Abstract
Although exogenous glycine betaine (GB) and cycloleucine (Cyc) have been reported to affect animal cell metabolism, their effects on plant growth and development have not been studied extensively. Different concentrations of exogenous glycine betaine (20, 40, and 60 mmol L−1) and cycloleucine (10, 20, and 40 mmol L−1), with 0 mmol L−1 as control, were used to investigate the effects of foliar spraying of betaine and cycloleucine on growth, photosynthesis, chlorophyll fluorescence, Calvin cycle pathway, abaxial leaf burr morphology, endogenous hormones, and amino acid content in eggplant. We found that 40 mmol L−1 glycine betaine had the best effect on plant growth and development; it increased the fresh and dry weight of plants, increased the density of abaxial leaf hairs, increased the net photosynthetic rate and Calvin cycle key enzyme activity of leaves, had an elevating effect on chlorophyll fluorescence parameters, increased endogenous indoleacetic acid (IAA) content and decreased abscisic acid (ABA) content, and increased glutamate, serine, aspartate, and phenylalanine contents. However, cycloleucine significantly inhibited plant growth; plant apical dominance disappeared, plant height and dry and fresh weights decreased significantly, the development of abaxial leaf hairs was hindered, the net photosynthetic rate and Calvin cycle key enzyme activities were inhibited, the endogenous hormones IAA and ABA content decreased, and the conversion and utilization of glutamate, arginine, threonine, and glycine were affected. Combined with the experimental results and plant growth phenotypes, 20 mmol L−1 cycloleucine significantly inhibited plant growth. In conclusion, 40 mmol L−1 glycine betaine and 20 mmol L−1 cycloleucine had different regulatory effects on plant growth and development.
Similar content being viewed by others
Introduction
Plant growth and development can be influenced by many factors, such as genetic factors1, growing environment2,3 and cultivation practices4. The primary (sugars, amino acids, nucleotides, etc.) and secondary metabolites (hormones, pigments, and toxins) synthesized by plants can also regulate their growth and development5,6. Indoleacetic acid (IAA), abscisic acid (ABA), and gibberellins (GA) can directly participate in and regulate plant growth and development, affecting plant morphological composition and various types of organ differentiation7,8. However, alkaloids and non-protein amino acids affect plant growth and development by influencing metabolic pathways9. As an important RNA modification, m6A methylation is widely involved in mRNA degradation10, splicing, export, stability10, and translation11, which in turn affects the synthesis and utilization of secondary metabolites in plant12. Studies on animal cells have shown that glycine betaine (GB) can affect intracellular m6A methylation modifications. However, this has not been reported for plants13,14,15.
Glycine betaine (GB) (N, N, N-trimethylglycine) is a secondary metabolite widely found in plants and animals and is an important osmoregulatory substance2. In plant cells, serine is synthesized into GB in the chloroplasts via betaine aldehyde, choline, and ethanolamine16. It is a safe and non-toxic class IV organic compound that stabilizes the structure and efficiency of PS II in chloroplasts, which in turn stabilizes or improves photosynthetic efficiency17. Similarly, when GB accumulates in plants, the nitrogen therein promotes seed germination and root growth, promoting plant growth18. Current research reports on betaine in plants have mainly focused on alleviating the effects of abiotic stresses in plants, such as salt19, drought20, and heavy metal21. This effect has also been verified in studies on mung beans22, tomatoes23, and zucchini24.
Studies have shown that exogenous spraying of GB can stabilize photosynthetic pigments, the net photosynthetic rate, and chlorophyll fluorescence, promote plant growth, increase leaf number, and improve chlorophyll content, etc.25,26. Betaine helps to maintain the structural and functional integrity of 1,5-bisphosphate ribulose carboxylase (RuBisCO), RuBisCO-activating enzyme (RCA), fructose-1,6-bisphosphatase (FBPase), FBP aldolase, and PRKase27. After betaine accumulates in plants under normal conditions, it can also increase the number of plant seeds and fruits, improve yield, and increase the number of flowers28. However, published results showed that eggplant did not accumulate betaine on its own when subjected to abiotic stresses; therefore, exogenous application of betaine was necessary29.
Cycloleucine (Cyc), a chemical inhibitor, has mostly been studied in animals and microorganisms. In animal cell assays, its main action was on methionine adenosyl transferase, which can reduce the level of S-adenosylmethionine in cells. It has been shown that high concentrations of cycloleucine are cytotoxic and can affect cell development30. In Escherichia coli cultures, adding cycloleucine to the medium can affect cell metabolism31. In recent years, most applications of cycloleucine have focused on m6A methylation in animals, and it has been widely used as a methylation inhibitor. However, its effects on plant growth and development have rarely been reported. In plants, the exogenous administration of cycloleucine strongly inhibits light-induced activation of catalase mRNA31. Plant growth was slowed, and chlorosis and even necrosis were observed after the exogenous application of cycloleucine during the cultivation of potato detoxification seedlings32, further confirming that cycloleucine is toxic not only to animal cells but also to plant cells.
Eggplant (Solanum melongena L.) is the fifth largest cash crop in solanaceous plants cultivated worldwide after potato, tomato, pepper, and tobacco33. Eggplants are rich in various nutrients and trace elements, such as plant polyphenols, which help protect cell membranes and enhance memory function in the brain34. The antioxidant content of eggplant also reduces the risk of cancer35 and prevents cardiovascular disease36. The antioxidant content of eggplant also reduces the risk of cancer and prevents cardiovascular diseases36. Most recent studies on the effects of exogenous GB on eggplant have focused more on abiotic stress amelioration and less on the growth and development of seedlings.
This study aimed to investigate the effects of exogenous spraying of different concentrations of glycine betaine and cycloleucine on the growth and development of eggplant seedlings and to enhance our understanding of the underlying mechanisms by which glycine betaine and cycloleucine influence plant growth and development. And to screen reasonable exogenous glycine betaine and cycloleucine concentrations for subsequent m6A methylation assays.
Results
Effect of exogenous glycinebetaine and cycloleucine on growth parameters in eggplant
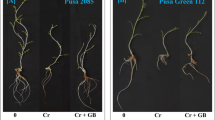
Exogenous GB promoted eggplant growth and flowering (Fig. 1A). Compared with 0 mmol L−1 (Table 1), exogenous GB at 40 mmol L−1 increased eggplant plant height, stem diameter, shoot fresh weight, shoot dry weight, plant fresh weight, and dry weight by 22.59%, 7.96%, 22.91%, 23.64%, 14.73%, and 19.34%, respectively (p < 0.05). However, after treatment with 20 mmol L−1 and 60 mmol L−1, the height, stem diameter, and shoot dry weight increased, but not significantly compared with the control (0 mmol L−1). Therefore, GB may promote the growth of eggplant seedlings at an optimal concentration of 40 mmol L−1.
Exogenous cycloleucine treatment directly resulted in stunted growth with increasing cycloleucine concentrations (Fig. 1B), deformed plant leaves, delayed flower bud differentiation, loss of apical dominance, and an increased number of lateral shoots (Figs. S1–S3). Compared with the control, the plant height, stem diameter, and fresh weights of shoots and roots were significantly decreased in the 10 mmol L−1, 20 mmol L−1, and 40 mmol L−1 treatments (p < 0.05).
Effect of exogenous glycine betaine and cycloleucine on the morphology of leaf abaxial hairs
Scanning electron microscopy was used to observe the morphology of the abaxial hair of eggplant leaves under different treatments. To observe the change in density of leaf abaxial hairs after exogenous glycine betaine treatment, we chose to magnify the leaf abaxial hairs by 40 times. In order to observe the morphological changes of leaf abaxial hairs after cycloleucine treatment, we chose to magnify the leaf abaxial hairs 110 times. After spraying with GB, the distribution density of leaf abaxial hairs increased with increasing GB concentration compared with the control (Fig. 2). Conversely, the morphology of abaxial leaf hairs was obvious changed in the cycloleucine treatment (Fig. 2 Cyc-0, Cyc-10, Cyc-20, and Cyc-40). The number of abaxial leaf hairs with needle-like projections was significantly less than that of the control, and some of the needle-like projections were curled and almost flattened.
Effect of exogenous glycine betaine and cycloleucine on abaxial hairs of eggplant leaves. GB-0: 0 mmol L−1; GB-20: 20 mmol L−1 glycine betaine; GB-40: 40 mmol L−1 glycine betaine; GB-60: 60 mmol L−1 glycine betaine. Cyc-0:0 mmol L−1; Cyc-10: 10 mmol L−1 cycloleucine; Cyc-20: 20 mmol L−1 cycloleucine; Cyc-40: 40 mmol L−1 cycloleucine.
Effect of exogenous glycine betaine and cycloleucine on gas exchange parameters and chlorophyll content in eggplant
The gas exchange parameters of eggplant plants treated with exogenous betaine are shown in Fig. 3. The Pn and Gs of plants treated with 20 mmol L−1, 40 mmol L−1, and 60 mmol L−1 GB were significantly higher than those of the 0 mmol L−1 (Fig. 3). The increase in these parameters also indicated that spraying exogenous betaine could promote plant growth (Fig. 1A, Table 1), but the Tr (transpiration rate) values of plants treated with 20 mmol L−1 GB decreased (p < 0.05). Compared to the 20 mmol L−1 and 40 mmol L−1 treatments, the Pn value of plants treated with 60 mmol L−1 GB decreased (p < 0.05), indicating that a high GB concentration was not favorable for the Pn value. Similarly, compared with plants treated with 0 mmol L−1 cycloleucine, the growth of plants treated with 10 mmol L−1, 20 mmol L−1, and 40 mmol L−1 cycloleucine was significantly inhibited (Fig. 1B), and Pn, Gs, and Tr values decreased (Fig. 3E,F,H). However, the Ci values of the plants treated with 40 mmol L−1 cycloleucine were higher than those of the other treatments.
The 40 mmol L−1 GB treatment significantly increased the content of chlorophyll a (Chl. a), chlorophyll b (Chl. b), and total chlorophyll (Chl. T) in the leaves by 33.15%, 53.23%, and 37.80%, respectively, compared with the control treatment (Table 2). However, there was no significant difference in the content of Chl. a, Chl. b, and Chl. T between the 60 mmol L−1 GB-treated plants and those with the control treatment. Exogenous cycloleucine treatment (all concentrations) significantly decreased the content of Chl. a, Chl. b, and Chl. T compared to the control (Table 2).
Effect of exogenous glycine betaine and cycloleucine on Calvin cycle enzyme activity in eggplant leaves
RuBisCO, 3-glyceraldehyde-phosphate dehydrogenase (GAPDH), fructose-1,6-bisphosphate aldolase (FBA), and transketolase (TK) enzyme activities increased significantly (p < 0.05) after exogenous GB treatment, with the highest enzyme activity in the 40 mmol L−1 GB treatment (Fig. 4). The FBPase activity of plants treated with 20 mmol L−1 GB was higher than that of all other treatments (Fig. 4C). Exogenous cycloleucine treatment also significantly inhibited the activities of RuBisCO, GAPDH, FBPase, and FBA (Fig. 4F–I) but increased TK enzyme activity (Fig. 4J). Plants treated with 20 mmol L−1 cycloleucine had much lower GAPDH, FBPase, and FBA enzyme activities than those in the other treatments (Fig. 4G–I).
Effect of exogenous glycine betaine and cycloleucine on chlorophyll fluorescence parameters in eggplant
Fv'/Fm' decreased after spraying with exogenous betaine (Fig. 5B) and increased with 40 mmol L−1 GB treatment (Fig. 5A). However, 60 mmol L−1 betaine decreased the Y(II) values (Fig. 5C). The qN value increased with increasing GB concentration. qP increased after spraying with 20 mmol L−1 betaine, but 1-qP decreased. Thus, GB spraying can alter qN and qP in eggplant plants and increase heat dissipation, leading to a change in Y(II).
After spraying exogenous cycloleucine, Fv/Fm, Y(II), Fv'/Fm,' qN, and 1-qP decreased (Fig. 5G–J,L), but qP increased, with the most pronounced effect in plants treated with 20 mmol L−1 cycloleucine (Fig. 5). This suggests that cycloleucine may affect the ability to use the captured electron energy for photochemical reactions, causing changes in the PS II excitation pressure and affecting leaf fluorescence parameters.
Effect of exogenous glycine betaine and cycloleucine on amino acids content
The 21 amino acids in eggplant treated with exogenous GB and cycloleucine are listed in Table 3. The top five amino acids in plants treated with exogenous GB were glutamate (143.26–335.10 ng g−1), aspartate (73.48–151.68 ng g−1), proline (33.96–130.13 ng g−1), serine (30.45–61.79 ng g−1), and alanine (43.88–52.63 ng g−1). Compared to 0 mmol L−1 (CK), glutamate, aspartate, serine, glutamine, lysine, valine, leucine, isoleucine, and methionine contents increased with increasing GB concentrations, whereas arginine, cystine, and glycine contents decreased (p < 0.05). In plants treated with 40 mmol L−1 GB, proline (130.13 ng g−1) and tryptophan (19.53 ng g−1) contents were significantly higher (p < 0.05) than the other treatments.
The amino acid contents of plants treated with exogenous cycloleucine were glutamate > proline > aspartate > serine > arginine > glutamine > lysine > asparagine > alanine > histidine > threonine > phenylalanine > tryptophan > valine > isoleucine > leucine > glycine > methionine > tyrosine > cysteine. The glutamate, proline, arginine, threonine, and glycine contents of plants treated with 20 mmol L−1 cycloleucine were higher than those of the other treatments (p < 0.05). The methionine content of cycloleucine-treated plants significantly increased with increasing cycloleucine concentrations.
Effect of exogenous glycine betaine and cycloleucine on endogenous hormone content
Plants treated with 40 mmol L−1 GB had the highest ABA content (4.11 ng g−1 FW), which increased by 3.79% and 4.58% (p < 0.05) compared with the 20 mmol L−1 and 60 mmol L−1 treatments, respectively (Fig. 6A). The IAA content increased with increasing betaine concentration (Fig. 6B). Compared with 0 mmol L−1, the IAA content of plants treated with 20, 40, and 60 mmol L−1 increased by 10.12%, 21.43%, and 20.24%, respectively (p < 0.05). The IAA/ABA ratio also increased with increasing betaine concentration (Fig. 6C).
The ABA content of plants sprayed with cycloleucine was significantly lower than that of plants sprayed with 0 mmol L−1; however, the differences between the 10, 20, and 40 mmol L−1 treatments were insignificant. Plants treated with 10 mmol L−1 cycloleucine had the highest IAA content (2.67 ng g−1 FW), and those treated with 20 mmol L−1 cycloleucine had the lowest IAA content. The plants treated with 10 mmol L−1 had the highest IAA/ABA value (Fig. 6F), significantly higher than the other treatments (p < 0.05).
Discussion
GB has been widely used to alleviate abiotic stress in plants and has shown good results19,21. GB mitigates abiotic stresses mainly by protecting cell membranes19, maintaining normal intracellular osmotic pressure20, reducing photosynthetic system damage, and stabilizing related enzyme activities23. We found that spraying exogenous GB on eggplant seedlings under normal conditions promoted plant growth, facilitated plant development, and improved photosynthesis in eggplants. In our study, the plant height, shoot fresh weight, and shoot dry weight of eggplant increased significantly after exogenous spraying with different GB concentrations, with 20 and 40 mmol L−1 being the most effective. It has been shown that when GB is applied exogenously, it is absorbed and quickly moves from the leaves to meristematic tissues, such as buds and stem tips, thus promoting flowering and growth37,38. Regarding plant appearance and morphology, 40 mmol L−1 GB significantly increased the flowering time of plants (Fig. 1A). However, 60 mmol L−1 betaine had some inhibitory effect on plant growth (Fig. 1A); plant height, stem thickness, root fresh weight, and root dry weight were slightly lower with 0 mmol L−1 (control). This indicates that exogenous GB can promote growth at low concentrations under normal conditions25,39.
Conversely, the plant height, stem diameter, shoot fresh weight, and root fresh weight of eggplant plants treated with different concentrations of exogenous cycloleucine decreased significantly with an increase in cycloleuvine concentration compared with 0 mmol L−1. These results indicated that the exogenous spraying of cycloleucine significantly inhibited the growth and development of eggplant plants. In terms of the plant phenotype, 10 mmo L−1 cycloleucine delayed the flowering time (Fig. S1), 20 mmol L−1 and 40 mmol L−1 cycloleucine directly caused stunted plant growth and development, and even led to the loss of apical dominance and an increase in the number of lateral shoots (Figs. S1, S2). These results are similar to those of Colombarin32 on potatoes, indicating that plant growth and development were inhibited after cycloleucine application.
Epidermal hairs on plant leaves can effectively reduce leaf water gain40, protect leaf flesh cells from damage41, and reduce pathogenic fungal attacks42. I In this experiment, the density of abaxial hairs on eggplant leaves increased significantly with increasing GB concentrations compared to the control treatment, and the morphology was sharper. However, treatment with cycloleucine spray resulted in a significant morphological change in eggplant leaf abaxial hair, which appeared flattened, and the number of individual hair-like branches decreased. In terms of magnification, 40 × magnification was required to observe leaf abaxial hairs of control plants and plants sprayed with GB, while 110 × magnification was required for cycloleucine-treated plants. This suggests that the morphology of abaxial hairs of leaves may be smaller after cycloleucine treatment.
Previous studies have shown that exogenous GB can significantly improve plant gas exchange properties43, stabilize photosynthetic pigments and chlorophyll fluorescence, and promote plant growth25. In this study, Pn and Gs increased and then decreased with increasing betaine concentration. It also validated the improved effect of GB on plant gas exchange parameters and indicated that the main factor of photosynthetic limitation could be the stomatal limitation24,39. Plants treated with 20 and 40 mmol L−1 GB showed a significant increase in Pn. In addition, according to Gs and Ci, the stomatal opening of leaves was significantly increased after betaine spraying, while Tr was the lowest in plants treated with GB at 20 mmol L−1 concentration and even significantly lower than CK. Combined with Pn, Gs and Ci, the main reason could be due to a decrease in the number of normally open stomata on the leaves, resulting in a decrease in Tr44.
Cycloleucine acts as a methylation inhibitor and affects photosynthesis in plants by inhibiting S-adenosylmethionine synthase and, thus, RNA methylation45. Gas exchange parameters were affected in plants sprayed with cycloleucine. Compared with the control plants, Pn, Gs, and Tr were reduced in cycloleucine-treated plants (Fig. 3E,F,H), whereas Ci levels were elevated. This suggests that the effect of cycloleucine on the gas exchange parameters may be achieved by affecting the uptake and utilization of CO2. This is because, as the concentration of cycloleucine increased, a rising and then decreasing trend was observed in Pn, and Gs, Ci, and Tr showed an increasing trend. This result suggests that cycloleucine may not affect the gas exchange capacity but rather the assimilation capacity of the photosynthetic system for CO2.
Key enzymes of the Calvin cycle (RuBisCO, GAPDH, FBA, FBPase, and TK) play an irreplaceable role in photosynthesis46,47. RuBisCO and FBPase play important roles in carbon fixation: reduction and RuBP regeneration, respectively. FBA and TK promote CO2 assimilation in leaves and carbon flow in the Calvin cycle, and GAPDH is a key enzyme in glycolysis47. The exogenous administration of GB has been shown to elevate the activity of key Calvin cycle enzymes, which is similar to our results48,49. We showed that exogenous spraying of GB increased RuBisCO, GAPDH, FBA, FBPase, and TK activity, whereas plants sprayed with cycloleucine showed decreased RuBisCO, GAPDH, FBA, FBPase, and TK enzyme activity. This suggests that GB can enhance the key activities of the Calvin cycle under normal conditions. Meanwhile, plants sprayed with cycloleucine had decreased activity of key Calvin cycle enzymes, which may be due to the effect of cycloleucine on RNA methylation32,50, affecting enzyme synthesis and transport, resulting in decreased enzyme activity. Compared to the control treatment, GB increased chlorophyll content (Table 2)38,51. The leaves of cycloleucine-treated plants turned yellow, and the chlorophyll content decreased. This indicates that cycloleucine may increase the rate of chlorophyll degradation and decrease the synthesis rate32,51.
Chlorophyll fluorescence provides insights into the cellular response to exogenous substances. It can quantify changes in the donor and acceptor sides of PSII reaction centers, thus providing information about energy uptake, utilization, and transport52. Compared to the control, GB treatment significantly increased Fv/Fm, qN, and qP (Fig. 5A,D,E) and significantly decreased Fv′/Fm′ (Fig. 5B). Exogenous GB affects Fv/Fm mainly by promoting electron transport from QA to QB in vesicle-like membranes53,54. Our results showed that exogenous GB elevated plant Fv/Fm (Fig. 5A). However, Fv'/Fm' decreased, indicating that spraying 40 mmol L−1 GB significantly promoted Fv/Fm compared to CK (p < 0.05), a result consistent with the findings in tomatoes54.
Similarly, qN increased because of dynamic photoinhibition (Fig. 5D). As qN increased, qP decreased (Fig. 5D,E), indicating a reduction in the actual photochemical rate of PSII55. Also, 1-qP decreased in plants treated with 20 and 40 mmol L−1 GB, indicating that GB could reduce the excitation stress faced by PSII, improve the efficiency of excitation energy capture (Fig. 5B), and inhibit the reduction in Y(II) (Fig. 5C). In contrast, cycloleucine was responsible for a significant decrease in Fv/Fm, Fv'/Fm,' Y(II), qN, and 1-qP (Fig. 5G–J,L), and a significant increase in qP (Fig. 5K). This indicates that chronic and dynamic photoinhibition are affected by cycloleucine spraying46,47. qN decreased while qP was enhanced, indicating that cycloleucine reduced heat dissipation and enhanced photosynthesis. Plants treated with 20 mmol L−1 cycloleucine showed increased Pn (Fig. 3E), which also verifies this result. The 1-qP value decreased significantly (Fig. 5L), also indicating that cycloleucine affected the PS II excitation pressure.
Amino acids are the basic units for synthesizing proteins and other metabolites. They are commonly involved in physiological and biochemical processes in plants, such as the regulation of osmotic pressure56, alteration of enzyme activity, regulation of stomatal opening, and ion transport57. Glutamate can serve as a carbon pool for δ-aminolevulinic acid during chlorophyll synthesis58, and δ-aminolevulinic acid is formed when glutamate reacts with tRNA, indicating the importance of glutamate in chlorophyll biosynthesis59. In this experiment, both betaine and cycloleucine applications significantly increased the glutamate content of the plants compared to that of the control (Table 3). GB spraying increased the chlorophyll content, verifying that exogenous GB elevates glutamate content60.
In contrast, treatment with cycloleucine increased glutamate content but decreased chlorophyll content. This suggests that spraying with cycloleucine may have inhibited glutamate utilization by the plants, leading to its formation and accumulation. Serine plays an active role in plant metabolic activities such as phospholipid biosynthesis, photorespiration, and biosynthesis of other amino acids61. The serine content of the plants sprayed with exogenous GB was significantly higher than that of the controls62. Meanwhile, the differences between plants treated with 20, 40, and 60 mmol L−1 GB were insignificant, indicating that the spraying of exogenous GB may have promoted serine synthesis. However, other reasons limited the increase in serine content.
Glycine is also a precursor of GB, which can be oxidized to form serine61,63. The increase in serine content and the decrease in glycine content after spraying exogenous GB may be due to the fact that exogenous GB inhibited glycine synthesis and promoted the conversion of glycine to serine. The glycine content in cycloleucine treatment increased and then decreased, whereas serine content increased. This indicates that the metabolic synthesis pathways of both serine and glycine were influenced by cycloleucine. Valine, leucine, and isoleucine can act as alternative electron donors in the mitochondrial electron transport chain64,65. After spraying exogenous GB, valine, leucine, and isoleucine levels increased with increasing GB concentration. The corresponding photosynthetic and chlorophyll fluorescence parameters improved44, indicating that GB promoted the synthesis and utilization of valine, leucine, and isoleucine66. However, photosynthetic and chlorophyll fluorescence parameters decreased with cycloleucine treatment, but leucine and isoleucine content increased, while valine content decreased. This suggests that cycloleucine may have inhibited the utilization of leucine and isoleucine but suppressed valine synthesis. Aspartate is a key amino acid for plant growth, development, and adaptation to environmental challenges, most likely through its interaction with plant hormones, such as growth hormones and ethylene67. The change in the aspartate content of both exogenous GB and cycloleucine-treated plants was inversely proportional to the IAA content (Fig. 6B,E; Table 3), which also verified the above conclusion68.
Aspartate, glutamate, alanine, and glycine support flowering, whereas threonine and phenylalanine inhibit it57,69. Combined with the plant phenotypes (Fig. 1A), aspartate, glycine, alanine, threonine, and phenylalanine contents were significantly increased after GB spraying compared to the control. However, flowering occurred earlier in plants treated with GB at 40 mmol L−1. This indicates that, although GB increases amino acid content, it also inhibits the action of threonine and phenylalanine, causing their formation and accumulation. Aspartic acid, glycine, alanine, threonine, and phenylalanine in cycloleucine-treated plants increased with increasing cycloleucine concentrations. However, spraying with cycloleucine significantly affected the reproductive growth of the plants (Fig. 1B, Fig. S1), indicating that as the plants took up cycloleucine, it directly affected the conversion and utilization of aspartic acid, glycine, alanine, threonine, and phenylalanine, causing their accumulation.
Methionine and threonine are precursors of isoleucine70,71. This conclusion is also verified by the fact that the trends of methionine, threonine, and isoleucine are the same regardless of whether GB or cycloleucine is sprayed. As for tryptophan, a synthetic precursor of IAA72, the trends of tryptophan and IAA contents in the plants were consistent after GB application. However, the IAA and tryptophan contents of plants sprayed with cycloleucine decreased. This indicates that cycloleucine affects tryptophan synthesis. Methionine can be used in cellular metabolism as a biomodulatory element for mRNA initiation, translation, protein composition, and S-adenosylmethionine (SAM)71,73. The high concentration of betaine used in this experiment catalyzed the increase in methionine content74.
In contrast, methionine content increased with increasing cycloleucine concentration, which worsened plant growth. This suggests that the effect of cycloleucine on methionine content may be consistent with the conversion and utilization of methionine in the metabolic pathway rather than the inhibition of methionine synthesis. Photorespiration is the main source of serine and glycine amino acids in photosynthetic tissues75,76. The increased serine and glycine content in plants sprayed with cycloleucine indicates enhanced photorespiration and increased energy loss in their leaves77,78.
Endogenous hormones play important roles in plant growth and development79,80. They participate in various physiological and biochemical processes in plant cells, tissues, and organs81, directly affecting plant growth and development82. GB treatment changes the hormone metabolic pathways in plants and affects the hormone content83. In this study, the leaf ABA content decreased (Fig. 6A), but the IAA content increased (Fig. 6B) after spraying with the exogenous GB. The ABA content in leaves affects the opening and closing of stomata, which in turn affects photosynthesis in the leaves84. After a decrease in ABA content (Fig. 6A), there was an increase in both the Pn and Gs (Fig. 3A,B), similar to the findings of Chen et al.85.
IAA promotes cell growth and differentiation86 and root development and plant growth87,88. After spraying exogenous GB, the IAA content increased and directly promoted plant growth and development (Fig. 1A), with the best effect observed for GB at 40 mmol L−1. After spraying cycloleucine, the ABA content decreased (Fig. 6D), but the IAA content increased and then decreased (Fig. 6E). This indicated that the endogenous hormone level of the plant decreased, and normal plant growth and development were inhibited (Fig. 1B)89,90. Changes in the hormone levels also resulted in delayed floral bud differentiation and flowering91,92.
In conclusion, the spraying of exogenous GB promoted the growth of eggplant seedlings, increased the fresh and dry weights of the plants, promoted the development of abaxial leaf hairs, promoted the differentiation of flower buds, shortened the flowering time, improved the photosynthetic and fluorescence parameters in eggplant plants, and increased the amino acid content, with the greatest effect at 40 mmol L−1 concentration of GB. Spraying exogenous cycloleucine at 20 mmol L−1 significantly inhibited the growth and development of eggplant, leading to a decrease in the gas exchange parameters of plant leaves, inhibiting the development of abaxial leaf hairs, leading to a decrease in phytohormone content, and affecting the conversion and utilization of amino acids in the plant.
Methods
Plant material and experimental treatments
The experiment was conducted from May 2021 to March 2022 in an artificial climate chamber (RDN-400E-4, Ningbo, Zhejiang) at the College of Horticulture, Gansu Agricultural University (36°05′ 39.86″ N, 103°42′ 31.09″ E). The test eggplant variety was 'Xingniang' (long eggplant, the main cultivar in Gansu Province), purchased from Jiuquan Xiahe Seed Co., Ltd. GB (purity > 99%, CAS: 107-43-7) and cycloleucine (purity > 98%, CAS: 52-52-8) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd.
The uniform-sized and full-textured eggplant seeds were selected, soaked in warm water at 55 °C for 30 min, and then put in germinating box at (28 ± 1) °C (day)/(18 ± 1) °C (night). After 96 h, germinated seeds were sown in plastic pots (10 cm × 10 cm) with a cultivation substrate of grass charcoal: vermiculite: perlite = 3:1:1. After germination, the seedlings were incubated in a climatic chamber at (28 ± 1) °C (day)/(18 ± 1) °C (night), with a light intensity of 30,000 lx. When the seedlings grew to two leaves, they were watered with ½ Hoagland's nutrient solution and water at intervals; when they grew to four leaves, they were watered with Hoagland's full nutrient solution and water at intervals. Before starting the spraying of exogenous substances, the weaker plants in each pot were removed, leaving only the better-growing plants. Uniformly sized plants were treated with different concentrations of exogenous GB and cycloleucine foliar sprays.
The experiment was conducted using a completely randomized design with three replicates. Three different betaine concentrations were used: 20, 40, and 60 mmol L−1, with 0 mmol L−1 as the control treatment. Three cycloleucine concentrations were also tested: 10, 20, and 40 mmol L−1, with 0 mmol L−1 as the control treatment. All treatments lasted for 3 days. The culture conditions at the end of treatment were the same as before. The growth physiology of 60-day-old seedlings (14 days after GB and cycloleucine treatment) was measured and analyzed using the indicators described below.
This study complies with local and national guidelines. Plant experiments were also performed in accordance with the relevant guidelines and regulations. As Solanum melongena L. is a commonly grown vegetable, no permission is required to collect it.
Growth index determination
Nine pots from each treatment were randomly selected to measure the plant height and stem diameter. Then, the plants were divided into shoot and root parts, the root substrate was rinsed, and the surface water was drained. After measuring the fresh weight of aboveground and belowground parts, they were placed in an oven at 105 °C for 30 min and then dried at 75 °C until constant weight93. The dried plants were crushed and ground for subsequent determination of other indices. All the remaining plants of each treatment were immediately frozen in liquid nitrogen and stored at − 80 °C in a refrigerator to determine other indexes.
Measurement of photosynthetic pigments, photosynthetic and gas exchange parameters, and chlorophyll fluorescence parameters
Random samples were collected from each treatment of eggplant plants to determine leaf chlorophyll content. Using the method of Wang, et al.94 and Parvaiz Ahmad et al.95: samples were collected with a 0.5 cm diameter leaf perforator, 0.1 g of leaf samples were accurately weighed, placed in a 20 mL stoppered test tube, 10 mL of 80% acetone was added, mixed thoroughly, sealed with paraffin film, and placed in a dark environment for 48 h to extract chlorophyll until the leaves turned white. The OD values of the extracts were measured at 663 nm and 645 nm using a UV-1780 UV spectrophotometer (Shimadzu Instruments (Suzhou) Co., Ltd., Suzhou, China). Chlorophyll content was calculated according to Arnon's96 method using the following equations:
V in the equation represents the total volume of the extraction solution, and m represents the sample mass.
When plants were grown for up to 60 days (day 14 after treatment), gas exchange parameters, including Pn, Ci, Gs, and Tr, were measured using a portable photosynthesis system (CIRAS-2, PP system). The second leaf of five plants was selected for a replicate of each treatment. The conditions set during measurement were as follows: leaf area, 1.7 cm2; chamber flow rate, 200 mL min−1; photosynthetically active irradiation, 1000 µmol m−2 s−1; carbon dioxide (CO2) concentration, 400 µmol mol−1; air temperature, 25 °C; and relative humidity, 75%46.
Three seedlings were randomly selected for each treatment to measure chlorophyll fluorescence parameters. After 30 min of dark acclimation, the third fully expanded functional leaf was cut and placed on a fluorimeter measuring table. Then, chlorophyll was measured using a modulated chlorophyll fluorescence imager (IMAGING-PAM; Heinz WaIz GmBH, Effeltrich, Germany). The parameters were set to a measurement light illumination intensity of 0.1 μmol m−2 s−1, photochemical light intensity of 81 μmol m−2 s−1, saturating pulsed light intensity of 2700 μmol m−2 s−1, and pulsed light time of 0.8 s. Saturating pulsed light was hit every 20 s for a total of 15 hits47. The initial fluorescence Fo and maximum fluorescence Fm in the dark were obtained by hitting the saturation pulse light, the maximum photochemical efficiency of PSII was calculated, and the steady-state fluorescence Fs was obtained under photochemical light after 300 s of continuous supply of photochemical light. Meanwhile, the maximum fluorescence yield Fm′ under light was obtained after 0.8 s of the saturation pulse light, and the actual photochemical efficiency Y(II) and photochemical burst coefficient qP were obtained97,98.
Morphological observation of leaf abaxial hairs
When plants were grown for 60 days, the middle part of their leaves was cut off in 5 × 5 mm pieces with a scalpel and immersed in 2.5% glutaraldehyde fixative for electron microscopy (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China). The dehydration method described by Yang, et al.46 was used. The fixative containing the samples was refrigerated at 4 °C for 24 h, washed four times with phosphate buffer (0.1 M PBS, pH 7.4) at 15 min intervals, and then dehydrated with ethanol (30, 40, 50, 60, 65, 70, 75, 80, 85, 90, 95%) in steps of 20 min each. Then, it was transferred to 100% ethanol for three immersion washes, 30 min each. In the end, the ethanol was replaced with tert-butanol (30, 50, 70, 80, 85, 90, 95, 100, 100, 100%). After drying, the samples were sprayed with gold using a gold sputter (Magnetron Sputter, MSP-1S; Vacuum Device, Tokyo, Japan), and the morphology of the leaf abaxial hairs was observed and photographed using a scanning electron microscope (SEM, S-3400N, Hitachi, Tokyo, Japan).
Calvin cycle key enzymes
The key enzymes of the Calvin cycle include RuBisCO, FBPase, FBA, GAPDH, and TK. The activities of these five enzymes were measured (three eggplant plants per treatment, n = 3) using an ELISA kit (Yaji Biotech, Shanghai, China). Leaf samples were completely ground with 0.05 mM Tris–HCl and 0.1 M phosphate buffer (pH 7.4) and centrifuged (4 °C, 3000×g, 15 min), and the supernatant was used to determine the enzyme activity. This method was performed according to the manufacturer's instructions. The amount of enzyme required to convert 1 µmol of the substrate in 1 min is called enzyme activity (U). U L−1 is the international unit of enzyme activity (U L−1 stands for enzyme activity per liter of enzyme preparation; U mL−1 stands for enzyme activity per mL of enzyme preparation).
Determination of endogenous hormones and amino acid content
For the determination of IAA and ABA content, 0.5 g of sample tissue was rapidly ground into powder using liquid nitrogen and packed into a 10 mL centrifuge tube with 5 mL of extraction solution (n-propanol: distilled water: hydrochloric acid = 2:1:0.002, V: V) and shaken for 30 min at 4 °C on a shaker at 100 rpm. The tube was removed, 2 mL of dichloromethane was added, and the mixture was shaken again for 30 min. Centrifuged in a refrigerated centrifuge (4 °C, 13,000 rpm, 5 min) after completion. The oily liquid was aspirated in a lyophilized bottle and stored at – 80 °C for 14 h before drying with a freeze dryer. The finished product was a white powder (it takes 24 h to obtain the finished product). Then, it was re-dissolved in 80% methanol aqueous solution, filtered through 0.22 μm organic membrane, and detected on the machine. Endogenous IAA and ABA contents were determined using high-performance liquid chromatography (Agilent 1100 series; Agilent Technologies, Santa Clara, CA, USA) with 10 μL of injected sample volume. A C18 inverse-phase column (ZORBAX SB-C18, 4.6 × 250 mm, 5 μm) was used at 30 °C with the mobile phase in acetonitrile: methanol: 0.6% acetic acid solution (5:50:45, V: V: V) at a flow rate of 1.0 mL min−1 and wavelengths of 218 nm (IAA) and 262 nm (ABA). The content of IAA and ABA was determined according to the method defined by Heidari et al.99.
An ultra-high liquid chromatography-mass spectrometry system (UPLC-MS, Agilent 1290-6460, LC/MS, Agilent Technologies) was used to determine the content of 21 free amino acids94. The method was as follows: 100 mg of fresh samples of each species were accurately weighed and extracted with 0.5 mol L−1 hydrochloric acid aqueous solution (1 mL). The solution was mixed by vortexing for 5 min, sonicated in a water bath at 25 °C for 20 min, and then centrifuged at 20,000×g for 20 min. Then, 250 µL of the supernatant was transferred to a liquid chromatography vial, diluted to 1 mL with 80% aqueous acetonitrile, and passed through an organic phase microporous membrane of 0.22 μm for determination.
The injection volume was 1 μL, and the column used was Agilent Infinity Lab Poroshell 120 HILIC-Z (2.1 × 100 mm). The mobile phase A was 20 mmol L−1 ammonium formate aqueous solution (pH = 3), mobile phase B was 20 mmol L−1 ammonium formate aqueous solution (pH = 3) and 90% acetonitrile aqueous solution V:V = 9:1. The mass spectrometry conditions were in ESI positive ionization mode with a desiccator temperature of 330 °C, a gas flow rate of 13.0 L min−1, an intrathecal gas temperature of 390 °C, an intrathecal gas flow rate of 12.0 L min−1, and capillary voltage of 1500 V.
Statistical analysis
All experimental data were analyzed using IBM Statistical Product and Service Solutions (SPSS) Statistics version 22.0 (IBM Corp., Armonk, NY, USA), and the statistical significance of treatment means was evaluated using Duncan's multiple range test (p < 0.05). All data are presented as mean ± SE. Data figures, correlation analysis, and principal component analysis were generated using Origin Pro 2021.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. This study complies with local and national guidelines. Plant experiments were also performed in accordance with the relevant guidelines and regulations.
References
Poor, P., Nawaz, K., Gupta, R., Ashfaque, F. & Khan, M. I. R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 41, 675–698. https://doi.org/10.1007/s00299-021-02675-8 (2022).
Hasanuzzaman, M. et al. Targeting glycinebetaine for abiotic stress tolerance in crop plants: Physiological mechanism, molecular interaction and signaling. Phyton 88, 185–221. https://doi.org/10.32604/phyton.2019.07559 (2019).
Kosar, F. et al. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant 172, 317–333. https://doi.org/10.1111/ppl.13155 (2021).
Xiong, C. et al. Plant developmental stage drives the differentiation in ecological role of the maize microbiome. Microbiome 9, 171. https://doi.org/10.1186/s40168-021-01118-6 (2021).
Kurepin, L. V. et al. Photosynthesis: Structures, Mechanisms, and Applications 185–202 (Springer, 2017).
Ahanger, M. A., Aziz, U., Alsahli, A. A., Alyemeni, M. N. & Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules https://doi.org/10.3390/biom10010042 (2019).
Bartwal, A., Mall, R., Lohani, P., Guru, S. K. & Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Regul. 32, 216–232. https://doi.org/10.1007/s00344-012-9272-x (2012).
Zhang, X. et al. Identification and responding to exogenous hormone of HB-KNOX family based on transcriptome data of Caucasian clover. Gene 828, 146469. https://doi.org/10.1016/j.gene.2022.146469 (2022).
Marchiosi, R. et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 19, 865–906. https://doi.org/10.1007/s11101-020-09689-2 (2020).
Shao, F. et al. A comparative analysis of differential N6-methyladenosine (m6A) modification between non-transgenic and LBD15 overexpressing Poplar 84 K plants. Tree Genet. Genomes https://doi.org/10.1007/s11295-021-01521-y (2021).
Du, X. et al. Global profiling of N(6) -methyladenosine methylation in maize callus induction. Plant Genome 13, e20018. https://doi.org/10.1002/tpg2.20018 (2020).
Liu, G., Wang, J. & Hou, X. Transcriptome-wide N(6)-methyladenosine (m(6)A) methylome profiling of heat stress in Pak-choi (Brassica rapa ssp. chinensis). Plants https://doi.org/10.3390/plants9091080 (2020).
Chen, J. N. et al. Regulation of m6A RNA methylation and its effect on myogenic differentiation in murine myoblasts. Mol. Biol. 53, 384–392. https://doi.org/10.1134/s002689331903004x (2019).
Wang, Z. et al. RNA sequencing reveals the regulation of betaine on chicken myogenesis. Animals https://doi.org/10.3390/ani12192508 (2022).
Yu, T. et al. Dynamic reprogramming and function of RNA N6-methyladenosine modification during porcine early embryonic development. Zygote 29, 417–426. https://doi.org/10.1017/S0967199420000799 (2021).
Hanson, A. D. Betaine synthesis from radioactive precursors in attached, water-stressed barley leaves. Plant Physiol. 66, 342–348. https://doi.org/10.1104/pp.66.2.342 (1980).
Chen, T. H. & Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 34, 1–20. https://doi.org/10.1111/j.1365-3040.2010.02232.x (2011).
He, C. et al. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica 177, 151–167. https://doi.org/10.1007/s10681-010-0263-3 (2010).
Dustgeer, Z. et al. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Notulae Bot. Horti Agrobotanici Cluj-Napoca https://doi.org/10.15835/nbha49112248 (2021).
Kathuria, H. et al. Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol. J. 7, 512–526. https://doi.org/10.1111/j.1467-7652.2009.00420.x (2009).
Ali, S. et al. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants https://doi.org/10.3390/plants9070896 (2020).
Jabeen, N. et al. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci. 62, 648–662. https://doi.org/10.1080/03650340.2015.1082032 (2015).
Rasheed, R. et al. Glycine betaine counteracts the inhibitory effects of waterlogging on growth, photosynthetic pigments, oxidative defence system, nutrient composition, and fruit quality in tomato. J. Hortic. Sci. Biotechnol. 93, 385–391. https://doi.org/10.1080/14620316.2017.1373037 (2017).
Yao, W., Xu, T., Farooq, S. U., Jin, P. & Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 144, 20–28. https://doi.org/10.1016/j.postharvbio.2018.05.007 (2018).
Tisarum, R., Theerawitaya, C., Samphumphuang, T., Singh, H. P. & Cha-um, S. Foliar application of glycinebetaine regulates soluble sugars and modulates physiological adaptations in sweet potato (Ipomoea batatas) under water deficit. Protoplasma 257, 197–211. https://doi.org/10.1007/s00709-019-01429-4 (2020).
Khan, M. U., Malik, R. N., Muhammad, S., Ullah, F. & Qadir, A. Health risk assessment of consumption of heavy metals in market food crops from Sialkot and Gujranwala Districts, Pakistan. Hum. Ecol. Risk Assess. 21, 327–337. https://doi.org/10.1080/10807039.2014.913445 (2015).
Diamant, S., Eliahu, N., Rosenthal, D. & Goloubinoff, P. Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276, 39586–39591. https://doi.org/10.1074/jbc.M103081200 (2001).
Ahanger, M. A., Gul, F., Ahmad, P. & Akram, N. A. Plant Metabolites and Regulation Under Environmental Stress 53–67 (Elsevier, 2018).
Chambers, F. et al. Glycine betaine and glycine betaine analogues in common foods. Food Chem. 83, 197–204. https://doi.org/10.1016/s0308-8146(03)00063-3 (2003).
Wang, X., Zhu, L., Chen, J. & Wang, Y. mRNA m(6)A methylation downregulates adipogenesis in porcine adipocytes. Biochem. Biophys. Res. Commun. 459, 201–207. https://doi.org/10.1016/j.bbrc.2015.02.048 (2015).
Feierabend, M. S. S. D. J. Post-transcriptional mechanisms control catalase synthesis during its light-induced turnover in rye leaves through the availability of the hemin cofactor and reversible changes of the translation efficiency of mRNA. Plant J. 31, 601–613. https://doi.org/10.1046/j.1365-313x.2002.01382.x (2002).
Colombarin, G. F. A. Correlation of potato virus S and virus M contents of potato meristem tips with the percentage of virus-free plantlets produced in vitro. Potato Res. 39, 129–140. https://doi.org/10.1007/bf02358213 (1996).
Ebrahimi, M., Souri, M. K., Mousavi, A. & Sahebani, N. Biochar and vermicompost improve growth and physiological traits of eggplant (Solanum melongena L.) under deficit irrigation. Chem. Biol. Technol. Agric. https://doi.org/10.1186/s40538-021-00216-9 (2021).
Kaushik, P. et al. Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res. Int. 102, 392–401. https://doi.org/10.1016/j.foodres.2017.09.028 (2017).
Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 63, 3323–3337. https://doi.org/10.1021/acs.jafc.5b00818 (2015).
Sharma, M. & Kaushik, P. Biochemical composition of eggplant fruits: A review. Appl. Sci. 11, 7078. https://doi.org/10.3390/app11157078 (2021).
Annunziata, M. G., Ciarmiello, L. F., Woodrow, P., Dell’Aversana, E. & Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 10, 230. https://doi.org/10.3389/fpls.2019.00230 (2019).
Khoshkharam, M., Shahrajabian, M. H. & Esfandiary, M. The effects of methanol and amino acid glycine betaine on qualitative characteristics and yield of sugar beet (Beta vulgaris L.) cultivars. Notulae Sci. Biol. 13, 10949. https://doi.org/10.15835/nsb13210949 (2021).
Zhang, T. P. et al. Glycinebetaine: A versatile protectant to improve rice performance against aluminium stress by regulating aluminium uptake and translocation. Plant Cell Rep. 40, 2397–2407. https://doi.org/10.1007/s00299-021-02780-8 (2021).
Hakeem, S., Ali, Z., Saddique, M. A. B., Habib-Ur-Rahman, M. & Trethowan, R. Leaf prickle hairs and longitudinal grooves help wheat plants capture air moisture as a water-smart strategy for a changing climate. Planta 254, 18. https://doi.org/10.1007/s00425-021-03645-w (2021).
Kong, D. et al. ZmSPL10/14/26 are required for epidermal hair cell fate specification on maize leaf. New Phytol. 230, 1533–1549. https://doi.org/10.1111/nph.17293 (2021).
Sallam, N. M. A. et al. Physiological and histopathological assessments of the susceptibility of different tomato (Solanum lycopersicum) cultivars to early blight disease. Eur. J. Plant Pathol. 160, 541–556. https://doi.org/10.1007/s10658-021-02263-2 (2021).
Ahmad, R. et al. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. Pollut. Res. Int. 27, 1101–1111. https://doi.org/10.1007/s11356-019-06761-z (2020).
Hamani, A. K. M. et al. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 21, 146. https://doi.org/10.1186/s12870-021-02892-z (2021).
Schmidt, M., Dehne, S. & Feierabend, J. Post-transcriptional mechanisms control catalase synthesis during its light-induced turnover in rye leaves through the availability of the hemin cofactor and reversible changes of the translation efficiency of mRNA. Plant J. 31, 601–613. https://doi.org/10.1046/j.1365-313x.2002.01382.x (2002).
Yang, Y. et al. Trehalose alleviates salt tolerance by improving photosynthetic performance and maintaining mineral ion homeostasis in tomato plants. Front. Plant Sci. 13, 974507. https://doi.org/10.3389/fpls.2022.974507 (2022).
Zhang, J. et al. Promoting pepper (Capsicum annuum) photosynthesis via chloroplast ultrastructure and enzyme activities by optimising the ammonium to nitrate ratio. Funct. Plant Biol. 47, 303–317. https://doi.org/10.1071/FP19149 (2020).
Huang, S., Zuo, T. & Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 251, 36. https://doi.org/10.1007/s00425-019-03330-z (2020).
Chen, T. H. H. & Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant, Cell Environ. 34, 1–20. https://doi.org/10.1111/j.1365-3040.2010.02232.x (2011).
Bokar, J. A. Fine-tuning of RNA functions by modification and editing. Top. Curr. Genet. 58, 141–177 (2005).
Huang, S., Zuo, T., Xu, W., Zhang, Y. & Ni, W. Improving albino tea quality by foliar application of glycinebetaine as a green regulator under lower temperature conditions. J. Agric. Food Chem. 69, 1242–1250. https://doi.org/10.1021/acs.jafc.0c06284 (2021).
Cheng, J., Du, X., Long, H., Zhang, H. & Ji, X. The effects of exogenous cerium on photosystem II as probed by in vivo chlorophyll fluorescence and lipid production of Scenedesmus obliquus XJ002. Biotechnol. Appl. Biochem. 68, 1216–1226. https://doi.org/10.1002/bab.2043 (2021).
Solymosi, D. et al. Cytochrome c M decreases photosynthesis under photomixotrophy in synechocystis sp. PCC 6803. Plant Physiol. 183, 700–716. https://doi.org/10.1104/pp.20.00284 (2020).
Wei, D. et al. Glycinebetaine mitigates tomato chilling stress by maintaining high-cyclic electron flow rate of photosystem I and stability of photosystem II. Plant Cell Rep. 41, 1087–1101. https://doi.org/10.1007/s00299-022-02839-0 (2022).
Hikosaka, K. Photosynthesis, chlorophyll fluorescence and photochemical reflectance index in photoinhibited leaves. Funct. Plant Biol. 48, 815–826. https://doi.org/10.1071/Fp20365 (2021).
Torres, M. A. & Dangl, J. L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8, 397–403. https://doi.org/10.1016/j.pbi.2005.05.014 (2005).
Khan, N. et al. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. https://doi.org/10.30848/pjb2020-2(24) (2020).
Liang, Y. et al. Photorespiration regulates carbon-nitrogen metabolism by magnesium chelatase D subunit in rice. J. Agric. Food Chem. 69, 112–125. https://doi.org/10.1021/acs.jafc.0c05809 (2021).
Zhi, T. et al. Loss of fumarylacetoacetate hydrolase causes light-dependent increases in protochlorophyllide and cell death in Arabidopsis. Plant J. 98, 622–638. https://doi.org/10.1111/tpj.14235 (2019).
Kang, Y. & Hwang, I. Glutamate uptake is important for osmoregulation and survival in the rice pathogen Burkholderia glumae. PLoS ONE 13, e0190431. https://doi.org/10.1371/journal.pone.0190431 (2018).
Rossi, S., Chapman, C., Yuan, B. & Huang, B. Improved heat tolerance in creeping bentgrass by γ-aminobutyric acid, proline, and inorganic nitrogen associated with differential regulation of amino acid metabolism. Plant Growth Regul. 93, 231–242. https://doi.org/10.1007/s10725-020-00681-6 (2021).
Molaei, S., Rabiei, V., Soleimani, A. & Razavi, F. Exogenous application of glycine betaine increases the chilling tolerance of pomegranate fruits cv Malase Saveh during cold storage. J. Food Process. Preserv. https://doi.org/10.1111/jfpp.15315 (2021).
Igamberdiev, A. U. & Kleczkowski, L. A. The glycerate and phosphorylated pathways of serine synthesis in plants: The branches of plant glycolysis linking carbon and nitrogen metabolism. Front. Plant Sci. 9, 318. https://doi.org/10.3389/fpls.2018.00318 (2018).
Aledo, J. C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 28, 1785–1796. https://doi.org/10.1002/pro.3698 (2019).
Heinemann, B. & Hildebrandt, T. M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 72, 4634–4645. https://doi.org/10.1093/jxb/erab182 (2021).
Chen, Y. et al. Analysis of amino acids in the roots of Tamarix ramosissima by application of exogenous potassium (K(+)) under NaCl stress. Int. J. Mol. Sci. 23, 9331. https://doi.org/10.3390/ijms23169331 (2022).
Li, H., Tang, X., Yang, X. & Zhang, H. Comprehensive transcriptome and metabolome profiling reveal metabolic mechanisms of Nitraria sibirica Pall. to salt stress. Sci. Rep. 11, 12878. https://doi.org/10.1038/s41598-021-92317-6 (2021).
Zhang, C. Y. et al. Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotox Environ Safe 190, 110090. https://doi.org/10.1016/j.ecoenv.2019.110090 (2020).
Tanaka, O. N., Emori, K., Takeba, G., Sato, K. & Sugino, M. Flower-inducing activity of lysine in Lemna paucicostata 6746. Plant Cell Physiol. 38, 124–128. https://doi.org/10.1093/oxfordjournals.pcp.a029141 (1997).
Joshi, V., Joung, J. G., Fei, Z. & Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 39, 933–947. https://doi.org/10.1007/s00726-010-0505-7 (2010).
Perveen, S. & Hussain, S. A. Methionine-induced changes in growth, glycinebetaine, ascorbic acid, total soluble proteins and anthocyanin contents of two Zea mays L. varieties under salt stress. JAPS 31, 0201 (2021).
Li, X. et al. Glycolate oxidase-dependent H2O2 production regulates IAA biosynthesis in rice. BMC Plant Biol. 21, 326. https://doi.org/10.1186/s12870-021-03112-4 (2021).
Hesse, H. & Hoefgen, R. Molecular aspects of methionine biosynthesis. Trends Plant Sci. 8, 259–262. https://doi.org/10.1016/S1360-1385(03)00107-9 (2003).
Sharma, A. et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules https://doi.org/10.3390/biom9070285 (2019).
Busch, F. A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 101, 919–939. https://doi.org/10.1111/tpj.14674 (2020).
Timm, S. The impact of photorespiration on plant primary metabolism through metabolic and redox regulation. Biochem. Soc. Trans. 48, 2495–2504. https://doi.org/10.1042/BST20200055 (2020).
Zimmermann, S. E. et al. The phosphorylated pathway of serine biosynthesis links plant growth with nitrogen metabolism. Plant Physiol. 186, 1487–1506. https://doi.org/10.1093/plphys/kiab167 (2021).
Joo, J. Y., Kim, M. S., Cho, Y. G., Fernie, A. R. & Sung, J. Transcriptional comparison of genes associated with photosynthesis, photorespiration, and photo-assimilate allocation and metabolic profiling of rice species. Int. J. Mol. Sci. 23, 8901. https://doi.org/10.3390/ijms23168901 (2022).
Chen, D., Shao, Q., Yin, L., Younis, A. & Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 9, 1945. https://doi.org/10.3389/fpls.2018.01945 (2018).
Raza, A. et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 13, 961872. https://doi.org/10.3389/fpls.2022.961872 (2022).
Bidabadi, S. S. & Jain, S. M. Cellular, molecular, and physiological aspects of in vitro plant regeneration. Plants https://doi.org/10.3390/plants9060702 (2020).
Hu, D., Wei, L. & Liao, W. Brassinosteroids in plants: Crosstalk with small-molecule compounds. Biomolecules https://doi.org/10.3390/biom11121800 (2021).
Ilyas, M. et al. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 40, 926–944. https://doi.org/10.1007/s00344-020-10174-5 (2021).
Wang, C. et al. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 226, 1042–1054. https://doi.org/10.1111/nph.16416 (2020).
Chen, Z. et al. The response of ABA and hydraulic indicator-mediated leaf gas exchange and nonstructural carbohydrate of Ginkgo biloba saplings to drought and rehydration. Acta Physiol. Plant. https://doi.org/10.1007/s11738-022-03397-4 (2022).
Mir, A. R., Siddiqui, H., Alam, P. & Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 26, 2503–2520. https://doi.org/10.1007/s12298-020-00914-y (2020).
Yang, L., You, J., Li, J., Wang, Y. & Chan, Z. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 72, 5599–5611. https://doi.org/10.1093/jxb/erab196 (2021).
Mehmood, A. et al. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 77, 225–235. https://doi.org/10.1007/s13199-018-0583-y (2018).
Ghosh, D., Gupta, A. & Mohapatra, S. Dynamics of endogenous hormone regulation in plants by phytohormone secreting rhizobacteria under water-stress. Symbiosis 77, 265–278. https://doi.org/10.1007/s13199-018-00589-w (2018).
Zhang, W. et al. Effects of low temperature at booting stage on sucrose metabolism and endogenous hormone contents in winter wheat spikelet. Front. Plant Sci. 10, 498. https://doi.org/10.3389/fpls.2019.00498 (2019).
Fang, S. et al. Chemical priming of seed alters cotton floral bud differentiation by inducing changes in hormones, metabolites and gene expression. Plant Physiol. Biochem. 130, 633–640. https://doi.org/10.1016/j.plaphy.2018.08.010 (2018).
Fan, H. et al. A comprehensive analysis of the floral transition in ma bamboo (Dendrocalamus latiflorus) reveals the roles of DlFTs involved in flowering. Tree Physiol. 42, 1899–1911. https://doi.org/10.1093/treephys/tpac035 (2022).
Noreen, S. et al. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 158, 244–254. https://doi.org/10.1016/j.plaphy.2020.11.007 (2021).
Wang, C. et al. Effect of methyl jasmonate treatment on primary and secondary metabolites and antioxidant capacity of the substrate and hydroponically grown Chinese chives. Front. Nutr. 9, 859035. https://doi.org/10.3389/fnut.2022.859035 (2022).
Ahmad, P., Ahanger, M. A., Alyemeni, M. N., Wijaya, L. & Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255, 79–93. https://doi.org/10.1007/s00709-017-1132-x (2018).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. https://doi.org/10.1104/pp.24.1.1 (1969).
Ivanov, D. A. & Bernards, M. A. Chlorophyll fluorescence imaging as a tool to monitor the progress of a root pathogen in a perennial plant. Planta 243, 263–279. https://doi.org/10.1007/s00425-015-2427-9 (2016).
Edwards, D. M. et al. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 79, 201–218. https://doi.org/10.1023/b:pres.0000015391.99477.0d (2004).
Heidari, P. et al. Exogenous EBR ameliorates endogenous hormone contents in tomato species under low-temperature stress. Horticulturae https://doi.org/10.3390/horticulturae7040084 (2021).
Acknowledgements
The Special Fund for Technical System of Melon and Vegetable Industry of Gansu Province, China (GARS-GC-1). The Natural Science Foundation of Gansu Province, China (Nos. 21JR7RA821).
Author information
Authors and Affiliations
Contributions
T.N., J.Z., J.X., and J.L. conceived and designed the experiments. T.N. analyzed the data. T.N. wrote the manuscript. T.N., J.Z., X.G., Y.G., and Y.C. were involved in the related discussion. H.M. organized the pictures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Niu, T., Zhang, J., Li, J. et al. Effects of exogenous glycine betaine and cycloleucine on photosynthetic capacity, amino acid composition, and hormone metabolism in Solanum melongena L.. Sci Rep 13, 7626 (2023). https://doi.org/10.1038/s41598-023-34509-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34509-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.