, the neurophysiological signatures supporting this effect are not well understood. Here, we ran two separate experiments and assessed behavioral and MEG responses in highly proficient Spanish-Basque bilinguals while they overtly name pictures in a mixed-language context. In the behavioral experiment, bilinguals were slower when naming items in switch relative to non-switch trials, and this switch cost was comparable for both languages (symmetrical). The MEG experiment mimicked the behavioral one, with switch trials showing more desynchronization than non-switch trials across languages (symmetric neural cost) in the alpha band (8–13 Hz). Source-localization revealed the engagement of right parietal and premotor areas, which have been linked to language selection and inhibitory control; and of the left anterior temporal lobe (ATL), a cross-linguistic region housing conceptual knowledge that generalizes across languages. Our results suggest that highly proficient bilinguals implement a language-independent mechanism, supported by alpha oscillations, which is involved in cue-based language selection and facilitates conceptually-driven lexical access in the ATL, possibly by inhibiting non-target lexical items or disinhibiting target ones.
Similar content being viewed by others
Introduction
Nowadays, bilingualism – mastery of more than one language1 – is not an exception but common practice in many societies. Some countries estimate the percentage of bilinguals to be above 65% and constantly growing [EuroStat, 2016]. In daily life, bilingual speakers seem to fluently switch from one language to another while avoiding cross-language interference. However, to achieve this apparent effortless behavior, bilinguals need to control their languages in use. Most of the experimental evidence from bilingual language control comes from studies using language-switching paradigms in which participants have to alternate between two languages in response to cues. For example, a bilingual might be instructed to name pictures in Language A when the picture is presented with a red cue but use Language B when the cue is blue. This includes switch trials (requiring alternations between the two languages) and non-switch trials (where participants stay in the same language). Typically, higher error rates and longer naming latencies are observed on the switch compared to non-switch trials, with the difference between them referred to as the switch cost effect (e.g.2).
Unbalanced bilinguals, who are more proficient in their dominant first language (L1) than in their weaker second language (L2), typically show larger switch costs when switching from the L2 to the L1 than vice versa2. One of the most prominent models of bilingual language control, the Inhibitory Control Model3,4, explains this paradoxical asymmetry via an inhibitory mechanism, in which the magnitude of the suppression is proportional to language proficiency and activation. Given that a more proficient language (L1) might be more active, more inhibition would be applied to suppress L1 interference during L2 naming and, hence, more effort/time would be required to release its residual effect when switching back to the L1. In other words, if there is a difference in proficiency between the two languages, the switch cost should be asymmetric because more inhibition is required to suppress the dominant L1.
On the other hand, this model predicts that for bilinguals showing similar proficiency in both languages, the switch cost should be symmetric, given that the amount of inhibition deployed to control for activation across languages should be equivalent. This prediction is indeed supported by behavioral data from highly proficient bilinguals showing symmetric switch costs when alternating between languages during picture-naming tasks in mixed-language contexts5,6,7,8,9,10.
From a neuroanatomical standpoint, numerous neuroimaging studies have explored the neural substrates supporting language switching in bilinguals. These studies have pointed to the involvement of cortical and subcortical regions related to language control but also to domain-general executive control11. Among these regions, the most commonly reported are the caudate, the anterior cingulate cortex (ACC), the supplementary motor area (SMA), the prefrontal cortex (PFC), the inferior frontal gyrus (IFG), and temporo-parietal areas comprising the supramarginal gyrus (SMG) and the superior frontal gyrus (SFG)3,8,9,12,13,14,15.
When considering the timing of switching in bilinguals, previous neurophysiological evidence from M/EEG studies focused on evoked responses (ERPs/ERFs) indicates that language control effects (i.e. switch vs. non-switch trials) emerge between ~ 200 ms and 600 ms after stimulus onset. This time window corresponds to the classical N2 and N400 components16,17. On the one hand, early N2 modulations have been proposed to reflect top-down control, possibly in the form of response inhibition or disengagement from the non-target language, with larger N2 responses for the switch as compared to non-switch trials, and with this effect being present only in the less dominant language18,19,20. On the other hand, N400 responses occurring later in time are thought to index semantic aspects of language processing. N400 modulations following switch trials have been suggested to reflect a greater demand in the integration of lexical and semantic representations21 or the effort necessary to overcome the inhibition of the previous language22. Earlier studies indicate that this component is sensitive to language proficiency, with individuals with low proficiency in their L2 showing deviant N400 responses23,24. Interestingly, recent evidence25 from balanced, highly proficient bilinguals shows similar N400 semantic responses across L1 and L2, suggesting that, as language proficiency improves, conceptual representations become semantically processed in the same way in the L1 and the L23,26.
However, classical time-locked ERP/ERF analysis is blind to information not phase-locked to the stimuli, resulting in less sensitivity when tapping into ongoing neurocognitive dynamics associated with bilingual language processing27,28. Relative to ERP/ERF, time–frequency analysis can better characterize the temporal dynamics of oscillations contained in the brain signal. Indeed, oscillatory activity is thought to play a key role in neural communication and to reflect distinct cognitive operations at different frequency bands29, providing a fine-grained characterization of neurophysiological mechanisms supporting cognition. Previous findings point to the involvement of two main oscillations in bilingual language control: theta (4–7 Hz) and alpha (8–13 Hz) rhythms17. On the one hand, theta power increases have been reported for L2 as compared to L1 switching during speech production in low proficient Chinese-English bilinguals with high inhibitory control abilities, possibly indexing cross-language interference at the lexical selection level30. Another study on word production in unbalanced Dutch-English bilinguals31, found theta power increases after participants selected the wrong language for speaking during cued language switching, reflecting a role for theta in the monitoring of speech errors. Similarly, in non-linguistic tasks in which participants have to deal with conflicting information (e.g. Go-no go, Flanker task), theta power increases have been observed in incongruous as compared to congruous trials32,33,34, thus supporting its broader involvement in executive control (e.g. conflict monitoring) under situations of increased cognitive demands.
On the other hand, oscillatory activity in the alpha frequency band (8–13 Hz) has been consistently linked to functional inhibition35,36,37. Under this view, alpha is considered a general mechanism that subserves various cognitive processes that use inhibitory control in tasks requiring interference suppression. When considering language control, bilinguals tend to exhibit overall higher alpha power than monolinguals38, with this power correlating with L1 and L2 experience-related measures. Furthermore, alpha oscillations have been linked to lexico-semantic access in highly proficient bilinguals39 and are thought to shape inhibition in semantic association networks, allowing the controlled retrieval of information from long-term memory36,40.
Altogether, these findings suggest that theta and alpha frequency bands might play a key role in bilingual language control. Nevertheless, only a few studies have attempted to investigate the oscillatory dynamics subserving this process during speech production30. Furthermore, to the best of our knowledge, no study has approached the topic while considering highly proficient bilinguals. To address this gap, we separately assessed behavioral and MEG responses in two independent groups of highly proficient Spanish-Basque bilinguals while they performed a picture-naming task requiring the utterance of nouns in a mixed-language context (i.e. alternating between languages within the same block depending on a color cue).
In line with previous evidence suggesting that highly proficient bilinguals show similar behavioral switch costs when switching from L1 to L2 and from L2 to L1, we expected symmetrical switch costs for Spanish and Basque. At the oscillatory level, we predicted similar differences between switch and non-switch trials across languages (i.e. symmetric neural costs) likely reflected in theta and alpha modulations between ~ 200 ms and ~ 600 ms, with this effect potentially engaging language control and more domain-general cognitive control regions.
Results
Online behavioral results
The 2-way ANOVA performed on the RTs obtained in the online experiment showed a main effect of Trial type (F1, 20 = 35.10, p < 0.0001, ηp2 = 0.64), suggesting that switch trials (Mean = 1.33 secs; SD = 0.251 secs) were named more slowly than non-switch trials (Mean = 1.24 secs; SD = 0.236 secs). No main effect of Language (F1, 20 = 3.18, p = 0.09, ηp2 = 0.14) nor the interaction between Trial type and Language (F1, 20 = 2.34, p = 0.14, ηp2 = 0.11) reached significance, suggesting that switch costs across Spanish (Mean = 0.106 secs) and Basque (Mean = 0.073 secs) were similar. See Fig. 1.
Because the lack of a significant interaction does not necessarily mean evidence for the absence of an effect, we conducted a Bayesian ANOVA that allows quantifying evidence for and against its presence. We used the JASP software41 with its default prior (Cauchy distribution, r = 0.5), and computed the inclusion Bayes Factor (BFincl) for each main effect and interaction. Briefly, BFincl indicates how much more likely the data are under models that include a particular predictor compared with those that do not, allowing to determine the relative strength of evidence for each factor on the dependent variable. A BFincl > 1 provides evidence for inclusion, whereas a BFincl < 1, indicates evidence for exclusion42.
Our analysis revealed overwhelming evidence in favor of the inclusion of the Trial type factor (BFincl = 3.996e + 6), indicating that models that include this factor are 3.996e + 6 times more likely than those that do not. However, we found evidence against inclusion for both Language (BFincl = 0.66) and the interaction between Trial type and Language (BFincl = 0.57). Overall, this supports the conclusion of the frequentist ANOVA showing that switching effects were similar across languages.
Finally, we ran a third analysis using linear mixed-effects models (LMMs) to model individual variation in the data. In this analysis, we included Language, Trial type, and their interaction as fixed effects and participants and items (images) as random effects. Overall, results from the LMMs were comparable to those obtained with the ANOVA. While the effect of Trial type was significant (F = 6.16, p = 0.03), the effect of Language (F = 0.319, p = 0.58) and the interaction between Language and Trial type did not reach significance (F = 0.003, p = 0.95).
Sensor level results
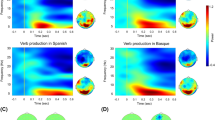
To analyze responses obtained in the MEG experiment, we used a cluster-based permutation approach to test for an effect of Language (Spanish vs. Basque) and Trial type (switch vs. non-switch). Then, following state-of-the-art pipelines for testing an interaction effect via means of a cluster-based permutation approach (www.fieldtriptoolbox.org/faq/how_can_i_test_an_interaction_effect_using_cluster-based_permutation_tests/); we subtracted switch and non-switch conditions within each language and compared the two differences. Overall, the effect of Language did not reach significance (all ps > 0.05) in any of the frequency bands (i.e. theta and alpha) or time windows (i.e. early and late). However, there was a significant effect of Trial type (Fig. 2A), as highlighted by a negative cluster in the alpha frequency band (8–13 Hz; Monte Carlo p = 0.01, two-tailed), showing stronger power decreases for the switch condition as compared to the non-switch one. This effect occurred in the late time window (i.e. 350-600 ms) and was localized in the right combined gradiometers (Fig. 2B). No Trial type effects were observed in the theta band. Finally, the Language x Trial type interaction did not reach significance (all ps > 0.05) in any of the frequency bands (i.e., theta and alpha) or time windows (i.e. early and late).
Oscillatory effects in bilingual speakers. (A) Topographic distribution plots for switch and non-switch conditions in the alpha frequency band (8–13 Hz) between 350 and 600 ms after object picture onset. (B) Time–frequency representation (TFR) showing the difference between switch and non-switch conditions across languages in the combined gradiometers highlighted by the significant alpha negative cluster. (C) Localization of alpha peaks (switch vs. non-switch) circumscribed to the time interval highlighted by the significant cluster. All plotted regions reached a p-value < 0.01.
Source localization of the MEG sensor-level results
Oscillatory effects at the sensor level were reconstructed at the source level on the frequency band and time window highlighted by the significant cluster (i.e. 8–13 Hz between 350 and 600 ms). Alpha peaks identified for the switching effect (switch vs. non-switch trials across languages) were localized in the right supramarginal gyrus (BA40), the right premotor/supplementary motor area (BA6), and the left anterior temporal lobe (BA38, see Fig. 2C).
Discussion
In the present study, we aimed to investigate the behavioral and oscillatory signatures supporting language control in highly proficient bilinguals by examining language switching during speech production. To this end, we ran two separate experiments in independent samples of highly proficient Spanish-Basque bilinguals and acquired behavioral and MEG responses during an overt picture naming task in a mixed-language context. Results from the behavioral experiment, revealed overall slower responses in switch as compared to non-switch trials (i.e. a switch cost). Importantly, this switch cost was comparable across the two languages, replicating previous findings of symmetrical switch effects in bilinguals with similar L1-L2 proficiency. Time–frequency results from the MEG experiment, revealed comparable neural switch costs in Spanish and Basque, showing significant power decreases in the alpha frequency band (8–13 Hz) between 350 and 600 ms after picture onset for switch compared to non-switch trials, irrespectively of the language at use. This effect was source-localized in domain-general (e.g. right SMG and PM/SMA) and language-specific (left ATL) control regions. Overall, we report behavioral and neuromagnetic evidence on the existence of a common (i.e., same for the two languages) control mechanism in highly proficient bilinguals, which supports cue-based language selection and controlled access to lexico-semantic representations during speech production.
When considering behavioral findings, bilingual speakers exhibited language switch costs (i.e. increased reaction times for switch vs. non-switch trials). This effect is well predicted by previous literature43, suggesting that switching involves an effort associated with system reconfiguration (i.e. choosing a different language from the one previously used). According to the IC model, one of the key mechanisms that underpin language switching is inhibition: bilingual speakers suppress the non-target language to properly produce a response in the target one3,4,44. According to this view, if L1 and L2 proficiency levels are balanced, symmetric switch costs should be observed, indicating that the amount of inhibition deployed to control for language interference is similar. Our findings of symmetric switch costs in Spanish and Basque align well with the IC model and with previous behavioral evidence on cued language switching5,6,7,8,9,10,45, suggesting the existence of comparable levels of control when bilinguals are highly proficient in both languages and acquire both during early childhood.
A similar pattern of responses was observed when considering MEG results, with bilinguals showing equivalent neural switch costs across languages (i.e. no effect of language nor interaction between language and trial type). Switch and non-switch conditions (collapsed across languages) significantly differed between 350 and 600 ms after picture onset, with alpha power (8–13 Hz) decreases being stronger for the switch compared to non-switch trials. From a broader standpoint, alpha oscillations have been proposed as a hallmark of inhibitory control and, in particular, as a fingerprint of controlled access to semantic knowledge stored in long-term memory36,37,46. In the language domain, alpha power decreases between ~ 300 ms and 500 ms after picture onset have been linked to the lexico-semantic processing of object-related knowledge during speech production in monolingual31,47 individuals. Furthermore, a similar spectro-temporal pattern has also been found in highly proficient bilinguals when naming pictures either in their L1 or L239. This aligns well with proposals suggesting that as language proficiency improves, L1 and L2 representations become semantically processed similarly3,26.
In the context of language switching, while there is evidence for a role of alpha power decreases in language control during comprehension48, to the best of our knowledge, this is the first study reporting its involvement during overt speech production.
Source localization of the alpha effect highlighted the involvement of language-specific (i.e. left ATL) and more domain-general executive control regions (i.e. right SMG and PMC/SMA). In highly proficient bilinguals, a similar activation pattern showing greater activity in the right SMG (BA40) and PMC/SMA regions (BA6) has been recently reported in neuroimaging studies for switch as compared to non-switch trials8,9,49. On the one hand, it has been proposed that bilateral inferior parietal cortices mediate language selection50. In particular, during switching, the right SMG's role would be biasing selection towards the target language – while its left counterpart would be responsible for biasing selection away from the language no longer in use. Interestingly, a recent study51 applying anodal transcranial direct current stimulation (tDCS) over the right parietal cortex shows a selective improvement in performance when switching to a recently inhibited task, supporting a broader role of this region in overcoming previous inhibition. Similarly, the SMA region has been implicated in proactive switching52, namely, in using contextual sensory cues hinting at the need for a change towards a new behavior. For instance, disruptive repetitive transcranial magnetic stimulation over the SMA selectively hampers individuals’ performance during the cue period that signals a switch trial53, suggesting that this region mediates cue-based prospective reconfiguration. This proposal aligns well with the cued language switching paradigm used in the present study, in which a color cue indicated to bilinguals whether a language change was required or not. Thus, the finding of parieto-prefrontal alpha oscillations mediating switching may reflect target language selection based on cue processing.
On the other hand, the left ATL (BA38) is proposed to play a key role in mapping concepts to words during speech production, with damage to this area resulting in specific deficits during conceptually-driven word retrieval54,55. Importantly, the ATL is thought to house language-invariant semantic representations in bilinguals39,56,57,58 and, in particular, object-related ones59,60,61. Indeed, it has been recently shown that highly proficient bilinguals recruit the ATL during object picture naming39. This finding aligns well with evidence suggesting that ATL involvement is critical when accessing conceptual knowledge from visual inputs62. Furthermore, the superior part of the left ATL exhibits strong connectivity with other areas highlighted by our source analysis (i.e. SMG and PM/SMA) during both task and rest63 and with the right parietal cortex during language switching in highly proficient bilinguals56. Of note, it has been shown64 that left ATL contribution to semantic processing is critical around 400 ms after stimulus onset, a time window that aligns well with our findings. Taken together, the source level results of the alpha effect suggest that the right parieto-prefrontal network may be involved in biasing language selection based on cue information. This network would facilitate conceptually-driven lexical access in the ATL, possibly by inhibiting non-target lexical items and/or disinhibiting target ones.
Limitations and avenues for further research
The present study is not without limitations. First, it is worth noting that, in our cued language switching paradigm, we used only one color cue per language. This leads to a potential confound between cue switching and language switching, which may explain our switch costs results (i.e. a language switch is also by definition a cue switch while a language non-switch is also by definition a cue non-switch). Nevertheless, based on previous studies10,65, we find this possibility unlikely. Indeed, it has been shown that even when cue-switch and language-switch are dissociated (e.g. by using multiple color cues per language), language-related switch costs are still substantial. Notably, one of these studies10 tested an equivalent group of highly proficient Spanish-Basque bilinguals and reported symmetrical switching costs similar to those found here, indicating that comparable results can be achieved when the cue and language switch are not confounded.
Second, another point that requires discussion is the fact that neuroimaging studies in highly proficient bilinguals have underscored the involvement of subcortical regions during language switching49. Here, we used MEG brain recordings, which are more suited to capture cortical activity. Furthermore, our source localization methodological approach is also biased towards detecting cortical regions underlying the sensor-level results. Thus, we cannot completely rule out that some subcortical regions may have also contributed to the observed effects, although our approach was not sensitive enough to detect them.
Third, our study was limited by the absence of a group of low proficient bilinguals. Including individuals with varying levels of language proficiency and diverse linguistic backgrounds in further studies could help capturing bilingualism as a spectrum of experiences that can differentially influence oscillatory brain dynamics. Therefore, future research should aim to address individual variability in L2 proficiency to provide a more comprehensive understanding of bilingual language control.
Finally, contrary to our expectations, no differences were observed in the theta frequency band (4–7 Hz). Theta power increases have been widely implicated in switch cost effects across language switching30,48,65 and task switching paradigms66,67,68,69. For instance, studies showing theta modulations during comprehension48 and, particularly, during speech production30, report power increases for switches into the L2 as compared to the L1. However, in the studies mentioned above, participants were low-proficiency bilinguals, and thus the executive control demands posed by cross-language interference may have been higher compared to bilinguals with more balanced proficiency levels in their L1 and L2. In line with this interpretation, it has been suggested that theta power increases may reflect higher executive control levels, possibly due to active monitoring of language selection errors31 or more demanding lexical search66. Thus, the absence of theta effects in our study likely reflects that mechanisms involved in monitoring potential speech errors and/or lexical access became tuned as proficiency increased. Future studies are required in order to disentangle this critical aspect.
Conclusions
The symmetrical switch and neural costs found in our study suggest that, during speech production, bilinguals with similar proficiency in their L1 and L2 recruit a common, language-independent control mechanism. This mechanism is supported by alpha oscillations sourced in parieto-prefrontal and ATL regions, which mediate language selection and facilitate conceptually-driven lexical access of the intended word.
Methods
Participants
Twenty-one Spanish-Basque bilingual speakers (M = 25.04 years; SD = 3.94, 16 females) were recruited through the BCBL Participa website (https://www.bcbl.eu/participa/) and received monetary compensation for their participation in the MEG study. Due to technical problems during behavioral data acquisition in the MEG experiment, the onset of verbal reaction times were lost for most of the participants. To compensate for this technical issue, we conducted the same experiment online in an independent sample of twenty-five Spanish-Basque bilinguals (M = 27.4 years; SD = 5.22, 9 females). This latter experiment was conducted online due to the restrictions imposed by the COVID-19 pandemic. Thus, it is worth noting that behavioral and MEG responses come from two independent samples of highly proficient Spanish-Basque bilinguals.
The sample size required for our 2 × 2 factorial design Language (Spanish and Basque) × Trial type (switch and non-switch) was determined with the MorePower software67. The expected effect size was set based on previous literature on behavioral switching in highly proficient bilinguals5,6,7,8,9 showing that reliable effects can be obtained using partial eta-squared (ηp2) values ranging between 0.25 and 0.5. We used an effect size of ηp2 = 0.35, an alpha level of 0.05 and a desired power of (1 − β) 85%, thus requiring at least 20 participants per group to yield adequate statistical power.
All participants were right-handed as assessed via the Edinburgh Handedness Inventory68 and had a normal or corrected-to-normal vision. None of the participants reported major medical, neurological, or psychiatric disorders. Prior to their inclusion in the study, all participants provided their written informed consent. The study protocol was approved by the Ethics Committee of the Basque Center for Cognition, Brain, and Language (BCBL) and was carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Before the experiments, all participants performed a language background questionnaire to collect detailed information about language age of acquisition and proficiency levels. Language proficiency was measured employing the Basque, English, and Spanish Test [BEST]69, using the semi-structured interview part of the test, which taps into fluency, lexical resources, grammatical aspects, and pronunciation (Likert-like scale with scores ranging from 1 to 5). The cut-off criteria for considering an individual as a highly proficient bilingual were scores ≥ 4 in their L2 (and L1). Furthermore, we used a composite score for assessing the percentage of language exposure in Spanish and Basque. The composite scores were calculated by averaging self-reported percentages of listening, writing, and speaking in each language mentioned above. These scores were further normalized using the min–max method. This method is commonly used for data normalization, and it preserves the relationships among the original data values. Higher scores indicate higher exposure to a language and are calculated as follows:
where v′ is a new, normalized value, v is the original value, min_A is the minimum value in the range, and max_A is the maximum one.
Overall, participants acquired their two languages in early childhood and had similar proficiency levels and exposure (see Table 1 for detailed information about group profiles).
Given that most of the participants across experiments (~ 72%) reported Spanish as their dominant language, we will refer to Spanish as L1 and Basque as L2.
Language switching task
Following previous switching paradigms5,8, we selected eight colorful hand-drawn pictures representing high-frequency objects as stimuli [Spanish/Basque names: "Perro"/"Txakurra" (dog); "Ventana"/"Leihoa" (window); "Oso"/"Hartza" (bear); "Gallina"/"Oiloa" (chicken); "Cuchillo"/"Labana" (knife); "Anillo"/"Eraztuna" (ring); "Camisa"/"Alkandora" (shirt); "Oreja"/"Belarria" (ear)]. The pictures were selected from a standardized battery developed by NEURE clinic® (https://www.neure.eu/) and matched in frequency. Frequencies for the Spanish words were calculated using the EsPal database70 and those for Basque words were calculated using the E-hirz database71. The mean frequency for Spanish words was 22.4 and for Basque 21.8 per million.
Participants were asked to overtly name the pictures in either Spanish or Basque depending on a color cue (e.g. red for Spanish and green for Basque) within the same block (i.e. mixed-language context). Participants were asked to name the pictures as fast and accurately as possible. Picture examples and trial structure are depicted in Fig. 3.
Examples of stimuli and experimental task. Each trial began with a fixation cross on the screen for 2 s followed by the picture presented for 3 s. Participants were requested to overtly name the observed item (e.g. “Camisa”) in either Spanish or Basque depending on a color cue (i.e. picture frame). ISI randomly varied between 2–3 secs.
A fixation cross appeared on the screen for ~ 2–3 secs. Then the picture was presented for 2 secs, and participants overtly named the observed item (e.g. "Camisa"). The picture remained on the screen for 2 secs regardless of the participants' voice onset. Inter-stimuli interval (ISI) randomly varied between ~ 2–3 secs. Reaction times and accuracy were collected online and stored for further processing offline.
Overall, there were two types of trials: (a) those in which participants had to switch between languages from one trial to another (switch trials), and (b) those in which participants had to name a picture in the same language as in the preceding trial (non-switch trials). Following the seminal work of2 in language switching during naming, the proportion of switch to non-switch trials was set to 30/70%. To control for this proportion, the list of the stimuli was pseudo-randomized. More specifically, a total of 336 pseudo-randomized trials were distributed into four conditions: (1) non-switch trials in Spanish (118 trials), (2) non-switch trials in Basque (118 trials), (3) switch trials in Spanish (50 trials), (4) switch trials in Basque (50 trials). Non-switch trials following a switch trial were eliminated to avoid the carry-over effects of switch trials; the first trial was also eliminated as it was impossible to quantify it as a switch or a non-switch trial. After the elimination, on average, 48 trials remained per condition.
The experiment was run at the BCBL facilities while magnetoencephalographic (MEG) signals were simultaneously recorded. The stimuli were presented on a white background in the center of a screen positioned ~ 1 m far from the participant. Participants' verbal responses were recorded using a high-resolution microphone. The online experiment was coded on Javascript using the jsPsych library and was run using the Cognition.run (https://www.cognition.run/) platform. Briefly, participants were instructed to sit in a quiet room to avoid distractions and to have a working microphone on their PCs. In addition, information about the browsers and operating systems required to ensure compatibility and avoid data loss was provided.
MEG and MRI data acquisition
MEG data were acquired in a magnetically shielded room at a sampling rate of 1000 Hz using a 306-channel (102 magnetometers and 204 planar gradiometers) Elekta Neuromag system (Helsinki, Finland). MEG signals were recorded at a 1 kHz sampling rate and online filtered at a bandwidth of 0.1–330 Hz. Participants' head position inside the helmet was continuously monitored throughout the experiment using 5 head-position indicator (HPI) coils. Six electrode pairs were used to measure horizontal and vertical ocular and cardiac activity. The standard fiducial landmarks (i.e. left and right pre-auricular points and nasion) plus ~ 300 additional points registered over the scalp and eyes/nose contours were digitalized and used to spatially align the MEG sensor coordinates to the native T1 high-resolution 3D structural MRI of each participant. T1s were acquired with a Siemens 3 T magnetom prismafit MR scanner (Siemens, Munich, Germany) in a separate session with the following parameters: echo time = 2.97 ms, non-switching time = 2530 ms, flip angle = 7° and field of view = 256 × 256 × 176 mm3, number of axial slices = 176, slice thickness = 1 mm, in-plane resolution = 1 mm × 1 mm.
Online behavioral assessment and preprocessing
Verbal responses acquired during the online experiment were recorded using the participant's hardware of choice (e.g. headphones, microphone, built-in microphone). For safety reasons and to make online data collection possible, the audio files were recorded as .webm files encoded in a base64 string. For processing speech data, a semi-automated open-source in-house software ("SPONGE") was developed using Python (https://github.com/Polina418/Audio_processing). The software was used to decode and convert the audio files into .wav format, semi-automatically detect speech onsets (with manual online correction), and perform speech recognition using Google Cloud Speech-to-Text API. Speech recognition results were manually corrected offline.
Reaction times were measured as the interval between picture presentation and the onset of the participant's verbal response, disregarding all background noise preceding the target response. Participants had a maximum of 3 s to give their responses. Trials in which the participant made a mistake or mumbled prior to the target word (e.g. "Hmmm dog") were excluded from further analysis (~ 3%). Four participants were removed from the online experiment due to the low quality of the audio recordings. Thus, the final analysis was performed on a reduced sample of twenty-one participants.
MEG data acquisition and preprocessing
Continuous data were preprocessed offline using the temporal extension of the signal space separation method (tSSS)72 implemented in Maxfilter 2.2 (Elekta-Neuromag). Briefly, tSSS subtracts external magnetic noise from the MEG recordings, corrects for head movements, and interpolates bad channels. Subsequent analyses were performed using the MatlabR2014B and FieldTrip toolbox version 20,170,91173. Recordings were down-sampled to 500 Hz and segmented into epochs time-locked to picture presentation from 1000 ms before image onset to 1000 ms after image onset.
A semi-automatic procedure was employed to remove epochs containing electromyographic artifacts, SQUID jumps, and flat signals. Finally, a fast independent component analysis (fast ICA) was used to identify components reflecting blinks and electrocardiographic artifacts74. Two participants were discarded from the final analysis due to a high number of blinking/muscular artifacts in the MEG recording (e.g. leading to > 40 kept trials in some conditions). Thus, the final MEG analysis was performed on a reduced sample of nineteen participants.
Data analysis
Behavioral analysis
Naming accuracy in all participants was at ceiling (Spanish Mean = 99.38%, SD = 1.14; Basque Mean = 99.49%, SD = 0.96); thus, statistical analyses were only performed on the RTs. RTs analyses were conducted on the correct trials. Individual RTs were subjected to a 2-way ANOVA with Language (Spanish, Basque) and Trial type (switch, non-switch) as within-subject variables. Furthermore, we paralleled the frequentist null-hypothesis significance testing with a Bayesian ANOVA approach to provide a more comprehensive assessment of the evidence for both the null and alternative hypotheses. This analysis was implemented in the JASP software41.
Finally, to properly model by-participant and by-item variation, we paralleled the ANOVA using LMMs analysis. As fixed effects, we entered Language, Trial type, and their interaction into the model. Apart from the fixed effects, the model included Participants and Items as random effects (random intercepts and random slopes). The two-level categorical predictors were coded as − 0.5 and 0.5 (i.e. Basque trials were coded as − 0.5 and Spanish trials as 0.5; non-switch trials were coded as − 0.5 and switch trials as 0.5). P-values were obtained using the Satterthwaite's method. The analysis was performed in R (R Core Team, version 4.1.3) using the lme4 package (version 1.1–29)75 and lmerTest package (version 3.1–3)76.
Time–frequency analysis
Time–frequency representations (TFR) were calculated on clean MEG data epochs in the theta (4–7 Hz) and the alpha (8–13 Hz) frequency bands. These frequency bands were selected based on a recent review covering findings regarding neural responses to bilingual language control in diverse tasks17. TFRs were obtained using a Hanning tapers approach and a fixed window length of 500 ms, advancing in 10 ms steps. Power was estimated separately for each orthogonal direction of a gradiometer pair and then combined, resulting in 102 measurement sensors. Magnetometers were discarded due to low signal-to-noise ratio. Power was calculated as the relative change with respect to a ~ 500 ms pre-stimulus baseline. We used cluster-based permutation tests for the statistical contrasts at the sensor level77. We averaged over frequency bins and time points in two specific time windows of interest to assess power differences: 100-350 ms and 350–600 ms after picture onset. These time windows were chosen based on data inspection and neurophysiological evidence from studies using speech production tasks39,47,78, suggesting that recordings not contaminated with articulatory activity can be safely acquired around these time windows.
The permutation p-value was calculated using the Monte-Carlo method with 1000 random permutations. The significance testing threshold was a p-value below 5% (two-tailed).
Source reconstruction
Source reconstruction was performed on the statistically significant effects observed at the sensor level. For each participant, individual T1-weighted MRI images were segmented into different brain tissues using the Freesurfer software. Co-registration between the MEG sensor space and participant's MRI coordinates was done by manually aligning the digitized head-surface and fiducial points to the outer scalp surface using MRIlab (Elekta Neuromag Oy, version 1.7.25). The forward model was calculated using the Boundary Element Method (BEM) implemented in the MNE suite (RRID: SCR_005972)79 for three orthogonal tangential current dipoles, placed on a homogeneous 5-mm grid covering the whole brain. The forward model was reduced to the two principal components of the highest singular value for each source, corresponding to sources tangential to the skull. All sensors (i.e. planar gradiometers and magnetometers) were used for source estimation, normalizing the signal of each sensor by its noise variance (i.e. 500 ms baseline period before picture onset). Brain source activity was calculated using a Linearly Constrained Minimum Variance (LCMV) beamformer approach80. The covariance matrix used to derive beamformer weights was estimated from the time–frequency window of the significant sensor-level effects and an equally-sized baseline period prior to picture onset. In order to perform group-level analysis, brain maps were transformed from the individual MRIs to the standard Montreal Neurological Institute (MNI) by applying a non-linear transformation using the spatial-normalization algorithm implemented in SPM8.
Comparisons between conditions were performed with the location-comparison method81. This method generates bootstrap group-averaged maps to build a permutation distribution of location difference between local maxima in the two conditions being compared and test the null hypothesis that this distance is zero. Local maxima are defined as sets of contiguous voxels displaying higher power than all other neighboring voxels. The threshold for statistical testing at p < 0.05 was computed as the 95-percentile of the permutation distribution. All supra-threshold local MEG peaks were interpreted as indicative of brain regions likely triggering the sensor-level effects. The coordinates of significant local power maxima were statistically compared across participants using t tests.
Data availability
All the data that support the findings of this study are available at https://osf.io/td7xn/. The code for data preprocessing and analysis are available on request from the corresponding author.
References
Fabbro, F. The bilingual brain: Cerebral representation of languages. Brain Lang. 79(2), 211–222 (2001).
Meuter, R. F. I. & Allport, A. Bilingual language switching in naming: Asymmetrical costs of language selection. J. Mem. Lang. 40, 25–40 (1999).
Abutalebi, J. & Green, D. Bilingual language production: The neurocognition of language representation and control. J. Neurolinguist. 20, 242–275 (2007).
Green, D. W. Mental control of the bilingual lexico-semantic system. Bilingualism 1, 67–81 (1998).
Calabria, M., Hernandez, M., Branzi, F. M. & Costa, A. Qualitative differences between bilingual language control and executive control: Evidence from task-switching. Front. Psychol. 2, 399 (2011).
Costa, A., Santesteban, M. & Ivanova, I. How do highly proficient bilinguals control their lexicalization process? Inhibitory and language-specific selection mechanisms are both functional. J. Exp. Psychol. Learn. Mem. Cogn. 32(5), 1057–1074 (2006).
Costa, A. & Santesteban, M. Lexical access in bilingual speech production: Evidence from language switching in highly proficient bilinguals and L2 learners. J. Mem. Lang 50, 491–511 (2004).
De Baene, W., Duyck, W., Brass, M. & Carreiras, M. Brain circuit for cognitive control is shared by task and language switching. J. Cogn. Neurosci. 27(9), 1752–1765 (2015).
Köpke, B. et al. Functional and structural differences in brain networks involved in language processing and control in highly proficient early and late bilinguals. J. Neurolinguist. 59(7), 100988 (2021).
de Bruin, A., Samuel, A. G. & Duñabeitia, J. A. Voluntary language switching: When and why do bilinguals switch between their languages?. J. Mem. Lang. 103, 28–43 (2018).
Abutalebi, J. & Green, W. D. Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Bilingualism 19(4), 689–698 (2016).
Hernandez, A. E., Martinez, A. & Kohnert, K. In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain Lang. 73(3), 421–431 (2000).
Branzi, F. M., Della Rosa, P. A., Canini, M., Costa, A. & Abutalebi, J. Language control in Bilinguals: Monitoring and response selection. Cereb. Cortex 26(6), 2367–2380 (2016).
Hernandez, A. E., Dapretto, M., Mazziotta, J. & Bookheimer, S. Language switching and language representation in Spanish-English bilinguals: An fMRI study. Neuroimage 14(2), 510–520 (2001).
Price, C. J., Green, D. W. & von Studnitz, R. A functional imaging study of translation and language switching. Brain 122(Pt 12), 2221–2235 (1999).
Moreno, E. M., Rodríguez-Fornells, A. & Laine, M. Event-related potentials (ERPs) in the study of bilingual language processing. J. Neurolinguist. 21, 477–508 (2008).
Tao, L., Wang, G., Zhu, M. & Cai, Q. Bilingualism and domain-general cognitive functions from a neural perspective: A systematic review. Neurosci. Biobehav. Rev. 125, 264–295 (2021).
Verhoef, K. M., Roelofs, A. & Chwilla, D. J. Electrophysiological evidence for endogenous control of attention in switching between languages in overt picture naming. J. Cogn. Neurosci. 22(8), 1832–1843 (2010).
Zheng, X., Roelofs, A., Erkan, H. & Lemhofer, K. Dynamics of inhibitory control during bilingual speech production: An electrophysiological study. Neuropsychologia 140, 107387 (2020).
Jackson, G. M., Swainson, R., Cunnington, R. & Jackson, S. R. ERP correlates of executive control during repeated language switching. Bilingualism 4(2), 169–178 (2001).
Chauncey, K., Grainger, J. & Holcomb, P. J. Code-switching effects in bilingual word recognition: A masked priming study with event-related potentials. Brain Lang. 105(3), 161–174 (2008).
Pellikka, J., Helenius, P., Makela, J. P. & Lehtonen, M. Context affects L1 but not L2 during bilingual word recognition: An MEG study. Brain Lang. 142, 8–17 (2015).
Ardal, S., Donald, M. W., Meuter, R., Muldrew, S. & Luce, M. Brain responses to semantic incongruity in bilinguals. Brain Lang. 39(2), 187–205 (1990).
Hahne, A. What’s different in second-language processing? Evidence from event-related brain potentials. J. Psycholinguist. Res. 30(3), 251–266 (2001).
Hut, S. C. A. & Leminen, A. Shaving bridges and tuning Kitaraa: The effect of language switching on semantic processing. Front. Psychol. 8, 1438 (2017).
Abutalebi, J. Neural aspects of second language representation and language control. Acta Physiol. (Oxf) 128(3), 466–478 (2008).
Braunstein, V. et al. Investigating the influence of proficiency on semantic processing in bilinguals: An ERP and ERD/S analysis. Acta Neurobiol. Exp. 72(4), 421–438 (2012).
Mouraux, A. & Iannetti, G. D. Across-trial averaging of event-related EEG responses and beyond. Magn. Reson. Imaging 26(7), 1041–1054 (2008).
Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 9(10), 474–480 (2005).
Liu, H., Liang, L., Zhang, L., Lu, Y. & Chen, B. Modulatory role of inhibition during language switching: Evidence from evoked and induced oscillatory activity. Int. J. Biling. 21(1), 57–80 (2015).
Piai, V. & Zheng, X. Speaking waves: Neuronal oscillations in language production. In Psychology of Learning and Motivation Vol. 71 (ed. Federmeier, K. D.) 265–302 (Academic Press, 2019).
Nigbur, R., Ivanova, G. & Stürmer, B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 122(11), 2185–2194 (2011).
van Steenbergen, H., Band, G. P. & Hommel, B. Reward valence modulates conflict-driven attentional adaptation: Electrophysiological evidence. Biol. Psychol. 90(3), 234–241 (2012).
Cohen, M. X. & Donner, T. H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 110(12), 2752–2763 (2013).
Jensen, O. & Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 4, 186 (2010).
Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16(12), 606–617 (2012).
Klimesch, W., Sauseng, P. & Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 53(1), 63–88 (2007).
Bice, K., Yamasaki, B. L. & Prat, C. S. Bilingual language experience shapes resting-state brain rhythms. Neurobiol. Lang. 1(3), 288–318 (2020).
Geng, S. et al. Oscillatory dynamics underlying noun and verb production in highly proficient bilinguals. Sci. Rep. 12(1), 764 (2022).
Luft, C. D. B., Zioga, I., Thompson, N. M., Banissy, M. J. & Bhattacharya, J. Right temporal alpha oscillations as a neural mechanism for inhibiting obvious associations. Proc. Natl. Acad. Sci. U.S.A. 115(52), E12144–E12152 (2018).
Team J. JASP (Version 0.17.1) [Computer software] (2023).
Keysers, C., Gazzola, V. & Wagenmakers, E. J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 23(7), 788–799 (2020).
Declerck, M. & Philipp, A. M. A review of control processes and their locus in language switching. Psychon. Bull. Rev. 22(6), 1630–1645 (2015).
Green, D. W. & Abutalebi, J. Language control in bilinguals: The adaptive control hypothesis. J. Cogn. Psychol. 25(5), 515–530 (2013).
Magezi, D. A., Khateb, A., Mouthon, M., Spierer, L. & Annoni, J. M. Cognitive control of language production in bilinguals involves a partly independent process within the domain-general cognitive control network: Evidence from task-switching and electrical brain activity. Brain Lang. 122(1), 55–63 (2012).
Klimesch, W., Freunberger, R. & Sauseng, P. Oscillatory mechanisms of process binding in memory. Neurosci. Biobehav. Rev. 34(7), 1002–1014 (2010).
AmorusoGeng, L. S. et al. Oscillatory and structural signatures of language plasticity in brain tumor patients: A longitudinal study. Hum. Brain Mapp. 42(6), 1777–1793 (2021).
Litcofsky, K. A. & Van Hell, J. G. Switching direction affects switching costs: Behavioral, ERP and time-frequency analyses of intra-sentential codeswitching. Neuropsychologia 97, 112–139 (2017).
Garbin, G. et al. Neural bases of language switching in high and early proficient bilinguals. Brain Lang. 119(3), 129–135 (2011).
Green, D. W. & Abutalebi, J. Understanding the link between bilingual aphasia and language control. Journal of Neurolinguistics 21, 558–576 (2008).
Sdoia, S., Zivi, P. & Ferlazzo, F. Anodal tDCS over the right parietal but not frontal cortex enhances the ability to overcome task set inhibition during task switching. PLoS One 15(2), e0228541 (2020).
Hikosaka, O. & Isoda, M. Switching from automatic to controlled behavior: Cortico-basal ganglia mechanisms. Trends Cogn. Sci. 14(4), 154–161 (2010).
Rushworth, M. F., Hadland, K. A., Paus, T. & Sipila, P. K. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J. Neurophysiol. 87(5), 2577–2592 (2002).
Schwartz, M. F. et al. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain 132(Pt 12), 3411–3427 (2009).
Mirman, D., Zhang, Y., Wang, Z., Coslett, H. B. & Schwartz, M. F. The ins and outs of meaning: Behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia 76, 208–219 (2015).
Zheng, B. et al. Semantic and attentional networks in bilingual processing: fMRI connectivity signatures of translation directionality. Brain Cogn. 143, 105584 (2020).
Correia, J. et al. Brain-based translation: fMRI decoding of spoken words in bilinguals reveals language-independent semantic representations in anterior temporal lobe. J. Neurosci. 34(1), 332–338 (2014).
Phillips, S. F. & Pylkkanen, L. Composition within and between languages in the Bilingual mind: MEG evidence from Korean/English Bilinguals. eNeuro https://doi.org/10.1523/ENEURO.0084-21.2021 (2021).
Buchweitz, A., Shinkareva, S. V., Mason, R. A., Mitchell, T. M. & Just, M. A. Identifying bilingual semantic neural representations across languages. Brain Lang. 120(3), 282–289 (2012).
Lambon Ralph, M. A., Pobric, G. & Jefferies, E. Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cereb. Cortex 19(4), 832–838 (2009).
Baldo, J. V., Arevalo, A., Patterson, J. P. & Dronkers, N. F. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex 49(3), 658–667 (2013).
Visser, M., Jefferies, E. & Lambon Ralph, M. A. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. J. Cogn. Neurosci. 22(6), 1083–1094 (2010).
Jackson, R. L., Hoffman, P., Pobric, G. & Lambon Ralph, M. A. The semantic network at work and rest: Differential connectivity of anterior temporal lobe subregions. J. Neurosci. 36(5), 1490–1501 (2016).
Jackson, R. L., Lambon Ralph, M. A. & Pobric, G. The timing of anterior temporal lobe involvement in semantic processing. J. Cogn. Neurosci. 27(7), 1388–1396 (2015).
Heikoop, K. W., Declerck, M., Los, S. A. & Koch, I. Dissociating language-switch costs from cue-switch costs in bilingual language switching. Bilingualism 19(5), 921–927 (2016).
Perez, G. et al. The Bilingual Lexicon, Back and Forth: Electrophysiological signatures of translation asymmetry. Neuroscience 481, 134–143 (2022).
Campbell, J. I. & Thompson, V. A. MorePower 6.0 for ANOVA with relational confidence intervals and Bayesian analysis. Behav. Res. methods 44(4), 1255–1265 (2012).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9(1), 97–113 (1971).
de Bruin, A., Carreiras, M. & Dunabeitia, J. A. The BEST dataset of language proficiency. Front. Psychol. 8, 522 (2017).
Duchon, A., Perea, M., Sebastian-Galles, N., Marti, A. & Carreiras, M. EsPal: One-stop shopping for Spanish word properties. Behav. Res. Methods 45(4), 1246–1258 (2013).
Perea, M. et al. E-Hitz: A word frequency list and a program for deriving psycholinguistic statistics in an agglutinative language (Basque). Behav. Res. Methods 38(4), 610–615 (2006).
Taulu, S. & Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 51(7), 1759–1768 (2006).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J. M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Jung, T. P. et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37(2), 163–178 (2000).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1), 1–48 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82(13), 1–26 (2017).
Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164(1), 177–190 (2007).
Quinones, I., Amoruso, L., Pomposo Gastelu, I. C., Gil-Robles, S. & Carreiras, M. What can glioma patients teach us about language (Re)organization in the bilingual brain: Evidence from fMRI and MEG. Cancers 13(11), 2593 (2021).
Gramfort, A. et al. MNE software for processing MEG and EEG data. Neuroimage 86, 446–460 (2014).
Van Veen, B. D., van Drongelen, W., Yuchtman, M. & Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 44(9), 867–880 (1997).
Bourguignon, M., Molinaro, N. & Wens, V. Contrasting functional imaging parametric maps: The mislocation problem and alternative solutions. Neuroimage 169, 200–211 (2018).
Acknowledgements
This research was supported by the Basque Government through the BERC 2018-2021 program and by the Spanish State Research Agency through BCBL Severo Ochoa excellence accreditation SEV-2015-0490, by the Ikerbasque Foundation, by a Juan de la Cierva Fellowship to LA (IJCI-2017-31373), by a Predoctoral grant from the Spanish government (BES-2017-081221) to PT and by the Plan Nacional RTI2018-096216-A-I00 (MEGLIOMA) to LA and RTI2018-093547-BI00 (LangConn) to MC and IQ, both funded by the Spanish Ministry of Economy and Competitiveness.
Author information
Authors and Affiliations
Contributions
L.A., P.T., I.Q., and M.C. designed the study. P.T. performed the experiment. L.A. and P.T. analyzed the data. L.A. wrote the original draft of the manuscript. L.A., P.T., I.Q., M.C., S.G., and A.B., reviewed and edited the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timofeeva, P., Quiñones, I., Geng, S. et al. Behavioral and oscillatory signatures of switch costs in highly proficient bilinguals. Sci Rep 13, 7725 (2023). https://doi.org/10.1038/s41598-023-34895-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34895-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.