Abstract
Coffee, next to water the most widespread beverage, is attributed both harmful and protective characteristics concerning cardiovascular health. This study aimed to evaluate associations of coffee consumption with cardiac biomarkers, echocardiographic, electrocardiographic parameters and major cardiovascular diseases. We performed a cross-sectional analysis of 9009 participants of the population-based Hamburg City Health Study (HCHS), enrolled between 2016 and 2018 median age 63 [IQR: 55; 69] years. Coffee consumption was classified into three groups: < 3 cups/day (low), 3–4 cups/day (moderate), > 4 cups/day (high). In linear regression analyses adjusted for age, sex, body mass index, diabetes, hypertension, smoking, and additives, high coffee consumption correlated with higher LDL-cholesterol (β = 5.92; 95% CI 2.95, 8.89; p < 0.001). Moderate and high coffee consumption correlated with lower systolic (β = − 1.91; 95% CI − 3.04, − 0.78; p = 0.001; high: β = − 3.06; 95% CI − 4.69, − 1.44; p < 0.001) and diastolic blood pressure (β = − 1.05; 95% CI − 1.67, − 0.43; p = 0.001; high: β = − 1.85; 95% CI − 2.74, − 0.96; p < 0.001). Different levels of coffee consumption did neither correlate with any investigated electrocardiographic or echocardiographic parameter nor with prevalent major cardiovascular diseases, including prior myocardial infarction and heart failure. In this cross-sectional analysis, high coffee consumption correlated with raised LDL-cholesterol levels and lower systolic and diastolic blood pressure. However, major cardiovascular diseases including heart failure and its diagnostic precursors were not associated with coffee consumption, connoting a neutral role of coffee in the context of cardiovascular health.
Similar content being viewed by others
Introduction
Coffee is one of the most widely consumed beverages around the world. Ever since consumption of coffee became vastly popular, the interest of its implications on health, and specifically the cardiovascular system, grew. First studies on coffee consumption and the risk of coronary artery disease (CAD) were already conducted in the 1960s leading to conflicting results1. Many studies have been published, attributing both protective and harmful characteristics to coffee in the context of the cardiovascular system2,3,4,5. Coffee is a complex liquid consisting of more than 1000 bioactive substances6. Most commonly, caffeine is regarded as the main driving component of mediating cardiovascular effects. Nevertheless, narrowing it down to a certain substance oversimplifies the versatile composition of coffee. Looking at coffee as a whole, several studies postulated a dose-dependent relationship of coffee consumption and cardiovascular diseases, e.g. low to moderate coffee consumption was shown to be associated with a reduced risk of heart failure whereas high coffee consumption reversed this trend7,8,9. However, an in-depth analysis of coffee consumption and its associations with cardiovascular diseases, especially heart failure and its possible precursors is lacking. Only few studies have evaluated the associations of coffee with cardiac functional parameters measured by echocardiography or electrocardiography10,11.
Trying to fill this gap, in the present study we analyze the association of coffee consumption and the cardiovascular system as a whole, integrating lifestyle-related behaviour, comorbidities, biomarkers, electrocardiographic and echocardiographic data, and finally major cardiovascular diseases in a large sample of the general population.
Methods
Study setting
Data from the first 10,000 participants from the Hamburg City Health Study (HCHS, www.hchs.hamburg) served as the base for this analysis. The HCHS (clinicaltrials.gov: NCT03934957), located in Hamburg, Germany, is an ongoing, prospective, single-centre, long-term, and randomly selected population-based cohort study which aims at investigating the interactions of socioeconomic risk factors, modern imaging techniques, physiological measurements, and clinical variables12. Our study population included a subset of the first 10,000 HCHS participants. Subjects with missing data on coffee consumption were excluded. Our final cohort comprised 9009 subjects (Fig. 1).
The research protocol of the study was approved by the HCHS steering board and the local ethics committee (PV5131, State of Hamburg Chamber of Medical Practitioners). All participants gave a written informed consent. The investigation conforms with the principles outlined in the Declaration of Helsinki.
Laboratory, clinical and questionnaire data
All measurements were conducted between 2016 and 2018 at a baseline visit at the HCHS Epidemiological Study Centre Hamburg-Eppendorf, Hamburg, following the published HCHS protocol12. Cholesterol levels were directly measured in blood samples drawn at the day of examination under fasting conditions. N-terminal pro-B-type natriuretic peptide (NT-proBNP) was measured in serum samples drawn at the day of examination and stored at − 80 °C in a dedicated blood biobank (immunoassay by Alere NT-proBNP for ARCHITECT, Abbott Diagnostics, measurement ranges between 8.2 and 35,000 ng/l). A digital 12-lead electrocardiogram (ECG) combined with a 2-min rhythm strip was acquired from each participant. The durations of wave intervals were calculated electronically and double-checked manually. Further ECG analysis, i.e., on conduction disturbances and underlying rhythm, was conducted by a trained physician. Arterial hypertension was defined as a systolic blood pressure > 140 mmHg, a diastolic blood pressure > 90 mmHg, or the use of antihypertensive drugs. For the assessment of medication, subjects were asked to bring their medication or a list of prescribed drugs to the day of their baseline visit. Before, during and after the baseline visit extensive self-completion questionnaires concerning nutrition, lifestyle, medical, and family history as well as health care research patterns, occupational history and environmental data were completed. Information on dietary intake was collected by validated questionnaires developed for the European Prospective Investigation into Cancer and Nutrition (EPIC) study. The participants were asked how many cups of coffee they drink regarding the last 12 months (1 cup equals 150 ml). Coffee consumption was categorized in the following categories: never, 1 per month, 2 per month, 1 per week, 4–6 per week, 1–2 per day, 3–4 per day, 5–6 per day, 7–8 per day, 9–10 per day, 11 or more per day. We then summarized the categories into 3 groups: < 3 cups/day, 3–4 cups/day, > 4 cups/day. Further questions concerning coffee consumption included additives like milk, sugar, and honey.
Medical history, smoking status, tea, and carbonated drinks consumption were detected by standardized, self-reported questionnaires. Atrial fibrillation was considered present if reported by questionnaire or 12-lead electrocardiogram or both. Diabetes mellitus was determined by a fasting glucose level of ≥ 126 mg/dl, or the use of antidiabetic drugs. CAD was defined as suffering from one or more of the following conditions: history of myocardial infarction, percutaneous coronary intervention (PCI) or coronary bypass surgery. The dichotomized variable PAD was derived from structured anamnesis data, self-based questionnaire, and baseline examination. All participants were asked if they had experienced any history of intermittent claudication, ischemic rest pain, or ischemic wound healing disorders. At the study center, the ankle-brachial-index (ABI) was measured in both legs and cut off for diagnosis were values < 0.9.
Transthoracic echocardiography
Transthoracic echocardiogram (TTE) examinations were systematically performed at the baseline visit using state-of-the-art cardiac ultrasound equipment (Siemens Acuson SC2000 Prime, Siemens Healthineers, Erlangen, Germany). Images were acquired and analysed by trained and internally certified medical professionals (cardiologists, sonographers) as previously published by our group. For continuous quality assessment, every 100th TTE exam was analysed twice. Left sided volumes and ejection fraction (LVEF) were calculated from the apical four- and two-chamber view using the method of disks summation. Left-sided diameters were measured in parasternal long-axis view. Mitral inflow pattern was assessed in apical four-chamber view by placing pulsed-wave (PW) Doppler sample volume between mitral leaflet tips. PW tissue Doppler imaging (TDI) e’ velocity was measured in apical four-chamber view by placing the sample volume at the lateral and septal basal regions. Tricuspid annular plane systolic excursion (TAPSE) was obtained by M-mode echocardiography in the apical four-chamber view.
Definition of heart failure
For the classification of subjects Heart Failure (HF) the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF were applied and modified13. HF was classified in two groups: heart failure with mildly-reduced and reduced ejection fraction (HF(m)rEF) as well as heart failure with preserved ejection fraction (HFpEF). Subjects had to show a combination of signs/symptoms, laboratory data, and echocardiographic criteria. Self-reported history of HF and/or the following medication were seen as equivalent if no symptoms or signs were detectable: betablockers, ACE-inhibitors (ACEi), angiotensin receptor blockers (ARB), angiotensin receptor neprilysin inhibitors (ARNI) mineralocorticoid receptor antagonists (MRA), Sodium-glucose Cotransporter-2 (SGLT2) inhibitors, and loop diuretics for HF(m)rEF and only loop diuretics for HFpEF. Oedema were evaluated by physical examination by medical professionals. Dyspnoea, history of HF, and medication were assessed by standardized self-reported questionnaires. All subjects with LVEF < 50% and symptoms or signs of HF were classified as HF with reduced and mildly reduced ejection fraction (HF(m)rEF), instead of differing between heart failure with mildly reduced ejection fraction (HFmrEF, LVEF 41–49%) and heart failure with reduced ejection fraction (HFrEF, LVEF < 40%). Subjects were classified in the HFpEF group if they showed LVEF ≥ 50%, symptoms or signs of HF, and either at least two or more echocardiographic signs of cardiac structural of functional abnormalities or the combination of NT-proBNP levels exceeding 125 ng/l (sinus rhythm) or 365 ng/l (atrial fibrillation) and at least one or more echocardiographic signs of cardiac structural of functional abnormalities. Echocardiographic signs of cardiac structural or functional abnormalities were defined as: left ventricular hypertrophy: LV mass indexed to BSA ≥ 95 g/m2 for women, ≥ 115 g/m2 for men, left atrial enlargement: left atrial volume index (LAVI) > 34 ml/m2 (sinus rhythm) and > 40 ml/m2 (atrial fibrillation), E/e’ ratio > 9, and tricuspid regurgitation velocity (Vmax) > 2.8 m/s. HF in general describes all subjects with either HF(m)rEF or HFpEF.
Statistical analyses
Continuous variables are presented as median and interquartile range, and categorical variables are presented as absolute numbers and percentages. Comparisons between the different coffee groups were performed using Kruskal–Wallis test or chi-squared test. For the analysis of the association between coffee consumption and continuous laboratory, echocardiographic, electrocardiographic outcome parameters as well as blood pressure we used multivariable linear regression models. Adjustment was performed for age, sex, BMI, diabetes, arterial hypertension, and smoking. For systolic and diastolic blood pressure, in line with Tobin et al.14, no adjustment for arterial hypertension was performed, instead values for treated individuals were imputed by adding 15 mmHg and 10 mmHg respectively to the measured blood pressure. Furthermore, logistic models were used for binary echocardiographic and electrocardiographic parameters as well as cardiovascular diseases. No correction for multiple testing was applied15. A p-value of < 0.05 was considered as statistically significant. All tests were two tailed. Data analysis was performed using R version 3.5.1.
Results
Study population
The included 9009 subjects (Fig. 1) of the first 10,000 HCHS participants showed the expected characteristics of a middle-aged European population. 4610 (51.2%) were women with a median age of 63 [IQR: 55; 69] years, and a median BMI of 26.01 [IQR: 23.5; 29.1] kg/m2.
Arterial hypertension was present in 5637 (62.6%) subjects, diabetes in 694 (7.7%) subjects. 1731 (19.3%) subjects were smokers. The median LVEF was 58.5% [IQR: 55.5, 61.8]. 8552 (94.9%) subjects consumed coffee. Of those, 5699 (63.3%) subjects consumed less than three cups of coffee per day (low), 2333 (25.9%) 3–4 cups per day (moderate) and 977 (10.8%) more than four cups per day (high). With a rising amount of coffee consumption, subjects were more likely to be men, younger, smokers, and showed higher LDL-levels and BMIs compared to those with lower coffee consumption. Moderate coffee consumers demonstrated the lowest prevalence of diabetes while no relevant interclass differences were observed for prior myocardial infarction, prevalent coronary artery disease (CAD), and peripheral artery disease (PAD) (Table 1).
Coffee consumption and biomarkers and common cardiovascular risk factors
In multivariable linear regression analysis adjusted for age, sex, BMI, diabetes, arterial hypertension, smoking, additives, and lipid-lowering drugs, high coffee consumption was associated with raised LDL-cholesterol levels indicated by a beta of 5.92 (95% CI 2.95, 8.89, p < 0.001) (Table 2, Supplementary Table 2).
Additionally, high coffee consumption demonstrated associations with total cholesterol with a beta of 4.78 (95% CI 1.56, 8.0; p = 0.004) and obesity (BMI ≥ 30 kg/m2) with an odds ratio (OR) of 1.32 (95% CI 1.08, 1.62; p = 0.008) (Tables 2 and 3, Supplementary Table 1).
Coffee consumption and ECG/TTE variables
No relevant associations of coffee consumption with ECG parameters were detected in regression analysis. In line, neither morphological nor functional echocardiographic parameters correlated with coffee consumption (Table 2).
Coffee consumption and blood pressure and cardiovascular diseases
In linear regression analysis, adjusted for age, sex, BMI, diabetes, smoking, and additives, moderate and high coffee consumption correlated with lower systolic (moderate: beta = − 1.91; 95% CI − 3.04, − 0.78; p = 0.001; high: beta = − 3.06; 95% CI − 4.69, − 1.44; p < 0.001) and diastolic blood pressure (moderate: beta = − 1.05; 95% CI − 1.67, − 0.43; p = 0.001; high: beta − 1.85; 95% CI − 2.74, − 0.96; p < 0.001) (Table 2, Supplementary Tables 5 and 6).
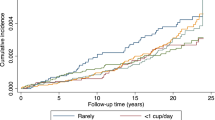
In contrast, coffee consumption showed no associations with CAD, and PAD. In our population, a total of 605 subjects were identified with the diagnosis of heart failure (Table 1). Nevertheless, neither heart failure in general nor differentiating into HFpEF and HF(m)rEF demonstrated significant associations with coffee consumption. In contrast, NT-proBNP was inversely associated with moderate (beta = − 0.06; 95% CI − 0.11, − 0.02; p = 0.005) and high (beta = − 0.09; 95% CI − 0.15, − 0.02; p = 0.013) coffee consumption (Table 2, Supplementary Table 4).
Simultaneous consumption of caffeine-containing drinks, dietary patterns, and sex-specific differences
In order to address potential confounding by black and green tea as well as caffeinated soft-drinks we performed sensitivity analyses, excluding all subjects with simultaneous consumption of coffee and green and black tea. Since the coincidence of coffee and tea consumption is extremely high, this led to significant reduction of sample size and statistical power (n = 1480). High coffee consumption still demonstrated a trend towards associations with LDL and an inverse trend towards associations with systolic and diastolic bp lacking statistical significance. (Supplementary Tables 132–156). To exclude potential bias caused be the consumption of certain food components, additional adjustment for specific diets (vegetarian diet, vegan diet) was performed revealing no changes in the detected associations of coffee consumption (Supplementary Tables 157–180).
Sex-specific stratification of all our multivariable regression analyses as well as sensitivity analyses separated by sex showed no differences regarding our key findings (Supplementary Tables 80–131).
Decaffeinated coffee consumption
From the overall cohort 807 subjects consumed decaffeinated coffee. Of those, 481 subjects consumed less than 3 cups/day, 241 subjects 3–4 cups/day, and 85 more than 4 cups/day. In linear regression analysis, adjusted for age, sex, BMI, diabetes, smoking, and additives, moderate and high decaffeinated coffee consumption correlated with lower diastolic (moderate: beta = − 2.05; 95% CI − 4.05, − 0.05; p = 0.045; high: beta − 3.79; 95% CI − 6.87, − 0.71; p < 0.001) and moderate decaffeinated coffee consumption with lower systolic blood pressure (moderate: beta = − 4.17; 95% CI − 7.88, − 0.45; p = 0.028; high: beta = − 5.01; 95% CI − 10.72, 0.69; p = 0.085) (Supplementary Tables 57–79). No further associations between decaffeinated coffee consumption and the assessed biomarkers, cardiovascular diseases, and ECG/TTE variables were detected.
Discussion
In this study we demonstrate that coffee consumption was not associated with altered cardiac function and morphology, heart failure, and most of its risk factors. However, we observed an association with higher LDL-cholesterol levels and an inverse association with systolic and diastolic blood pressure.
Coffee is a complex liquid containing a multitude of compounds that could affect cardiovascular health including caffeine and polyphenols16,17. Whereas in earlier studies, detrimental effects of coffee consumption on cardiovascular health were promoted, recent studies favor a neutral or positive effect of moderate coffee consumption2,3,4,5.
The number of studies investigating associations of coffee consumption with echocardiographic parameters are scarce. Acute coffee intake seems to have no impact on cardiac function measured by echocardiography in healthy subjects18. Yet, in patients with CAD, coffee intake led to a decrease in left ventricular function, as well as a mild diastolic dysfunction possibly mediated by vasoconstriction and missing cardiac reserve in these patients10. In our community-based study, we did not depict relevant correlations of systolic or diastolic function with coffee consumption. In contrast, the CARDIA study indicated that low-to-moderate daily coffee consumption from early adulthood to middle age was associated with better LV systolic and diastolic function11. Additionally, several studies have suggested a favorable cardiovascular outcome and less heart failure for low- to moderate coffee consumption7,9,19. Accordingly, we observed a weak inverse association of coffee consumption with NT-proBNP. However, in our cross-sectional study there were no relevant associations of coffee consumption with heart failure or echocardiographic and electrocardiographic detectable HF precursors.
In line with most studies, we did not detect associations of coffee consumption with neither atrial fibrillation nor any other measured ECG time interval20. Only few studies addressed the topic of coffee consumption and ECG changes. In young healthy adults, moderate caffeine consumption showed no effect on the PR, QRS, QT and QTc intervals21,22. Supportive of these findings, we were not able to depict any associations between coffee consumption and ECG variables. Nevertheless, some studies reported beneficial effects of coffee consumption on atrial fibrillation23. Although caffeine induces the release of metanephrines and raises calcium sensitivity of the myocardium, our study showed no association of coffee consumption and atrial fibrillation24,25.
In line with previous observations, moderate and high coffee consumption was associated with increasing LDL-cholesterol levels26. Several studies on coffee consumption and lipids proposed that diterpenes, which are highly prevalent in unfiltered coffee, are the main drivers of a coffee-mediated increase in cholesterol levels27,28. In vitro, diterpenes mediated a reduction of LDL receptor activity29. Since the LDL receptor is responsible for the endocytic process of Apo B- and Apo E-containing lipoproteins, its suppression consequently leads to an extracellular accumulation of cholesterol. However, possible coffee-induced elevations of LDL-cholesterol were not accompanied by a rise in the prevalence of cardiovascular diseases such as coronary artery disease or peripheral artery disease.
Data on the effect of coffee on blood pressure are inconsistent30. Whereas several studies demonstrated an association of coffee consumption with elevated blood pressure, other studies were not able to reproduce any influence of coffee consumption on blood pressure31,32. Another meta-analysis even demonstrated a linear association between increasing coffee consumption and decreased risk of hypertension33. Possible explanations for these contradicting results might be attributed to differences in population genetics. Caffeine is mainly metabolized by Cytochrome P450 1A2. Variations in the CYP1A2 allele lead to a slower metabolization of caffeine and are associated with an increased risk for hypertension34. However, even the consumption of decaffeinated caffeine showed the same negative association with systolic and diastolic blood pressure, challenging the role of caffeine as the main driver of the described associations. The positive association with LDL-cholesterol and negative association with blood pressure might support the hypothesis of counterbalancing effects of coffee consumption on cardiovascular health.
Limitations
The HCHS includes a sample from the middle-aged population of the German city of Hamburg with subjects mainly of Caucasian ascend. Accordingly, translations of our results to other ethnic groups should be done with caution. Since the amount of subjects suffering from heart failure was limited (n = 293 subjects), we decided to alter ESC HF Guidelines and consider HFrEF and HFmrEF as a joint HF(m)rEF group. This brings up the need for further studies, with larger sample sizes of subjects suffering from HF, allowing the distinction of HFmrEF and HFrEF.
As our study design is cross-sectional, only descriptions of associations but no causal claims can be made. Furthermore, all subjects have to answer the questionnaires by memory. Being asked about the last 12 months' behaviors and habits can always be distorted by either wrong recollection or deliberate misinformation.
Coffee is a highly complex beverage containing more than 1000 compounds acting as myriad bioactive substances. Conclusions about which substance, e.g. caffeine, derived antioxidants or diterpene alcohols, is responsible for the investigated effects, cannot be made.
Finally, coffee consumption might be associated with certain dietary patterns. While our regression analysis accounted for major dietary factors, the possibility of undetected confounding by additional nutritive and non-nutritive components cannot be completely ruled out.
Conclusion
Our study provides new data on the associations of coffee consumption with cardiovascular health: LDL-cholesterol was positively, systolic and diastolic blood pressure inversely associated. Coffee consumption was not associated with cardiovascular diseases or altered cardiac structure or function suggesting possibly counterbalancing, neutral effects of coffee on cardiovascular health.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
Paul, O. et al. A longitudinal study of coronary heart disease. Circulation 28, 20–31 (1963).
Wilhelmsen, L., Rosengren, A., Eriksson, H. & Lappas, G. Heart failure in the general population of men: Morbidity, risk factors and prognosis. J. Intern. Med. 249, 253–261 (2001).
Schocken, D. D. et al. Prevention of heart failure. Circulation 117, 2544–2565 (2008).
Mukamal, K. J. et al. Coffee consumption and mortality after acute myocardial infarction: The Stockholm Heart Epidemiology Program. Am. Heart J. 157, 495–501 (2009).
Mostofsky, E., Rice, M. S., Levitan, E. B. & Mittleman, M. A. Habitual coffee consumption and risk of heart failure a dose-response meta-analysis. Circ. Heart Fail. 5, 401–405 (2012).
Zulli, A. et al. Caffeine and cardiovascular diseases: Critical review of current research. Eur. J. Nutr. 55, 1331–1343 (2016).
Ding, M., Bhupathiraju, S. N., Satija, A., Van Dam, R. M. & Hu, F. B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129, 643–659 (2014).
Larsson, S. C., Drca, N., Jensen-Urstad, M. & Wolk, A. Coffee consumption is not associated with increased risk of atrial fibrillation: Results from two prospective cohorts and a meta-analysis. BMC Med. 13, 207 (2015).
Wang, Y. et al. Coffee consumption and the risk of heart failure in Finnish men and women. Heart 97, 44–48 (2011).
Yaylali, Y. T., Yaylali, O. & Kirac, S. Impact of caffeine ingestion on ventricular function in coronary artery disease. Int. J. Cardiol. 163, 337–339 (2013).
Nwabuo, C. C. et al. Coffee and tea consumption in the early adult lifespan and left ventricular function in middle age: The CARDIA study. ESC Heart Fail. 7, 1510–1519 (2020).
Jagodzinski, A. et al. Rationale and design of the Hamburg city health study. Eur. J. Epidemiol. https://doi.org/10.1007/s10654-019-00577-4 (2019).
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 00, 1–128 (2021).
Tobin, M. D., Sheehan, N. A., Scurrah, K. J. & Burton, P. R. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat. Med. 24, 2911–2935 (2005).
Bender, R. & Lange, S. Adjusting for multiple testing–when and how?. J. Clin. Epidemiol. 54, 343–349 (2001).
Chrysant, S. G. Coffee consumption and cardiovascular health. Am. J. Cardiol. 116, 818–821 (2015).
Ferruzzi, M. G. The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol. Behav. 100, 33–41 (2010).
Casiglia, E. et al. Haemodynamic effects of coffee and caffeine in normal volunteers: A placebo-controlled clinical study. J. Intern. Med. 229, 501–504 (1991).
Beller, E. et al. Significant impact of coffee consumption on MR-based measures of cardiac function in a population-based cohort study without manifest cardiovascular disease. Nutrients https://doi.org/10.3390/nu13041275 (2021).
Kim, E.-J. et al. Coffee consumption and incident tachyarrhythmias: Reported behavior, mendelian randomization, and their interactions. JAMA Intern. Med. https://doi.org/10.1001/jamainternmed.2021.3616 (2021).
Ammar, R., Song, J. C., Kluger, J. & White, C. M. Evaluation of electrocardiographic and hemodynamic effects of caffeine with acute dosing in healthy volunteers. Pharmacotherapy 21, 437–442 (2001).
Zhang, Y. et al. Coffee, alcohol, smoking, physical activity and QT interval duration: Results from the Third National Health and Nutrition examination Survey. PLoS ONE https://doi.org/10.1371/journal.pone.0017584 (2011).
Bodar, V., Chen, J., Gaziano, J. M., Albert, C. & Djoussé, L. Coffee consumption and risk of atrial fibrillation in the physicians’ health study. J. Am. Heart Assoc. 8, 1–6 (2019).
Robertson, D. et al. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N. Engl. J. Med. 298, 181–186 (1978).
Rasmussen, C. A. J., Sutko, J. L. & Barry, W. H. Effects of ryanodine and caffeine on contractility, membrane voltage, and calcium exchange in cultured heart cells. Circ. Res. 60, 495–504 (1987).
Jee, S. H. et al. Coffee consumption and serum lipids: A meta-analysis of randomized controlled clinical trials. Am. J. Epidemiol. 153, 353–362 (2001).
Godos, J. et al. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 65, 925–936 (2014).
Grosso, G., Godos, J., Galvano, F. & Giovannucci, E. L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 37, 131–156 (2017).
Rustan, A. C., Halvorsen, B., Huggett, A. C., Ranheim, T. & Drevon, C. A. Effect of coffee lipids (cafestol and kahweol) on regulation of cholesterol metabolism in HepG2 cells. Arterioscler. Thromb. Vasc. Biol. 17, 2140–2149 (1997).
Guessous, I., Eap, C. B. & Bochud, M. Blood pressure in relation to coffee and caffeine consumption. Curr. Hypertens. Rep. 16, 1–9 (2014).
Jee, S. H., He, J., Whelton, P. K., Suh, I. & Klag, M. J. The effect of chronic coffee drinking on blood pressure: A meta-analysis of controlled clinical trials. Hypertension 33, 647–652 (1999).
Steffen, M., Kuhle, C., Hensrud, D., Erwin, P. J. & Murad, M. H. The effect of coffee consumption on blood pressure and the development of hypertension. J. Hypertens. 30, 2245–2254 (2012).
Grosso, G. et al. Long-term coffee consumption is associated with decreased incidence of new-onset hypertension: A dose–response meta-analysis. Nutrients 9, 890 (2017).
Palatini, P. et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J. Hypertens. 27, 1594–1601 (2009).
Acknowledgements
The authors acknowledge the participants of the Hamburg City Health Study, the staff at the Epidemiological Study Centre, cooperation partners, patrons and the Deanery from the University Medical Centre Hamburg.
Funding
Open Access funding enabled and organized by Projekt DEAL. The HCHS is supported by the Innovative medicine initiative [grant number 116074], by the Foundation Leducq [grant number 16 CVD 03], by the euCanSHare grant agreement [Grant number 825903-euCanSHare H2020], and the Deutsche Forschungsgemeinschaft [grant number TH1106/5-1; AA93/2-1]. Furthermore, it is supported by the participating institutes and departments from the University Medical Centre Hamburg-Eppendorf, which contribute with individual and scaled budgets to the overall funding. Technical equipment is provided by SIEMENS according to a contract for 12 years, the Schiller AG on a loan basis for six years, and Topcon on a loan basis from 2017 until 2022. The Hamburg City Health Study is additionally supported by an unrestricted grant (2017 to 2022) by Bayer. Project-related analyses are supported by Amgen, Astra Zeneca, BASF, Deutsche Gesetzliche Unfallversicherung (DGUV), Deutsches Krebsforschungszentrum (DKFZ), Deutsches Zentrum für Herz-Kreislauf-Forschung (DZHK), Deutsche Stiftung für Herzforschung, Novartis, Seefried Stiftung, and Unilever. The study is further supported by donations from the “Förderverein zur Förderung der HCHS e.V.”, TePe® (2014) and Boston Scientific (2016). A current list of the supporters is online available on www.uke.de/hchs. Sponsor funding has in no way influenced the content or management of this study.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: S.B., J.-P.W.; data collection: J.-P.W., B.-C.Z.; statistical analysis: K.B., F.O.; analysis and interpretation of results: J.S., J.N., G.A., T.B., C.M., C.-A.B., C.W., R.T., J.-P.W.; draft manuscript preparation: J.S., J.N., J.-P.W. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
SB reports honoraria from Abbott, Siemens, Thermo Fisher, and Roche, outside of the submitted work. RT reports speaker honoraria/consulting honoraria from Abbott, Amgen, Astra Zeneca, Roche, Siemens, Singulex and Thermo Scientific BRAHMS, outside the submitted work. The remaining authors do not have any conflict of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Senftinger, J., Nikorowitsch, J., Borof, K. et al. Coffee consumption and associations with blood pressure, LDL-cholesterol and echocardiographic measures in the general population. Sci Rep 13, 4668 (2023). https://doi.org/10.1038/s41598-023-31857-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31857-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.