Abstract
In this study, genomic and plasmid characteristics of Escherichia coli were determined with the aim of deducing how mcr genes may have spread on a colistin withdrawn pig farm. Whole genome hybrid sequencing was applied to six mcr-positive E. coli (MCRPE) strains isolated from pigs, a farmworker and wastewater collected between 2017 and 2019. Among these, mcr-1.1 genes were identified on IncI2 plasmids from a pig and wastewater, and on IncX4 from the human isolate, whereas mcr-3 genes were found on plasmids IncFII and IncHI2 in two porcine strains. The MCRPE isolates exhibited genotypic and phenotypic multidrug resistance (MDR) traits as well as heavy metal and antiseptic resistance genes. The mcr-1.1-IncI2 and IncX4 plasmids carried only colistin resistance genes. Whereas, the mcr-3.5-IncHI2 plasmid presented MDR region, with several mobile genetic elements. Despite the MCRPE strains belonged to different E. coli lineages, mcr-carrying plasmids with high similarities were found in isolates from pigs and wastewater recovered in different years. This study highlighted that several factors, including the resistomic profile of the host bacteria, co-selection via adjunct antibiotic resistance genes, antiseptics, and/or disinfectants, and plasmid-host fitness adaptation may encourage the maintenance of plasmids carrying mcr genes in E. coli.
Similar content being viewed by others
Introduction
Colistin (polymyxin E) is a high priority antimicrobial that is a treatment of choice for infections with multidrug resistant Enterobacteriaceae. The extensive use of colistin in livestock has encouraged the rapid spread of plasmid mediated mcr genes encoding colistin resistance. Several reports have suggested that farm animals can be a source of mcr-1 that spreads to humans1,2. The mcr-1 gene is the most common member of the mcr gene family and has been found in bacteria from many ecological niches3. Multiple plasmid types, particularly the IncX4, IncI2, and IncHI2 plasmids, have been found to contain mcr-1 and mcr-3 genes4,5. Additionally, the use of biocides or other antibiotics may result in co-selection of mcr genes and/or cross-resistance to colistin6,7. The frequent recovery of mcr-positive bacteria, particularly E. coli, from humans, animals, and the environment is very problematic. Despite numerous cases of colistin resistant Enterobacteriaceae being reported from livestock and humans in Thailand, detailed genomic characterization of mcr-positive E. coli, particularly amongst livestock isolates, is still limited. In this study, six mcr-positive E. coli strains with multi-drug resistance traits8 were subjected to whole genome sequencing (WGS) and characterization of the plasmids for better understanding of mcr genes dissemination between pigs and the farm environment.
Results
Genomic characterization of mcr positive E. coli
The six MCRPE strains submitted for whole genome sequencing had colistin MIC values of 4–8 mg/L and had different PFGE profiles and ST types8. These MCRPE strains transferred mcr-1 and mcr-3 genes to recipient E. coli J53 at conjugative transfer frequency rate of 1.7–2 × 10−4. Their genome sizes ranged from 4 to 4.8 Mb. Detailed information about the colistin resistant E. coli, including their serotypes detected by Serotype finder 2.0 is presented in Table 1. The mcr-1.1 gene was detected in three of the six strains, one each from a human (CP52E, 2017), a wastewater sample (CPWW7, 2017) and a pig (CPA1200, 2019), and these showed 100% identity to mcr-1 in KP347127, the first mcr-1 gene that was identified in China2. Unfortunately, the mcr-1 gene and other mcr genes were not found after in silico analysis in the E. coli of wastewater origin from 2018 (CPWWCT), even though it had tested positive previously by PCR and was phenotypically resistant during screening. Seemingly, this strain had lost the mcr-1 plasmid during sub-culturing. Analysis of the plasmids in this E. coli strain showed that the IncX1 plasmid harboured various mobile genetic elements together with various AMR genes (supplementary Fig. 1).
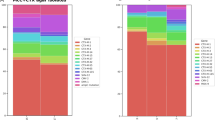
Two of the porcine strains were mcr-3 positive: CPE35 (Pig, 2017) contained mcr-3.2 on the IncFII plasmid while CPF6 (Pig, 2018) contained mcr-3 variants mcr-3.2 and mcr-3.5 on IncHI2 and IncFII, respectively. None of the MCRPE strains carried mcr genes on their chromosomes. All the mcr-1.1 genes found in this study were located either on IncI2 or IncX4 plasmids. Several plasmids, including both phenotypically known and unnamed plasmids were detected in all MCRPE strains. Moreover, all the plasmid replicon types detected in the MCRPE strain of human origin were also found in strains from pigs and wastewater (Fig. 1).
Antibiotic resistance genes and virulence genes
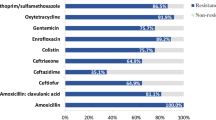
A total of 31 ARGs were identified amongst the six strains, with those encoding resistance to aminoglycosides, beta-lactams, cephalosporins, fluoroquinolones, trimethoprim, macrolides, chloramphenicol, sulfonamides and tetracyclines (Table 1). Genes encoding resistance to the disinfectant; hydrogen peroxide and quaternary ammonium compounds were detected in the two porcine E. coli strains collected from 2018 and 2019 and in both strains of wastewater origin. Apart from the plasmids pCPE35-IncFII (pig, 2017) and pCPF6-IncHI2 (pig, 2018), the other plasmids harbouring mcr in this study contained only colistin resistance genes. Moreover, the genomes of four MCRPE strains (except for CPE35 and CPWW7) contained genes conferring resistance to the heavy metals copper, silver and zinc (supplementary Table 1). On the other hand, CPWW7 carried mercury resistance genes on the plasmid as well as various aminoglycosides resistance genes. Genes encoding a multidrug resistance efflux pump including emrD, mdtM and mdfA were also detected on the chromosome of MCRPE strains.
All six MCRPE carried genes encoding various virulence factors (Table 1), with the majority of these being present on the chromosomes. Virulence genes of different pathotypes encoding virulence factors such as adherence factors, flagellar associated proteins, fimbrial adhesin proteins, hlyE, hlyF (haemolysin), type III secretion system related factors, toxins (astA, enteroaggregative heat-stable toxin, EAST-1), and siderophore receptors (fyuA) were detected. The most common virulence genes detected were associated with type III secretion systems, adhesion, and haemolysis (hlyE).
Genomic insights into the mcr-1 and mcr-3 positive E. coli strains
The mcr-genes were detected in IncX4 (n = 1), IncHI2 (n = 1), IncI2 (n = 2) and IncFII (n = 2). The ~ 33 kb pCP52E-IncX4 plasmid harboured by E. coli of human origin did not contain resistance genes other than mcr-1.1 on the same plasmid, and no ISApl1 elements were found flanking the mcr-1.1 gene. Typical plasmid backbone features such as genes encoding type IV secretion system (T4SS) proteins and toxin-antitoxin system components (HicA-HicB) were present. A structural comparison of pCP52E-IncX4 against other reference plasmids that contained mcr-1 is shown in Fig. 2a. The IncX4-mcr-1 plasmid in this study shared 95% coverage and 99.95% identity with pCSZ4 (GenBank no. KX711706) from an E. coli isolate of porcine origin from China.
Sequence alignment and circular comparison of mcr-1 plasmids. Panel (a): Sequence alignment of pCP52E-IncX4 (human, 2017) plasmid with pCSZ4 (GenBank no. KX711706) and pHNSHP45 (GenBank no. KP347127). The outer circle with red arrows denotes annotation of the plasmid pCP52E-IncX4. Panel (b): Sequence alignment of pCPA1200-IncI2 (pig, 2019) plasmid with pCPWW7-IncI2 (wastewater, 2017) and pHNSHP45 (GenBank no. KP347127) and pmcr1_IncI2 (GenBank no. KU761326). The outer circle with red arrows denotes annotation of the plasmid pCPA1200-IncI2.
The mcr-1.1-IncI2 plasmids of pCPA1200-IncI2 (pig, 2019) and pCPWW7-IncI2 (wastewater, 2017) shared high similarity, including 99% coverage and 100% identity. A structural comparison of pCPA1200-IncI2 with pCPWW7-IncI2 and pHNSHP45 (IncI2 plasmid reported from China) is shown in Fig. 2b. Moreover, these IncI2 plasmids contained numerous conjugation related genes such as T4SS, and pilus modification and conjugative transfer system protein genes. The ~ 60 kb IncI2-mcr-1.1 plasmids showed the same genetic structure as ISApl1-mcr-1-pap2, with loss of downstream ISApl1. A comparison of the genetic environment of the mcr-1.1 cassette from IncI2 and IncX4 plasmids from this study and the references plasmids is presented in Fig. 3.
Comparison of the genetic environment of the mcr-1.1 gene from MCRPE in this study with references plasmids. The grey area indicates the blast identities, and the percentage of identity is indicated in the legend. Open arrows represent coding sequences (green for mcr-1.1, blue for PAP2, purple for ISApl1 and yellow for other genes). The arrow size is proportional to the gene length. The image was generated using EasyFig with default parameters.
The phylogenetic tree constructed for the mcr-1.1-harbouring IncI2 plasmids of different origins identified six distinct clades (Fig. 4). The IncI2 plasmids from this study, pCPWW7-IncI2 and pCPA1200-IncI2, were in the same clonal lineage and related to the plasmid pSCZE4 from a pig (China), p25 from a dog (Eucador) and pGD16-131 from a chicken (China). Moreover, IncI2 plasmids in this study also were found to be clonally related to pHNSHP45 (pig, China), pMRY15-1312 (cow, Japan) and pJS021 (pig, Thailand), showing that highly related mcr-1-IncI2 plasmids from different sources are distributed globally.
Phylogenetic analysis of 22 mcr-1-harbouring IncI2 plasmids. The mcr-1-carrying IncI2 plasmids from this study (red colour) pCPWW7-IncI2 from wastewater and pCPA1200-IncI2 from a pig and other mcr-1-carrying IncI2 plasmids of E. coli deposited in the GenBank database. Sequences were aligned using MAFFT with default values and the phylogenetic tree was constructed by using the neighbour-joining method with the MEGA 10 software.
The ~ 83 kb IncFII plasmids which carried mcr-3.2 in pCPE35-IncFII (pig, 2017) and mcr-3.5 in pCPF6-IncFII (pig, 2018) had 92% coverage with 99% identity. These IncFII plasmids harboured conjugation related transfer protein genes (tra). Moreover, the pCPE35-IncFII plasmid contained genes encoding microcin producing protein (McmM) while pCPF6-IncFII contained genes for a MDR efflux pump protein (Tap) (Fig. 5a). The pCPF6-IncHI2 (pig, 2018) plasmid showed high identity with the IncHI2 plasmid pWJ1 (GenBank no. KY924928) from a Chinese porcine E. coli isolate (Fig. 5b). Genetic arrangements in the vicinity of mcr-3.2 comprised TnAs2–mcr-3.2–dgkA–ISKpn40. In contrast, mcr-3.5 on the pCPF6-IncFII plasmid was flanked by TnAs2-mcr-3.5-dgkA-IS26 (Fig. 6). The plasmid pCPF6-IncHI2 contained multiple resistance genes against aminoglycosides, tetracycline and extended spectrum beta lactamases (ESBL). Moreover, a disinfectant resistance gene (qacC) and an integron (Intl1) were also detected downstream of the mcr-3.2 gene on the same plasmid. A comparison of the genetic environment surrounding the mcr-3 cassette in the IncFII and IncHI2 plasmids from this study and the reference plasmids are shown in Fig. 6. A phylogenetic tree constructed based on core genome sequences of mcr-3 carrying IncFII plasmids from this study and from 15 IncFII plasmids deposited in the GenBank database identified two distinct subclades, as shown in Fig. 7. Interestingly, the IncFII plasmids from this study together with IncFII plasmids from Asian countries (China, Hong Kong, Vietnam, Thailand) were found to be closely related and branched as a group in the phylogenetic tree. On the other hand, IncFII plasmids from Europe and the USA were different and were found in distinct subclades from the pCPF6-IncFII and pCPE35-IncFII plasmids of this study. The accession numbers and detailed information for the references plasmids is provided in (supplementary Table 2).
Sequence alignment and circular comparison of mcr-3 plasmids. Panel (a): Sequence alignment of pCPE35-IncFII (pig, 2017) mcr-3.2 plasmid with pCPF6-IncFII (pig, 2018) mcr-3.5 plasmid (this study) and the reference plasmids pECQ4552 (GenBank no. CP077064.1) and pBJ114-141 (GenBank no. MF679146). The outer circle with red arrows denotes annotation of the plasmid pCPE35-IncFII. The black bar represents the multidrug efflux pump (Tap) from pCPF6. Panel (b): Sequence alignment of pCPF6-IncHI2 (pig, 2018) mcr-3.2 plasmid with pWJ1 (GenBank no. KY924928). The outer circle with red arrows denotes annotation of the plasmid pCPF6-IncHI2.
Comparison of the genetic environment of mcr-3 genes from MCRPE isolates from this study with references plasmids. The grey area indicates the blast identities, and the percentage of identity is indicated in the legend. Open arrows represent coding sequences (green for antimicrobial resistance genes, blue for dgkA (orf), purple for mobile genetic elements and yellow for other genes). The arrow size is proportional to the gene length. The image was generated using EasyFig with default parameters.
Phylogenetic analysis of mcr-3 IncFII plasmids from this study with 15 IncFII plasmids of E. coli deposited in the GenBank database. The mcr-3-carrying IncFII plasmids from this study pCPE35-IncFII and pCPF6-IncFII from pigs are shown in red. Sequences were aligned using MAFFT with default values and the phylogenetic tree was constructed by using the neighbour-joining method with the MEGA 10 software.
Discussion
The six E. coli strains selected for WGS in this study were found to have resistance genes against various antibiotic classes in both their chromosomes and plasmids. Genes conferring resistance to disinfectants and biocides were also detected in the MCRPE strains. Biocides are often present in agricultural products and feed additives, and their stability in the environment acts to prolong exposure and selective pressure on bacteria9. Notably, bacterial resistance to the above compounds could favour co-selection and co-expression of various antibiotic resistance genes that also may be present10.
The MCRPE in this study also possessed various virulence genes. Notably, strain CPE35 from a pig belonged to ST10 with serotype O101: H9, which has been reported to be associated with animal and human disease11. This serotype has been reported in Shiga toxin-producing E. coli (STEC) from humans and in enterotoxigenic E. coli (ETEC) from calves with diarrhoea in Europe12,13. Serotype O128: H12, which was identified in the MCRPE from a human (CP52E) in this study, has been associated with ETEC as well as in enteropathogenic E. coli (EPEC)12. These findings support the likelihood that healthy pigs may be carriers for MDR bacteria which also may harbour various virulence genes, and which may be disseminated to humans.
The epidemic plasmids IncI2 and IncX4 were the source of the mcr-1.1 genes identified in this study. IncI2 plasmids largely have been detected in various mcr-1 cases from different hosts around the world14. On the other hand, the IncX4 plasmid type has been reported as the dominant mcr-1-carrier in isolates from healthy humans in China3 and in carbapenem and mcr-1 co-carrying Enterobacteriaceae from clinical patients across Thailand15. The IncX4 plasmids have been reported to be genetically less variable and relatively smaller than IncI2 plasmids16,17. IncI2 replicon type plasmids are known to have the strongest competitive and fitness advantage in the host bacterium when compared to other plasmid types such as IncHI2 or IncX4 plasmids14,18. These considerations also applied in the current study where the mcr-1-IncI2 plasmid was still found in E. coli isolates recovered long after the colistin ban was implemented at the start of 2017. A previous study also found that mcr-1 bearing IncI2 plasmids were present in E. coli from fattening pigs that had no exposure to colistin14. It was noteworthy that the IncI2 plasmids contained more conjugation transfer system genes than genes associated with replication, which suggests that they are more adapted to spread from host to host than to undergo replication19. Therefore, the absence of antibiotic selective pressure might have a neglectable effect on the persistence or conjugative rate of such plasmids that have low fitness cost.
In comparison, mcr-3 genes were detected on IncFII and IncHI2 plasmids. The mcr-3 gene was first identified in a IncHI2 plasmid in China5, and mcr-3 mediated IncP and IncFII plasmids previously have been reported in E. coli in Thailand20. The MDR plasmid pCPF6-IncHI2-mcr-3.2 (pig, 2018) from this study contained resistance genes against tetracycline, aminoglycosides, chloramphenicol, cephalosporin, disinfectant, as well as colistin. Various antimicrobial resistance genes located on the same plasmid could enhance the persistence and co-selection of mcr genes21. Therefore, aside from colistin withdraw, continual monitoring of other antimicrobial use during the pig production cycle is needed to improve control of colistin resistance in pig farms.
The occurrence of highly similar plasmids in different lineages of MCRPE isolates collected from different sources and at different times implies that the associated plasmids have been widely disseminated through the farm. It is concerning that colistin resistance plasmids could persist with relative ease among genotypically distinct E. coli strains from different niches, and it implies that the transmission of these plasmids might be extremely hard to control. Moreover, mcr-1 is mobilized by a composite transposon called Tn6330, where the mcr-1 with a putative open reading frame (PAP2 like protein) is flanked by two ISApl1 insertion sequences22. In the IS30 family, ISApl1 performs a ‘copy-out, paste-in’ mechanism and is highly active23. Therefore, the mcr-1 genes from E. coli recovered from pigs and wastewater in this study were mobilizable and able to be persisted even after 2 years without colistin exposure. In agreement with our results, significantly more mcr-1 cases with attached insertion sequence ISApl1 have been found amongst animal isolates than in human isolates24. These findings are consistent with animals being a primary source for mcr-1 bearing bacteria that are transmitted to humans.
In this study, there were too few isolates examined to determine which of the mcr genes were most likely to persist after colistin withdrawal. A previous in vitro experiment showed that plasmids carrying mcr-3 have greater stability than mcr-1 plasmids in the absence of colistin, with mcr-3 having a lower fitness cost25. On the other hand, another study found that certain E. coli strains were more likely to eliminate mcr-3 genes than mcr-1 genes in vitro, with or without exposure to colistin16. Therefore, the persistence and fitness cost of the mcr-1 and mcr-3 genes in bacteria might differ depending upon the plasmid as well as on the host genetic background. In previous studies, mcr-3.2 positive E. coli of bovine origin26 and mcr-3.1 bearing isolates of porcine origin5 were found to have genetic environment TnAs2-mcr-3.2-dgkA-ISKpn40, which occurred on both IncFII and IncHI2 plasmids in this study. ISKpn40 belongs to the IS3 family and was first identified in an E. coli strain from a pig, whereas the IS6 family of IS26 which was detected on the mcr-3.5-pCPF6-IncFII plasmid facilitates mobilization of resistance genes in Gram-negative bacteria27. Although the genetic environments of mcr-3 determinants are variable, the core structure of TnAs2-mcr-3-dgkA, accompanied with other mobile elements or resistance genes, is highly conserved16.
A previous in vitro study found that in the absence of antibiotic selective pressure, IncFII plasmids were unstable and outcompeted by plasmid-free cells14. Notably, the cost of the plasmid is increased according to its metabolic load, such as expression of biomolecules or energy-rich compounds, as well as introduction of an efflux pump28. This is consistent with our results where IncFII plasmids encoded either bacteriocin producing proteins or efflux pump proteins. On the other hand, the IncHI2 plasmid found in this study was a MDR plasmid accompanied by several mobile genetic elements. Such kinds of MDR plasmid may present a fitness burden and may be prone to deletion of the mcr-3 gene or the whole plasmid from the bacteria.
The metabolic cost of a plasmid increases relatively with increasing levels of resistance genes on the plasmid and the level of their phenotypic expression rather than on the size of the plasmid itself29,30,31. This phenomenon could be related to our finding that the mcr-1 bearing plasmids were colistin mono-resistant plasmids with high stability traits. Moreover, the moderate MICs (4–8 mg/L) for colistin, and the fitness benefit expressed by mcr-1-bearing IncI2 and IncX4 plasmids further helps to explain their long-term persistence in the farm after colistin withdrawal. Hence, not only cessation of colistin selective pressure, but also the characteristics of mcr bearing plasmids and co-selection by other antibiotic usage and farm management need to be considered when attempting to control colistin resistant bacteria in the population.
In conclusion, the mcr bearing plasmids could still be transmitted between hosts on the farm for some time after colistin selective pressure was removed. According to our findings, even without antibiotic selective pressure, resistance plasmids with little or no fitness burden on the bacterial hosts could persist in the population. Moreover, the presence of resistance genes against various antimicrobials and disinfectants could help to co-select for colistin resistant bacteria. Undoubtedly, the need to minimize the use of antibiotics as well as continuous monitoring of the antimicrobial resistance profiles of bacteria in livestock farms are essential for AMR control.
Materials and methods
Sampling and strain selection
The strains studied were obtained in a previously reported investigation conducted in the central region of Thailand8. In that study MCRPE isolates (n = 170) from pigs, wastewater, and workers on a pig farm where routine prophylactic colistin use had been withdrawn at the start of 2017 were obtained over a three-year period and longitudinally monitored. Six of these multi-drug resistant MCRPE strains with different clonal types based on pulsed field gel electrophoresis and similar plasmid replicon types found by PCR were selected for the current molecular study. The strains comprised three from pigs, two from wastewater and one from a farm worker. The strains were isolated in mid-2017 (n = 3), 2018 (n = 2) and 2019 (n = 1). A more detailed description of the origin of the six strains is presented in Table 1.
Bacterial identification and antimicrobial susceptibility testing
The MCRPE strains were identified as E. coli using IMViC biochemical tests and MALDI-TOF MS32. The mcr-1-5 genes were detected by multiplex PCR, as previously reported33. The minimum inhibitory concentration (MIC) for colistin was determined by using the broth microdilution technique, and an MIC value of ≥ 4 mg/L was considered to indicate colistin resistance (CLSI, 2021). Antimicrobial susceptibility testing for mcr positive E. coli strains was performed by using the AST-GN 38 test kit in a Vitek2 apparatus (BioMérieux, France)34.
Plasmid conjugation
Conjugation was performed by broth mating technique to confirm mcr genes were located on conjugative plasmids, and their transferability rate35. The E. coli J53 strain which is resistant to sodium azide (MIC > 512 mg/L) and susceptible to colistin (MIC < 2 mg/L) were applied as recipient strain. Transconjugants were cultured on LB agar (Oxoid) plates containing colistin (2 mg/L) and sodium azide (100 mg/L). The presence of mcr genes in transconjugants were determined by PCR as described above.
DNA preparation and whole genome sequencing
The genomic DNA of the E. coli strains was extracted by using the ZymoBIOMICS DNA Miniprep Kit (Zymo Research, USA) according to the manufacturer’s instructions. The extracted DNA was subjected to quantity checking using a Qubit Fluorometer. The samples then were submitted for sequencing using the Illumina NovaSeq PE150 platform and MinION (Oxford Nanopore Technologies for long read sequencing).
Sequence analysis
The paired-end reads were quality filtered to remove adapters and low-quality sequences with quality scores < 30 by using Trimmomatic v.0.36.536. The related bioinformatic analyses were performed on the European Galaxy server (https://usegalaxy.eu). Reads assembly were perform by using the Unicycler hybrid assembly (Galaxy Version 0.4.8.0) with default settings37. Sequences were analyzed for species identification (KmerFinder 2.1), Multilocus Sequence Type (MLST 1.6), virulence factors (VirulenceFinder 1.2), antimicrobial resistance (ResFinder 2.1), plasmids (PlasmidFinder 1.2) and mobile elements finders (v1.0.3) using the Center for Genomic Epidemiology (CGE) pipeline26. Acquired antimicrobial resistance genes (ARGs), and E. coli virulence factors were also identified using ABRicate on the Galaxy server (Galaxy Version 1.0.1). The databases used in this platform were CARD Resistance Gene Identifier38 and ARG-ANNOT (Antibiotic Resistance Gene-ANNOTation)39 databases, while for virulence genes the VFDB databases were used40. The genomes of MCRPE isolates were annotated by the NCBI Prokaryotic Genomes Annotation Pipeline (PGAP) and Prokka (Prokaryotic genome annotation) (Galaxy Version 1.14.6)41. Screening for biocide resistance genes was carried out on the BacAnt server42 using the antibacterial biocide and metal resistance gene databases ResDB and BacMet43.
Plasmid sequences were obtained using plasmid finder and annotated by Prokka (Galaxy Version 1.14.6)41. The contigs of mcr variants were compared with reference sequences using BLAST. The annotated plasmids carrying mcr genes were compared with reference plasmids belonging to the same Inc groups using BLAST ring image generator (BRIG)44, and the genetic context of mcr-1 and mcr-3 contigs were visualized by using Easyfig (http://mjsull.github.io/Easyfig/)45. All the reference plasmids and sequences used in the study were recovered from the NCBI database.
Since highly similar mcr bearing plasmids of the IncI2 and IncFII plasmids were detected in isolates from pigs and wastewater recovered in different isolation years, a phylogenetic comparison was performed on the IncI2 and IncFII plasmids from this study with reference plasmid sequences from GenBank. A phylogenetic tree of the mcr-1.1-carrying IncI2 plasmids from this study was constructed by comparing with 20 mcr-1-carrying IncI2 plasmids deposited in the GenBank database. The sequences were aligned using MAFFT (Galaxy Version 7.505 + galaxy0)46 and a phylogenetic tree was obtained by using the neighbour-joining method in MEGA 10 software using1000 times bootstrap values. Likewise, phylogenetic trees of the two mcr-3 carrying IncFII plasmids were generated by comparing with 15 E. coli IncFII plasmids deposited in the GenBank database.
Nucleotide sequence accession numbers
The complete nucleotide sequences of the six MCRPE strains CP52E, CPE35, CPWW7, CPF6, CPWWCT and CPA1200 were deposited in GenBank under the accession numbers CP075731, CP075722, CP075716, CP075737, CP076575 and JAHKSR000000000, respectively. The plasmids containing mcr genes were: pCP52E-IncX4 (accession number-NZ_CP075733.1), pCPA1200-IncI2 (accession number- JAHKSR010000004.1), pCPWW7-IncI2 (accession number- NZ_CP075719.1), pCPE35-IncFII (accession number- NZ_CP075741.1), pCPF6-IncFII (accession number- CP075741.1) and pCPF6-IncHI2 (accession number- CP075738.1).
Data availability
The datasets generated and/or analysed during the current study are available in the National Library of Medicine repository BioProject: PRJNA735516 and PRJNA731849.
References
Cheng, P. et al. Prevalence and characteristic of swine-origin mcr-1-positive Escherichia coli in Northeastern China. Front Microbiol 12, 712707. https://doi.org/10.3389/fmicb.2021.712707 (2021).
Liu, Y. Y. et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis 16, 161–168. https://doi.org/10.1016/S1473-3099(15)00424-7 (2016).
Shen, C. et al. Dynamics of mcr-1 prevalence and mcr-1-positive Escherichia coli after the cessation of colistin use as a feed additive for animals in China: A prospective cross-sectional and whole genome sequencing-based molecular epidemiological study. The Lancet Microbe 1, e34–e43. https://doi.org/10.1016/S2666-5247(20)30005-7 (2020).
Wang, Q. et al. Expanding landscapes of the diversified mcr-1-bearing plasmid reservoirs. Microbiome 5, 70. https://doi.org/10.1186/s40168-017-0288-0 (2017).
Yin, W. et al. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. MBio 8, e00543-17. https://doi.org/10.1128/mBio.00543-17 (2017).
Wand, M. E., Bock, L. J., Bonney, L. C. & Sutton, J. M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 61, e01162-16. https://doi.org/10.1128/AAC.01162-16 (2017).
Xu, F., Zeng, X., Hinenoya, A. & Lin, J. mcr-1 confers cross-resistance to bacitracin, a widely used in-feed antibiotic. mSphere 3, e00411-18. https://doi.org/10.1128/mSphere.00411-18 (2018).
Khine, N. O. et al. Longitudinal monitoring reveals persistence of colistin-resistant Escherichia coli on a pig farm following cessation of colistin use. Front. Vet. Sci. 9, 845746. https://doi.org/10.3389/fvets.2022.845746 (2022).
Seiler, C. & Berendonk, T. U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 3, 399. https://doi.org/10.3389/fmicb.2012.00399 (2012).
McNeilly, O., Mann, R., Hamidian, M. & Gunawan, C. Emerging concern for silver nanoparticle resistance in Acinetobacter baumannii and other bacteria. Front. Microbiol. 12, 652863. https://doi.org/10.3389/fmicb.2021.652863 (2021).
He, W. Y. et al. Clonal spread of Escherichia coli O101: H9-ST10 and O101: H9-ST167 strains carrying fosA3 and blaCTX-M-14 among diarrheal calves in a Chinese farm, with Australian Chroicocephalus as the possible origin of E. coli O101: H9-ST10. Zool. Res. 42, 461–468. https://doi.org/10.24272/j.issn.2095-8137.2021.153 (2021).
Contrepois, M., Bertin, Y., Pohl, P., Picard, B. & Girardeau, J.-P. A study of relationships among F17 a producing enterotoxigenic and non-enterotoxigenic Escherichia coli strains isolated from diarrheic calves. Vet. Microbiol. 64, 75–81. https://doi.org/10.1016/S0378-1135(98)00253-3 (1998).
Begaud, E., Mondet, D. & Germani, Y. Molecular characterization of enterotoxigenic Escherichia coli (ETEC) isolated in New Caledonia (value of potential protective antigens in oral vaccine candidates). Res. Microbiol. 144, 721–728. https://doi.org/10.1016/0923-2508(93)90036-2 (1993).
Wu, R. et al. Fitness Advantage of mcr-1-bearing IncI2 and IncX4 plasmids in vitro. Front. Microbiol. 9, 331. https://doi.org/10.3389/fmicb.2018.00331 (2018).
Paveenkittiporn, W., Kamjumphol, W., Ungcharoen, R. & Kerdsin, A. Whole-genome sequencing of clinically isolated carbapenem-resistant Enterobacterales harboring mcr genes in Thailand, 2016–2019. Front Microbiol 11, 586368. https://doi.org/10.3389/fmicb.2020.586368 (2020).
Li, R. et al. Comprehensive genomic investigation of coevolution of mcr genes in Escherichia coli strains via nanopore sequencing. Glob. Chall. 5, 2000014. https://doi.org/10.1002/gch2.202000014 (2021).
Rozwandowicz, M. et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother 73, 1121–1137. https://doi.org/10.1093/jac/dkx488 (2018).
Li, W. et al. mcr expression conferring varied fitness costs on host bacteria and affecting bacteria virulence. Antibiotics (Basel) 10, 872. https://doi.org/10.3390/antibiotics10070872 (2021).
Meinersmann, R. J. The biology of IncI2 plasmids shown by whole-plasmid multi-locus sequence typing. Plasmid 106, 102444. https://doi.org/10.1016/j.plasmid.2019.102444 (2019).
Tansawai, U. et al. Emergence of mcr-3-mediated IncP and IncFII plasmids in Thailand. J. Glob. Antimicrob. Resist. 24, 446–447. https://doi.org/10.1016/j.jgar.2021.02.006 (2021).
Vines, J. et al. Transmission of similar mcr-1 carrying plasmids among different Escherichia coli lineages isolated from livestock and the farmer. Antibiotics (Basel) 10, 313. https://doi.org/10.3390/antibiotics10030313 (2021).
Snesrud, E., McGann, P. & Chandler, M. The Birth and demise of the ISApl1-mcr-1-ISApl1 composite transposon: The vehicle for transferable colistin resistance. MBio 9, e02381-17. https://doi.org/10.1128/mBio.02381-17 (2018).
Snesrud, E. et al. Analysis of serial isolates of mcr-1-positive Escherichia coli reveals a highly active ISApl1 transposon. Antimicrob. Agents Chemother. 61, e00056-17. https://doi.org/10.1128/AAC.00056-17 (2017).
Wang, Y. et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: An epidemiological and clinical study. Lancet Infect. Dis. 17, 390–399. https://doi.org/10.1016/S1473-3099(16)30527-8 (2017).
Yang, Q. E. et al. Compensatory mutations modulate the competitiveness and dynamics of plasmid-mediated colistin resistance in Escherichia coli clones. ISME J. 14, 861–865. https://doi.org/10.1038/s41396-019-0578-6 (2020).
Alba, P. et al. Molecular epidemiology of mcr-encoded colistin resistance in Enterobacteriaceae from food-producing animals in Italy revealed through the EU harmonized antimicrobial resistance monitoring. Front. Microbiol. 9, 1217. https://doi.org/10.3389/fmicb.2018.01217 (2018).
Partridge, S. R., Kwong, S. M., Firth, N. & Jensen, S. O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31, e00088-27. https://doi.org/10.1128/CMR.00088-17 (2018).
Carroll, A. C. & Wong, A. Plasmid persistence: Costs, benefits, and the plasmid paradox. Can. J. Microbiol. 64, 293–304. https://doi.org/10.1139/cjm-2017-0609 (2018).
Sandegren, L., Lindqvist, A., Kahlmeter, G. & Andersson, D. I. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J. Antimicrob. Chemother. 62, 495–503. https://doi.org/10.1093/jac/dkn222 (2008).
San Millan, A. et al. Multiresistance in Pasteurella multocida is mediated by coexistence of small plasmids. Antimicrob. Agents Chemother. 53, 3399–3404 (2009).
Vogwill, T. & MacLean, R. C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach. Evol. Appl. 8, 284–295. https://doi.org/10.1111/eva.12202 (2015).
Singhal, N., Kumar, M., Kanaujia, P. K. & Virdi, J. S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 6, 791. https://doi.org/10.3389/fmicb.2015.00791 (2015).
Rebelo, A. R. et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill 23, 17–00672. https://doi.org/10.2807/1560-7917.ES.2018.23.6.17-00672 (2018).
Khine, N. O. et al. Multidrug resistance and virulence factors of Escherichia coli harboring plasmid-mediated colistin resistance: mcr-1 and mcr-3 genes in contracted pig farms in Thailand. Front. Vet. Sci. 7, 582899. https://doi.org/10.3389/fvets.2020.582899 (2020).
Wang, M. et al. Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob. Agents chemother. 47, 2242–2248 (2003).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. https://doi.org/10.1093/bioinformatics/btu170 (2014).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595. https://doi.org/10.1371/journal.pcbi.1005595 (2017).
McArthur, A. G. et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 57, 3348–3357. https://doi.org/10.1128/AAC.00419-13 (2013).
Gupta, S. K. et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 58, 212–220. https://doi.org/10.1128/AAC.01310-13 (2014).
Chen, L., Zheng, D., Liu, B., Yang, J. & Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis–10 years on. Nucleic Acids Res. 44, D694-697. https://doi.org/10.1093/nar/gkv1239 (2016).
Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069. https://doi.org/10.1093/bioinformatics/btu153 (2014).
Hua, X. et al. BacAnt: A Combination annotation server for bacterial DNA sequences to identify antibiotic resistance genes, integrons, and transposable elements. Front. Microbiol. 12, 649969. https://doi.org/10.3389/fmicb.2021.649969 (2021).
Pal, C., Bengtsson-Palme, J., Rensing, C., Kristiansson, E. & Larsson, D. G. BacMet: Antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 42, D737-743. https://doi.org/10.1093/nar/gkt1252 (2014).
Alikhan, N. F., Petty, N. K., Ben Zakour, N. L. & Beatson, S. A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genomics 12, 402. https://doi.org/10.1186/1471-2164-12-402 (2011).
Sullivan, M. J., Petty, N. K. & Beatson, S. A. Easyfig: A genome comparison visualizer. Bioinformatics 27, 1009–1010. https://doi.org/10.1093/bioinformatics/btr039 (2011).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. https://doi.org/10.1093/molbev/mst010 (2013).
Acknowledgements
We wish to acknowledge the farm veterinarian Dr. Tawat Pongpan for participating in sample collections.
Funding
Funding for the study was received from the Second Century Fund (C2F batch 5/2022), Chulalongkorn University, Thailand, Royal Golden Jubilee Ph.D. (RGJPHD) program, The Agricultural Research Development Agency (ARDA) [CRP6205031110], the CHE-TRF Senior Research Fund (RTA6280013), Thailand Science Research and Innovation, and Pathogen Bank, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand and the 90th Anniversary of Chulalongkorn University Scholarship.
Author information
Authors and Affiliations
Contributions
N.K. and N.P.* performed the methods, data collection, analysis and prepared the manuscript.. Whole genome sequencing was performed by T.W. and P.J. Sequence analysis and bioinformatic analysis was performed by N.K. D.H. and N.P.* critically revised the drafted manuscript. All authors reviewed the manuscript and read and approved the final manuscript. *Indicated the Corresponding author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khine, N.O., Wongsurawat, T., Jenjaroenpun, P. et al. Comparative genomic analysis of Colistin resistant Escherichia coli isolated from pigs, a human and wastewater on colistin withdrawn pig farm. Sci Rep 13, 5124 (2023). https://doi.org/10.1038/s41598-023-32406-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32406-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.