Abstract
Urinary tract infection (UTI) is one of the most common bacterial infections in women; about 50% of women get during their life time. Moreover, it is a common health problem in patients with gynecological pathologies, which increases the chance of acquiring infection. The aim of this study was to determine the bacterial profile that causes UTI and their antibiotic susceptibility pattern among admitted gynecological cases. A cross-sectional study was conducted in south west Ethiopia region. A total of 386 patients admitted with gynecological cases were recruited by sequential sampling technique and structured questionnaire was used to collect socio-demographic and risk factor-related data. About 10 ml freshly voided midstream and catheterized urine specimens were collected using sterile containers. Identification of isolate was done using culture characteristics, gram staining, and a series of biochemical tests. The antibiotic susceptibility test was performed as per the Kirby–Bauer disc diffusion technique. The data obtained were entered into EpiData Version 3.1 and analyzed using SPSS Version 25. A P value of less than 0.05 was used as a level of significance. In this study, the overall prevalence of UTI was 25.4%. Escherichia coli was the most frequently isolated bacteria, which accounted for 38 (37.6%), followed by Klebsiella species 22 (21.8%), CONS 14 (13.9%), Staphylococcus aureus 10 (9.9%), Enterobacter species 6 (5.9%), Citrobacter species 5 (4.9%), Proteus mirabilis 4 (4%), and Pseudomonas aeroginosa 2(2%). Histories of UTI (AOR = 1.977, 95% CI 1.06, 3.68, P = 0.032) and catheterization (AOR = 2.38, 95% CI 1.28, 4.45, P = 0.006) were found to be statistically associated with significant bacteriuria. Gram-negative isolates showed a high level of resistance, 88.3% for ampicillin and 66.2% for tetracycline, and a relatively low level of resistance against ceftazidime, 22.1%, and meropenem, 3.9%. Gram-positive uropathogens showed a high level of resistance to penicillin, 91.6%, whereas all isolates were sensitive 100.0% to nitrofurantoin. Furthermore, 80 (79.2%) of the isolates had multidrug resistance, and 16 (26.7%) of both E. coli and Klebsiella spp. produced Extended spectrum β-lactamase (ESBL). In this study, a high prevalence of uropathogenic bacteria and multidrug resistance for commonly prescribed drugs were observed with a significant number of ESBL producers. Therefore, screening admitted gynecological patients, especially for those who have history of catheterization and UTI, by urine culture and antimicrobial susceptibility testing is important.
Similar content being viewed by others
Introduction
Urinary tract infection (UTI) is the most frequent human-acquired bacterial infection which affects about 150 million people all over the world each year1. It involves anywhere in the urinary tract, including the kidney, ureter, bladder, and urethra. It is one of the most common bacterial infections in women2, more than 50% of women get during their life time3. This can be due to the ascent of a number of organisms into the bladder is easier than in men because of the relatively short urethra, the absence of bactericidal prostatic secretion, and the ease of contamination of the urinary tract with fecal flora4.
In patients with gynecological problems, blockage of urine flow5, anatomical and physiological changes6, incomplete bladder emptying, frequent bladder infections, incontinence of urine and stool, and catheterization after surgical procedures may further predispose them to urinary tract infections7,8. During and after gynecological surgery, bladder drainage by transurethral foleys catheter is common practice used to monitor urine output and prevent post-operative urinary retention, which is associated with an increased risk of UTIs in patients, and the daily risk of acquisition of bacteriuria when an indwelling catheter is in-situ is 3–7%9,10. It is known that, UTI poses a high risk of morbidity, mortality, and significant health care costs11. Moreover, among patients with gynecological problem, it has been a matter of morbidity, anxiety, and long hospital stays, which occurs more frequently during the postoperative period12.
In developing countries including Ethiopia, management of UTIs is usually empirical which may contributes for the emergency and spread of antimicrobial resistant strain which is a leading cause of treatment failure in UTI13. As a result, clinicians are left with very limited drug choices for the treatment of urinary tract infections. One of the leading antimicrobial resistance mechanisms for many UTI causing Gram-negative bacteria is extended-spectrum β-lactamase enzyme production that hydrolyzes the β-lactam ring of antimicrobials, which gives bacterial resistance to commonly prescribed antibiotics including penicillins; first, second and third-generation cephalosporins, aztreonams14.
Generally, most admitted gynecological cases need prolonged hospitalization and more intensive nursing care like prolonged bladder catheterization, which may contribute to the development of urinary tract infections that require extensive treatment with antibiotics. Therefore, this study was conducted to determine the bacterial etiologic agents of uropathogens and evaluate their in vitro susceptibility pattern to commonly used antibiotics among admitted gynecological cases at tertiary hospital in southwest Ethiopia.
Methods
Study design, setting, and population
A cross-sectional study was conducted among gynecological cases admitted from September 16 to December 30, 2021 at Jimma University Medical Center, South West Ethiopia. The study area is located 348 km away from the capital city, Addis Ababa. The annual average admission of the centre is over 20,000 patients. The service delivery sites are the outpatient department, gynecology ward, maternity and labor ward, and operation rooms. All gynecological cases admitted to the gynecology ward during the study period were included in the study population, and those treated with antibiotics within 15 days of the study's start date were excluded.
Sample size and sampling techniques
The sample size was calculated using a single population proportion formula \(n = \frac{{\left( {Z^{\frac{a}{2}} } \right)P\left( {1 - P} \right)}}{{d^{2} }}\), where n = number of sample size, Z is the statistics corresponding to a 95% level of confidence (1.96), d = margin of error, and P = is the assumed prevalence of uropathogenic bacteria among gynecologic cases (50%). The infection prevalence was assumed to be 50% because the current status of uropathogenic bacterial infection in the area is unknown. Therefore, the sample size was adjusted to 384 gynecological cases. A sequential sampling method was used to find study participants.
Data collection methods
Information on demographic variables was collected from each patients with gynecolological cases by a face-to-face interview using a structured questionnaire. Clinical data was gathered through a review of patients' medical records and consultation with a gynecologist.
Specimen collection
After obtaining informed consent or assent from study subjects and/or parental/guardian, about 5–10 ml of urine was collected from patients by following aseptic technique and clean catch mid-stream urine (MSU) using a sterile screw-capped, wide-necked container. For those patients on a catheter, the sample was collected by aseptic techniques: cleansing the catheter port with alcohol and allowing drying time, and then aspirating the urine from the indwelling catheter with a sterile syringe. The container was labeled with the date, the name, and a code number. After collection, the specimen was immediately delivered to the microbiology laboratory for laboratory investigation. The specimens were processed within 2 h of collection. In the event of a delay in processing, the urine specimens were refrigerated at 4 °C until they were processed.
Culture and identification techniques
The collected urine sample was inoculated onto 5% Blood Agar, Mannitol Salt agar and MacConkey agar plates (Oxoid Ltd., Bashingstore Hampaire, UK) by streak plate methods following the standard microbiological techniques and procedures15. After incubating the plates aerobically at 37 °C for 24 h, they were inspected for the presence or absence of bacterial growth. If colonies were found, they were counted and multiplied by the reciprocal of the loop’s volume, or 1000. Counting colonies yielding bacterial growth of ≥ 105 cfu/ml with pure growth were considered as significant bacteriuria, and negative cultures contained no growth or mixed urogenital flora (more than two isolates). For cultures containing two types of colonies, sub-culture for further identification and antimicrobial susceptibility testing. For catheter collected urine samples, the presence of symptoms or signs compatible with UTI with no other identified source of infection along with ≥ 103 colony-forming units/ml and the presence of ≥ 105 cfu/ml in a single catheter urine specimen in a patient without symptoms compatible with UTI is recommended as representing significant bacteriuria16,17.
All positive urine cultures showing significant bacteriuria were further identified by their physical characteristics such as colony morphology, odor, swarming, and presence of hemolysis on their respective media, Gram-reaction, and pattern of biochemical reactions using the standard procedures15. The gram-negative rods were identified with the help of a series of biochemical tests, namely Citrate, Oxidase, Sulphur Indole Motility (SIM) media, Kligler’s Iron Agar (KIA), lysine decarboxylase, lactose fermentation, urea hydrolysis18. Gram-positive cocci were identified based on their Gram reaction, mannitol fermentation, catalase and coagulase tests15.
Antimicrobial susceptibility testing (AST)
The Kirby–Bauer disc diffusion method was employed for antibiotic susceptibility testing as recommended by Clinical Laboratory Standards Institute (CLSI) 2020 guidelines19. When a pure culture with significant bacteriuria was obtained, a loopful of bacteria was taken from a colony and transferred into a tube containing 5 ml of sterile normal saline (0.85% NaCl) and mixed gently until it formed a homogenous suspension. The turbidity of the suspension was adjusted to an optical density equivalent to 0.5 McFarland standards. The inoculated plates were left at room temperature to dry for 3–5 min while the Petridish lids were in place.
The following antimicrobial discs with their respective concentration were used for susceptibility testing and all the antimicrobials used for the study were obtained from Oxoid Ltd in the following concentrations: ciprofloxacin (CIP, 5 µg), trimethoprim-sulfamethoxazole (SXT, 1.25/23.75 µg), nalidixic acid (NA, 30 μg), meropenem (MEM, 10 μg ), cefotaxime (CTX, 30 µg), ceftazidime (CAZ, 30 µg), tetracycline (TTC, 30 µg) nitrofurantoin (F, 30 µg), norfloxacin (NOR, 10 µg), ceftriaxone (CRO, 30 µg), amoxicillin-clavulanic acid (AMC, 20/10 µg), ampicillin (AMP, 10 µg), gentamicin (CN, 10 µg), erythromycin (E, 15 µg), penicillin (PEN, 10 µg), clindamycin (DA, 10 µg) and chloramphenicol (C, 30 µg). Among these, nalidixic acid (NA), meropenem (MEM, 30 μg), cefotaxime (CTX, 30 µg), ceftazidime (CAZ, 30 µg), tetracycline (TTC, 30 µg) can be used only for gram-negative bacteria, whereas erythromycin (E, 15 µg), penicillin (PEN, 10 µg) and clindamycin (DA, 10 µg) can be used only for gram-positive bacteria; however, the rest of the antibiotics were used for both isolates. The diameter of the zone of inhibition around each disc was measured to the nearest whole millimeter (mm) by using a ruler, and the isolate was classified as sensitive, intermediate, or resistant according to the standardized table supplied by CLSI, 202019.
Detection of extended spectrum β-lactamase (ESBL)
ESBL-producing Escherichia coli and Klebsiella spp. were first screened for ESBL production by the phenotypic method and then confirmed by the phenotypic confirmatory test as per CLSI guidelines 202019.
Phenotypic screening for ESBL production
The ESBL screening test was performed by the standard disk diffusion method using ceftazidime (30 µg), cefotaxime (30 µg), and ceftriaxone (30 µg) (Oxoid, UK). More than one antibiotic disc was used for screening to improve the sensitivity of ESBL detection19. The three antibiotic discs were placed on Muller–Hinton agar and incubated at 37 °C for 18–24 h. These breakpoints for suspicion of ESBL production were: ≤ 25 mm for ceftriaxone (30 g), ≤ 22 mm for ceftazidime (30 g), and ≤ 27 mm for cefotaxime (30 g)19.
Phenotypic confirmation of ESBL producers
Confirmation of suspected ESBL producers was done by using the double-disk synergy (DDS) method. On Muller Hinton agar, an Amoxicillin/clavulanic acid (20/10 g) disc was placed in the center of the plate, and ceftazidime (30 g) and cefotaxime (30 g) discs were placed 15 mm apart, center to center. The plate was incubated at 37 °C for 18–24 h. A ≥ 5 mm increase in the diameter of the zone of inhibition for either of the cephalosporin-clavulanate disk combinations versus the zone diameter of the respective cephalosporin disk was considered positive, and the isolate was interpreted as an ESBL producer as recommended by CLSI guidelines 202019.
Data processing and analysis
Data were entered using EpiData version 3.1 and exported to SPSS version 25 software. Descriptive statistics were used to summarize socio-demographic data, bacterial profile and susceptibility patterns of isolates. Bivariate logistic regression was employed to look for associations between the outcome variable and each independent variable; and those variables significant at a P value of less than 0.25 in the bivariate regression were then selected for the multivariate analysis model. The corresponding variables with a P value ≤ 0.05 at a 95% confidence interval were then considered statistically significant.
Quality assurance
The legibility of the filled questionnaire and any labeling errors were confirmed immediately. Laboratory analyses were carried out using standard operating procedures. Culture media were tested for sterility and performance by incubating 5% of the batch at 35–37 °C overnight and observing the media for microbial growth. Those media which showed growth was discarded and replaced by a new sterile batch. Standard reference strains of Staphylococcus aureus (ATCC25923), E. coli (ATCC25922) and Pseudomonas aeruginosa (ATCC27853) were used during culture and antimicrobial susceptibility testing. Escherichia coli ATCC 25922 was used as an ESBL-negative whereas K. pneumoniae ATCC 700603 was used as an ESBL-positive reference strain19.
Ethical consideration
The study protocol was evaluated and approved by the Institutional Review Board of Jimma University (Ref.: IRB000144/2020), and ethical clearance was obtained. The support letter was obtained from Jimma University School of Medical Laboratory Science and then submitted to JMC, gynecology ward and all methods were performed in accordance with relevant guidelines and regulations. After adequately explaining the objectives and purpose of the study, written informed consent was obtained from all the study subjects and/ or assent were obtained from study subject less than 18 years and/or guardians before data and sample collection. All data obtained in the course of the study was kept confidential. Positive cases were referred to the attending clinician as soon as possible for their better management.
Ethics approval and consent to participate
Ethical permission for this study was approved by Jimma University, Research and Ethics Committee of the School of Medical Laboratory. All participants were voluntary and each supplied informed consent.
Result
Socio-demographic characteristics
A total of 386 patients admitted with gynecological cases were included in the present study. The mean age of the study participants was 31.9 ± 11.5 years, with an age range of 15–70 years. About 153 (39.6%) of study participants were in the age range of 25–34 years, and the majority were married (315, or 81.6%), high school (1–8; 35.2%) in their educational status, and urban dwellers (50.5%) in their residence. Based on their parity, the majority of 61.7% of the study participants were multiparous. Approximately 50.5% and 28% of the study participants had a history of catheterization and UTI, respectively (Table 1).
The prevalence of UTIs and the types of bacteria isolated
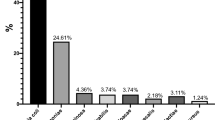
Of the 386 urine specimens analyzed, 98 had significant bacteriuria, with an overall prevalence of 25.4%. A total of 101 bacterial isolates (bacterial species belonging to seven genera) were identified from the study participants. The prevalence of bacteriuria among patients with gynecological cases who had undergone gynecologic surgery was 73 (31.12%). Of these, 15 (20.5%) underwent emergency surgery and 58 (79.5%) underwent elective surgery. Among patients who had undergone non-surgical procedures the prevalence was 25 (16.4%) and 32 (34.4%) of the isolates were from symptomatic cases. The prevalence of asymptomatic bacteriuria was 66 (22.5%). Of the total 101 isolates, the majority (77, or 76.2%) were gram-negative organisms, while 24 (23.8%) were gram-positive bacteria. Escherichia coli was found to be the most frequent isolate 38 (49.4% of the gram-negatives, 37.6% of all isolates), followed by Klebsiella spp. 22 (21.8%) and CoNS 14 (13.9%). The other isolated organisms were S. aureus 10 (9.9%), Enterobacter spp. 6 (5.9%), Citrobacter spp. 5 (4.9%), P. mirabilis 4 (4%), and P. aeroginosa 2(2%). Of all, mixed pathogens were isolated from 3 (3.1%) patients (Fig. 1).
Associated risk factors of UTI
Twelve independent variables were considered during the bivariate analysis of risk factors for significant bacteriuria. In multivariate analysis, histories of UTI (AOR = 1.977, 95% CI 1.06, 3.68, P = 0.032) and catheterization (AOR = 2.38, 95% CI 1.28, 4.45, P = 0.006) were found to have a statistically significant association with significant bacteriuria (Table 2).
Antimicrobial susceptibility pattern of bacterial uropathogens
Gram negative bacteria
In this study, gram-negative uropathogens showed high levels of resistance to 68 (88.3%) for ampicillin and 51 (66.2%) for tetracycline. However, all gram-negative bacterial isolates showed a relatively low level of resistance against nitrofurantoin 21 (27.3%), norfloxacin 18 (23.4%), ceftazidime 17 (22.1%), and meropenem 3 (3.9%) (Table 3).
Gram-positive bacteria
Out of the tested antibiotics, gram-positive bacterial isolates showed a high level of resistance to penicillin (91.6%) and trimethoprim-sulfamethoxazole (66.7%). On the other hand, all gram-positive isolates showed full sensitivity to 100.0% nitrofurantoin. Moreover, gram-positive isolates showed sensitivity towards clindamycin 79.2%, ciprofloxacin 75.0%, norfloxacin 87.5%, and amoxicillin-clavulanic acid 91.6% (Table 4).
Multidrug resistance pattern of the isolates
Among the total isolates (n = 101), overall, 96 (95.0%) bacterial isolates were resistant to at least one antimicrobial agent, whereas 87 (86.1%) isolates were resistant to ≥ 2 antimicrobials. Multidrug resistance (defined as non-susceptible to ≥ 1 agent in ≥ 3 antimicrobial categories)20 was seen in 80 (79.2%) of all isolated bacterial uropathogens. 62 (80.5%) of gram-negative and 18 (75%) of gram-positive bacteria showed multidrug resistance for the tested antimicrobial drugs. Escherichia coli and K. pneumoniae were found to be highly resistant to most of the antibiotics tested (Table 5).
ESBL-producing uropathogens
The isolates were E. coli 38(49.3%), K. pneumoniae 21 (27.3%), Enterobacter spp. 6(7.8%), P. mirabilis 4(5.2%), Citrobacter spp. 5(6.5%), P. aeruginosa 2(2.6%), and K. oxytoca 1(1.3%). In this study, E. coli and Klebsiella spp. were the most frequently isolated bacteria, and the methods were validated for them. Of the 60 g-negative isolates of E. coli and Klebsiella spp., 16 (26.7%) were positive for ESBL production, with 10 (26.3%) of the E. coli isolates and 6 (27.3%) of Klebsiella spp. All ESBL-producing isolates showed 100% resistance to ceftriaxone, cefotaxime, and ceftazidime, 87.5% of drugs. However, all the ESBL-producing uropathogens were sensitive to meropenem at 100% (Table 5).
Discussion
In this study, the overall prevalence of significant bacteriuria among admitted gynecological cases was found to be 25.4%, which was in agreement with the findings of the previous studies conducted in India 24%21, Nigeria, 26.2%22 and USA 22.4%23. However, our finding was higher than other studies reported from Norway, 16.3%24 and USA, 11.3%25. But, our finding was lower than the reports from Ethiopia, 58.1%26 and Nigeria, 76.1%27. This inconsistency in prevalence might be due to differences in the sample size, methods employed, standard of personal hygiene, predisposing factors, and study population. Furthermore, the prevalence of uropathogenic bacteria observed in our study is still high compared to those reported in developed countries 6%28 and this might be due to a difference in the level of health-care development29.
In this study, gram-negative bacteria isolates were more prevalent with 77 (76.2%) than gram-positive bacteria isolates with 24 (23.8%). This could be due to the presence of a unique structure in gram-negative bacteria that helps with the attachment to the uroepithelial cells and prevents bacteria from being washed away by urine, allowing for multiplication and tissue invasion30.
E. coli was the most frequent etiological agent of UTI, accounting for up to 37.6% of isolated cases. This finding is in agreement with the findings from Ethiopia, 35.7%31, Nigeria, 34.2%32 and India, 37.3%33. The possible explanation for this high isolation rate of E. coli in the present finding could be due to the significant abundance of E. coli in the rectal area, which in turn via contamination ascends through genitalia to the urinary tract and causes UTI and it could also be due to E. coli having various enhanced virulence factors specific for colonization and invasion of the urinary epithelium, such as P-fimbriae and S-fimbriae adherence factors which mediate the attachment of E. coli to viginal and uroepithelial cells34.
Patients with gynecological cases who underwent gynecologic surgery had 72 (30.9%) positive cultures, whereas 26 (17%) of the 153 patients who underwent non-surgical procedures had significant bacteriuria, indicating that patients with gynecological cases who underwent gynecologic surgery were more affected than those who did not. This might be due to the close proximity of the rectum to the operating field and the use of urinary catheters, which aid in emptying the bladder during the procedure, which has a strong association with the development of UTI35.
This study's findings also show that a history of UTI has a strong association with significant bacteriuria, as shown in Table 2. Similar finding were reported from Ethiopia26,36. This is possibly due to the presence of resistant bacterial strains from those who had a previous history of UTI after poor diagnosis and ineffective treatment.
The prevalence of uropathogenic bacteria in admitted gynecological cases with a history of catheterization was also significantly higher than those without a history of catheterization, which is almost similar to other reports where catheterization is the most important risk factor for the development of catheter associated bacteriuria in Northern America37 and Ethiopia26 this is possibly due to catheterization, which could cause urethral mucosa injury when staying for a long time and might induce hematogenous bacterial spread in the urinary tract, the formation of biofilm by gram negative bacteria, and contamination during the insertion of catheters38,39.
Symptoms were not associated with the significant bacteriuria in this study. From 93 patients with gynecological pathologies who complain of having symptoms that suggest symptomatic urinary tract infection, only 32/63 (34.4%) were found to have culture confirmed urinary tract infection. Similar findings were also reported in Ethiopia26. Symptomatic patients whose urine cultures did not show significant growth might be due to several different microorganisms that can cause UTIs, including protozoan parasites, fungi, and viruses, even though bacteria are the major causative organisms40.
In this study, 26.7% of the gram-negative (E. coli and Klebsiella spp.) isolates were found to be ESBL producers. ESBL production of Klebsiella isolates was 27.3%, which is slightly higher than previous findings in Jimma, Ethiopia of 23.5%41. The rise in the prevalence of ESBL-producing uropathogens in the current study might be due to the fact that our study participants were all hospitalized, since hospitalization was identified as the strongest independent risk factor for ESBL42.
In this study, among gram-negative bacteria, the highest resistance was shown to ampicillin 88.3%, followed by tetracycline 66.2%. The reason may be due to the continuous use of these drugs for many years. High level of resistance to beta-lactam ring containing antibiotics could happen because of the presence of extended spectrum β-lactamase in these strains. On the other hand, about 74/77 (96.1%) of isolates were sensitive to meropenem. This might be due to the unavailability of this drug in the area. In addition, lower resistance (higher rate of sensitivity) was observed against 51/77 (66.2%) nitrofurantoin, 55 (71.4%) norfloxacin and 58/77 (75.3%) ceftazidime. The possible justification for such low-level resistance might be attributable to the infrequent prescription of these drugs. Hence, they could be considered as alternative options in the treatment of UTI.
Among the gram-negatives, the predominant isolate was E. coli, which is sensitive to nitrofurantoin 81.6%, ceftriaxone 78.9% and norfloxacin 76.3%. This was consistent with findings in Addis Ababa, Ethiopia43, while high resistance was shown to ampicillin (31; 81.6%) and tetracycline (27; 71.1%), which is in line with studies from Dire-Dawa, Ethiopia44 and Gondor, Ethiopia45. In this study, Klebsiella spp. showed a high rate of sensitivity to norfloxacin (14; 66.7%) and ceftriaxone (14; 66.7%), which is in line with a study done at Gondor, Ethiopia45. Also, the isolates showed high resistance to ampicillin 22 (100%) and amoxicillin-clavulanic acid 17 (77.3%), which is similar to the study done by Belete et al.46. The possible explanation of ampicillin and amoxicillin-clavulanic acid resistance might be due to their extensive use in health facilities, as this may augment the selection of uropathogens harboring the β-lactamase enzyme, which can hydrolyze penicillin.
Gram-positive bacteria were relatively resistant to penicillin (91.6%) and trimethoprim-sulfamethoxazole (66.7%). This might be due to the easy availability and indiscriminate use of these drugs, which could lead to an increase in resistance. On the contrary, all tested gram-positive isolates showed sensitivity to nitrofurantoin 100.0%. The reason for the effectiveness of this drug might be due to its narrow range of clinical indications, which results in less usage.
According to the international standard for the definition of drug resistance20, multidrug resistance (non-susceptible to ≥ 1 agent in ≥ 3 antimicrobial categories) was observed in 79.2% of the total isolated bacterial uropathogens, which was comparable with the finding from North East Ethiopia of 80.4%46. However, our finding was lower than previously reported MDR prevalence in Ethiopia (95–100%)44,45 and Kenya 96.0%47. In contrast, the result of this study was higher than studies from other documented results in Ethiopia, 74.0%48. The possible reason for this rise in MDR might be the repeated, inappropriate, and incorrect use of antimicrobial agents in empirical treatment, which in turn raises the prevalence of resistant microorganisms in the community44. It could also be due to multiple resistant genes that can develop on the mobile genetic elements49 and plasmids bearing genes-encoding ESBLs, which frequently also carry genes encoding resistance to other antimicrobial agents14.
All ESBL-producing isolates showed 100.0% sensitivity to meropenem. Therefore, carbapenems are considered the drugs of choice in the treatment of severe infections caused by ESBL-producing bacteria50. All of the ESBL positive isolates showed a high level of resistance; 100.0% to ampicillin, sulphamethoxazole-trimethoprim, ceftriaxone, cefotaxime, and 87.5% to ceftazidime. The treatment of choice for ESBL-producing bacteria is very limited14. Such findings indicate that the use of these antibiotics for the treatment of urinary tract infections caused by ESBL-positive strains may not produce the desired effect and may result in a significant amount of treatment failure. Conversely, ESBL-positive strains can respond better to carbapenem drugs such as meropenem, which could be a better treatment option.
Limitations of the Study was, it focused on admitted patients until the time of sample collection, before discharge and didn’t investigated infections that may have developed after the patients were discharged. In addition, molecular techniques for ESBL confirmation and pathogen identification were not carried out.
Conclusions
The overall prevalence of UTI among admitted gynecological cases was 25.4%. Escherichia coli was the most dominant pathogen, which accounted for 37.6% of the isolates, followed by Klebsiella spp. The history of UTI and history of catheterization had a statistically significant association with the uropathogenic bacterial isolates. This study also showed that Nitrofurantoin and Norfloxacin were the drugs of choice for both gram-negative and gram-positive bacteria, whereas ampicillin and trimethoprim-sulfamethoxazole were less effective for the management of UTI among our study participants. While meropenem and clindamycin were found effective against gram-negative and positive bacteria isolated, respectively, a significant number of ESBL producers (26.7%) and an alarmingly high multi-drug resistance have been shown in most of the bacterial isolates (79.2%). Therefore, screening admitted gynecological patients, especially for those who have history of catheterization and UTI, by urine culture and antimicrobial susceptibility testing. Furthermore, antibiotic stewardship program should start at the hospital to reduce the observed antibiotic resistance and prevent further complications.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- ATCC:
-
American Type Culture Collection
- BAP:
-
Blood agar plate
- CFU:
-
Colony forming unite
- CLSI:
-
Clinical Laboratory Standard Institute
- CoNS:
-
Coagulase negative staphylococci
- COR:
-
Crude odds ratio
- DDS:
-
Double-disk synergy
- ESBL:
-
Extended spectrum B-lactamase
- JUMC:
-
Jimma University Medical Center
- KIA:
-
Kligler’s iron agar
- MSU:
-
Mid-stream urine
- QC:
-
Quality control
- SIM:
-
Sulphur indole motility
- SOP:
-
Standard operating procedure
- SPSS:
-
Statistical package for social science
- UK:
-
United Kingdom
- UTI:
-
Urinary tract infection
- WHO:
-
World Health Organization
References
Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. 28(1), 1–13 (2014).
Al-Badr, A. & Al-Shaikh, G. Recurrent urinary tract infections management in women: A review. Sultan Qaboos Univ. Med. J. 13(3), 359 (2013).
Bergamin, P. A. & Kiosoglous, A. J. Non-surgical management of recurrent urinary tract infections in women. Transl. Androl. Urol. 6(Suppl 2), S142 (2017).
El-Naggar, W., Hassan, R., Barwa, R., Shokralla, S. & Elgaml, A. Molecular diagnosis of gram negative bacteria in urinary tract infections. Egypt. J. Med. Microbiol. 19(1), 93 (2010).
Kilpatrick, C. C. Cervical Myomas. MSD Manual (2019).
Dewhurst, J. Dewhurst’s Textbook of Obstetrics and Gynaecology (Wiley, 2012).
Salvatore, S. et al. Urinary tract infections in women. Eur. J. Obstet. Gynecol. Reprod. Biol. 156(2), 131–136 (2011).
Sutkin, G. et al. Symptomatic urinary tract infections after surgery for prolapse and/or incontinence. Int. Urogynecol. J. 21, 955–961 (2010).
Guggenbichler, J. P., Assadian, O., Boeswald, M. & Kramer, A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials–Catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenh. Interdiszip. 6(1), 1–19 (2011).
Nero, D. C., Lipp, M. J. & Callahan, M. A. The financial impact of hospital-acquired conditions. J. Health Care Finance 38(3), 40 (2012).
Marques, L. P. J. et al. Epidemiological and clinical aspects of urinary tract infection in community-dwelling elderly women. Braz. J. Infect. Dis. 16(5), 436–441 (2012).
Akter, J., Seraji, A., Nahar, L., Khan, S. I. & Ahsanullah, M. R. Prevalence of urinary tract infection due to urinary catheterization in obstetric and gynaecological operations. Bangladesh J. Med. Sci. 18(4), 696–702 (2019).
Girma, A. & Aemiro, A. The bacterial profile and antimicrobial susceptibility patterns of urinary tract infection patients at Pawe General Hospital, Northwest Ethiopia. Scientifica https://doi.org/10.1155/2022/3085950 (2022).
Rawat, D. & Nair, D. Extended-spectrum β-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2(3), 263 (2010).
Ogodo, A. C., Agwaranze, D. I., Daji, M. & Aso, R. E. Microbial techniques and methods: Basic techniques and microscopy. In Analytical Techniques in Biosciences (eds Egbuna, C. et al.) 201–220 (Amsterdam, 2022).
Karah, N. et al. Guideline for urine culture and biochemical identification of bacterial urinary pathogens in low-resource settings. Diagnostics 10(10), 832 (2020).
CDC. Urinary Tract Infection (Catheter Associated Urinary Tract Infection [CAUTI] and Non-Catheter Associated Urinary Tract Infection [UTI] and Other Urinary System Infection (USI) Events). Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf. Accessed 6 March 2023 (2023 ).
Dura, S. Biochemical Tests for Identification of Gram-Negative Bacteria. Available from: www.thesciencenotes.com/biochemical-tests-for-identification-of-gram-negativ. Accessed 5 March 2023 (2020)
Institute: CCaLs. Performance Standards for Antimicrobial Susceptiblity testing (CLSI supplement M100, 2020).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281 (2012).
Sabharwal, E. R. Antibiotic susceptibility patterns of uropathogens in obstetric patients. N. Am. J. Med. Sci. 4(7), 316 (2012).
Ghatak, D. A study of urinary fistulae in Sokoto, Nigeria. J. Indian Med. Assoc. 90(11), 285–287 (1992).
Harmanli, O. H., Cheng, G. Y., Nyirjesy, P., Chatwani, A. & Gaughan, J. P. Urinary tract infections in women with bacterial vaginosis. Obstet. Gynecol. 95(5), 710–712 (2000).
Schiøtz, H. Postoperative bacteriuria and urinary tract infections in gynecological patients. Tidsskr. Nor. Laegeforen. Tidsskr. Prakt. Med. Raekke 116(2), 246–248 (1996).
Dawson, M. L., Cramer, M. S., Thompson, D. R. & Vakili, B. Microbiological analysis of urine cultures in women after pelvic reconstructive surgery. Curr. Urol. 11(4), 212–217 (2017).
Dereje, M., Woldeamanuel, Y., Asrat, D. & Ayenachew, F. Urinary tract infection among fistula patients admitted at Hamlin fistula hospital, Addis Ababa, Ethiopia. BMC Infect. Dis. 17(1), 150 (2017).
Adeoye, I. S., Oladeinde, O., Uneke, J. & Adeoye, J. An assessment of asymptomatic bacteriuria among women with vesico-vaginal fistula in South-Eastern Nigeria. Nepal J. Epidemiol. 1(2), 64–69 (2011).
DiMarco, C., Croak, A., DiMarco, D. & Klingele, C. Urinary tract infections after gynecologic surgery. Infect. Dis. Obst. Gynecol. 12(3/4), 207 (2004).
R Douglas Scott, I., Culler, S. D. & Rask, K. J. Understanding the economic impact of health care-associated infections: A cost perspective analysis. J. Infus. Nurs. 42(2), 61–69 (2019).
Bublitz, D. C. et al. Epidemiology of pathogenic enterobacteria in humans, livestock, and peridomestic rodents in rural Madagascar. PLoS ONE 9(7), e101456 (2014).
Ejerssa, A. W., Gadisa, D. A. & Orjino, T. A. Prevalence of bacterial uropathogens and their antimicrobial susceptibility patterns among pregnant women in Eastern Ethiopia: Hospital-based cross-sectional study. BMC Womens Health 21(1), 291 (2021).
Ekwedigwe, K. et al. Prevalence and antimicrobial susceptibility of asymptomatic bacteriuria among women with pelvic organ prolapse in Abakaliki, South-East Nigeria. BMC Womens Health 18(1), 1–5 (2018).
Rupakala, B., Lasune, S., Prakash, R. & Nagarathanamma, R. Postoperative Catheter induced bacteriuria in obstetrics and gynaecological cases. Int. J. Reprod. Contracept. Obstet. Gynecol. 6, 1965–1968 (2017).
Kot, B. Virulence factors and innovative strategies for the treatment and control of uropathogenic Escherichia coli. In Escherichia coli-Recent Advances on Physiology, Pathogenesis and Biotechnological Applications (ed. Samie, A.) (InTech, 2017).
Schiøtz, H. Urinary tract infections and bacteriuria after gynecological surgery: Experience with 24-hour Foley catheterization. Int. Urogynecol. J. 5(6), 345–348 (1994).
Emiru, T., Beyene, G., Tsegaye, W. & Melaku, S. Associated risk factors of urinary tract infection among pregnant women at Felege Hiwot Referral Hospital, Bahir Dar, Bahir Dar, North West Ethiopia. BMC Res. Notes 6, 1–6 (2013).
El-Nashar, S. A. et al. Urinary tract infection after hysterectomy for benign gynecologic conditions or pelvic reconstructive surgery. Obstet. Gynecol. 132(6), 1347–1357 (2018).
Brusch, J. L. Catheter-Related Urinary Tract Infection (UTI) [Available from: https://emedicine.medscape.com/article/2040035-overview (2021).
Pallett, A. & Hand, K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J. Antimicrob. Chemother. 65(suppl3), 25–33 (2010).
Imam, T. H. Overview of Urinary Tract Infections (UTIs) University of Riverside School of Medicine. Available from: https://www.merckmanuals.com/home/kidney-and-urinary-tract-disorders/urinary-tract-infections-utis/overview-of-urinary-tract-infections-utis (2020).
Abayneh, M., Tesfaw, G. & Abdissa, A. Isolation of extended-spectrum β-lactamase-(ESBL-) producing Escherichia coli and Klebsiella pneumoniae from patients with community-onset urinary tract infections in Jimma University Specialized Hospital, Southwest Ethiopia. Can. J. Infect. Dis. Med. Microbiol. https://doi.org/10.1155/2018/4846159 (2018).
Al-Jamei, S. A., Albsoul, A. Y., Bakri, F. G. & Al-Bakri, A. G. Extended-spectrum β-lactamase producing E. coli in urinary tract infections: A two-center, cross-sectional study of prevalence, genotypes and risk factors in Amman, Jordan. J. Infect. Public Health 12(1), 21–25 (2019).
Wabe, Y. A., Reda, D. Y., Abreham, E. T., Gobene, D. B. & Ali, M. M. Prevalence of asymptomatic bacteriuria, associated factors and antimicrobial susceptibility profile of bacteria among pregnant women attending Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Ther. Clin. Risk Manag. 16, 923 (2020).
Derese, B., Kedir, H., Teklemariam, Z., Weldegebreal, F. & Balakrishnan, S. Bacterial profile of urinary tract infection and antimicrobial susceptibility pattern among pregnant women attending at Antenatal Clinic in Dil Chora Referral Hospital, Dire Dawa, Eastern Ethiopia. Ther. Clin. Risk Manag. 12, 251 (2016).
Alemu, A. et al. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res. Notes 5(1), 197 (2012).
Belete, M. A. Bacterial profile and ESBL screening of urinary tract infection among asymptomatic and symptomatic pregnant women attending antenatal care of Northeastern Ethiopia region. Infect. Drug Resist. 13, 2579 (2020).
Onyango, H. A., Ngugi, C., Maina, J. & Kiiru, J. Urinary tract infection among pregnant women at Pumwani Maternity Hospital, Nairobi, Kenya: Bacterial etiologic agents, antimicrobial susceptibility profiles and associated risk factors. Adv. Microbiol. 8(03), 175 (2018).
Assefa, A., Asrat, D., Woldeamanuel, Y., Abdella, A. & Melesse, T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiop. Med. J. 46(3), 227–235 (2008).
Gillespie, S. & Bamford, K. Medical Microbiology and Infection at a Glance (Wiley, 2012).
Ruppé, É., Woerther, P.-L. & Barbier, F. Mechanisms of antimicrobial resistance in gram-negative bacilli. Ann. Intensive Care 5(1), 21 (2015).
Acknowledgements
We are thankful to our data collectors for their excellent data collection. We are grateful to the entire study participants for their kind cooperation and to Jimma University for logistical and material support.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
S.T.: Conception of the research idea, carried out the experiment work, analyzed the data, and wrote the manuscript. Z.S., M.M., R.T., T.B.: supervision and review of the manuscript. T.D. and Y.A.: helped data collection and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teferi, S., Sahlemariam, Z., Mekonnen, M. et al. Uropathogenic bacterial profile and antibiotic susceptibility pattern of isolates among gynecological cases admitted to Jimma Medical Center, South West Ethiopia. Sci Rep 13, 7078 (2023). https://doi.org/10.1038/s41598-023-34048-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34048-4
This article is cited by
-
Unraveling the antimicrobial efficacy and chemical fingerprinting of medicinal plants against the WHO’s prioritized pathogens
Bulletin of the National Research Centre (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.