Abstract
To investigate the prognostic value of systemic inflammation and insulin resistance in women with breast cancer with different body mass index (BMI). This multicenter, prospective study included 514 women with breast cancer. Multivariate survival analysis showed that patients with high C-reactive protein (CRP), high CRP to albumin ratio (CAR), high lymphocyte to CRP ratio (LCR), high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (LHR), and high triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-c) were significantly associated with worse prognosis. The mortality rate of patients with both high CAR and high LHR or both low LCR and high LHR were 3.91-fold or 3.89-fold higher than patients with both low CAR and low LHR or both high LCR and low LHR, respectively. Furthermore, the combination of LCR and LHR significantly predicted survival in patients within the high BMI group. The CRP, CAR, LCR, LHR, and TG/HDL-c were associated with poor survival in women with breast cancer. The combination of CAR and LHR or LCR and LHR could better predict the prognostic outcomes of women with breast cancer, while the combination of LCR and LHR could better predict the prognosis of those patients with overweight or obese patients.
Similar content being viewed by others
Introduction
The 2022 cancer statistics for the United States show that from 2014 to 2018, female breast cancer incidence continued to increase (by 0.5% annually), and the number of new female breast cancer cases was 287,850 (31%), ranking as the most prevalent new cancer in women. Female breast cancer had the second highest mortality rate with 43,250 (15%) deaths1. In China, the incidence of female breast cancer still the highest among women2. Inflammation and insulin resistance (IR) play important roles in a variety of chronic diseases, including cancer3. Cancer is generally considered an inflammatory disease, and systemic inflammation is often a hallmark of cancer and a major driver of metabolic alterations in cancer patients4,5. The production of acute-phase proteins, such as C-reactive protein (CRP), is considered an accurate measure of systemic inflammation and pro-inflammatory cytokine activity6. Glucose intolerance is the earliest identified metabolic abnormality in cancer patients7, resulting in a type II diabetic state with IR8. Glicksman et al. showed that about 37% of cancer patients have a diabetic glucose tolerance curve9. The characteristics of IR in cancer patients are distinct from those in type II diabetic patients, in which normal fasting blood glucose is associated with high, normal, or low insulin levels10, manifested by increased hepatic glucose production and gluconeogenesis, possibly due to intracellular gluconeogenesis11. The redistribution of glucose to supply energy needs can lead to hypoglycemia, which in turn, leads to an increase in compensatory hormonal signaling or glucagon.
In recent years, obesity has become the most common metabolic disease worldwide, and its incidence has rapidly increased12. Unfortunately, obesity is fast becoming an epidemic in developed and many developing countries13. Overweight or obesity is associated with an increased risk of recurrence or death in patients with breast cancer14,15. Some obesity-related cancers, such as those of the breast and internal organs, occur in or near fat depots. This suggests that altered fat biology, typically found in the context of elevated BMI, locally contributes to the development of several cancers16. Obesity-induced inflammation or inflammatory disturbances are a major feature of adipose tissue dysfunction17. In fact, adipose tissue is not only a storehouse of excess energy in the form of triacylglycerols (TAGs), but is also an active endocrine organ secreting different peptides called adipocytokines18. The production and expression of inflammatory adipocytokines, such as interleukin (IL)-6, tumor necrosis factor α (TNF-α), and monocyte chemoattractant protein 1, are increased in obese and insulin-resistant subjects19. Compared with lean people, adipose tissue in obese subjects was inflamed by inflammatory macrophages20. Macrophages are important and key contributors to adipocyte inflammation21. Inflammatory macrophages typically accumulate within adipose tissue, and this accumulation leads to localized inflammation. This local inflammation leads to multiple metabolic disturbances, including atherosclerosis and systemic inflammation22. In addition, CRP, another inflammatory marker, is elevated in the serum of individuals with higher BMI23. IR is a common pathological condition in obese patients with impaired insulin action in adipose tissue. During IR, insulin is significantly increased in the circulation to avoid hyperglycemia24. Therefore, insulin is included in the study as a hormone, and insulin levels are often increased in the setting of obesity24. This hyperinsulinemia is associated with BMI25.
Some reports showed that CRP alone26,27,28 or in combination with other inflammatory markers, such as the CRP to albumin ratio (CAR)29,30, were associated with poor prognosis in breast cancer. Recently, Lymphocyte to C-reactive Protein Ratio (LCR) has been reported to be related to cancer prognosis31,32,33, but there is no relevant report on the relationship between LCR and breast cancer prognosis. Studies showed that elevated insulin levels and hyperinsulinemia are associated with poor prognosis in breast cancer patients34,35. Some simple and feasible IR surrogate indicators reported earlier have attracted attention relative to the homeostasis model assessment of IR (HOMA-IR)36. These IR indicators included fasting triglyceride glucose (TyG) index37,38, low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (LDL-c/HDL-c, LHR)39, triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-c)38, and total cholesterol to high-density lipoprotein cholesterol ratio (TC/ HDL-c)38. Thus, in this study, we aimed to select the optimal IR index in breast cancer, and select the best combination of inflammation index and IR index in breast cancer patients with different body mass index (BMI). Finally, we selected the best combination of inflammation and IR indicators for combined survival analysis and selected the best combination of indicators to predict the survival of patients with breast cancer with different BMI. This study aimed to analyze the prognostic value of systemic inflammation and IR markers in women with breast cancer, as well as their distribution and ability to predict survival in different BMI subgroups.
Results
Baseline characteristics
After excluding 3 male breast cancer cases and 27 missing TNM stage data, a total of 514 women with breast cancer were included in our study. The detailed flow chart is showed in Fig. 1. Their mean age was 53.72 ± 10.87 years, and the population’s mean BMI was 24.36 kg/m2. Comparing the baseline differences between patients in different BMI groups (low BMI group, BMI < 24 kg/m2 vs. high BMI group, BMI ≥ 24 kg/m2), the age (54.65 vs. 52.75, P = 0.047), BMI (27.23 vs. 21.34, P < 0.001), CRP (3.02 vs. 2.78, P = 0.020), CAR (0.07 vs. 0.06, P = 0.029), TyG (4.55 vs. 4.51, P = 0.008), LHR (2.40 vs. 2.09, P < 0.001), TG/HDL-c (1.72 vs. 1.34, P < 0.001), and TC/HDL-c (4.15 vs. 3.61, P < 0.001) were all higher in the patients in high BMI group than those in low BMI group. However, the LCR (6408.0 vs. 5254.9, P = 0.029) was higher in the patients in the low BMI group than those in high BMI group. Table 1 shows the baseline characteristics of the women with breast cancer. The median follow-up time for patients was 43.1 (40.7–49.6) months, and the 5-year overall mortality rate was 70 (18%), resulting in 41.4 mortality events per 1000 patient-year.
Differences in the distribution of inflammation and IR markers in different BMI subgroups
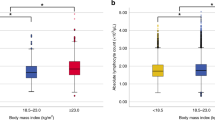
The distribution curves for systemic inflammation-related indicators in different BMI subgroups showed that the CRP, CAR, and LCR values in the high BMI group were significantly higher than those in the low BMI group (All P < 0.05) (Fig. 2A–C). Similarly, we analyzed the differences in the distribution of different IR indicators in different BMI subgroups and found that TyG, LHR, TG/HDL-c, and TC/HDL-c were all highly distributed in the high BMI group compared with the low BMI subgroup patients (All P < 0.05) (Fig. 2D–G).
The distribution of systemic inflammatory indicators and IR makers stratified by BMI in women with breast cancer. (A) CRP; (B) CAR; (C) LCR; (D) TyG; (E) LHR; (F) TG/HDL-c; (G) TC/HDL-c. Notes IR insulin resistance, BMI body mass index, CRP C-reactive protein, LCR lymphocyte to C-reactive protein ratio, CAR C-reactive protein to albumin ratio, TyG triglyceride-glucose index, LHR LDL-c/HDL-c ratio, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol.
Prognostic AUC curves and survival analysis correlated with systemic inflammatory markers and IR markers
To select the optimal inflammatory index and IR index in female breast cancer, we drew the prognostic area under the curve (AUC) curves of inflammatory index and IR index, respectively. The results showed that the predictive ability of LCR and CAR was better than that of CRP among different inflammatory indicators, while among different IR indicators, TyG showed the worst predictive ability of prognosis, compared with LHR, TG/HDL-c, and TC/HDL-c (Fig. 3).
The prognostic AUC curves of systemic inflammatory indicators and IR makers in female breast cancer. (A) Systemic inflammatory indicators of CRP, CAR, and LCR; (B) IR makers of TyG, LHR, TG/HDL-c, and TC/HDL-c. Notes AUC area under the curve, IR insulin resistance, CRP C-reactive protein, LCR lymphocyte to C-reactive protein ratio, CAR C-reactive protein to albumin ratio, TyG triglyceride-glucose index, LHR LDL-c/HDL-c ratio, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol.
The survival curves of CRP, CAR, and LCR in women with breast cancer showed that patients with high CRP, high CAR, or high LCR had a worse prognosis than patients with low CRP(P = 0.0025), low CAR (P < 0.001), or low LCR (P < 0.001), respectively (Fig. 4A–C). In addition, survival curves showed that compared with patients with low TyG, low LHR, or low TG/HDL-c, patients with high TyG (P = 0.03), high LHR (P = 0.017), or high TG/HDL-c (P = 0.018) had worse prognosis, respectively. However, there was no significant difference in survival between patients with low TC/HDL-c or high TC/HDL-c (P = 0.085) (Fig. 5A–D).
Multivariate survival analysis of systemic inflammatory indicators in women with breast cancer indicated that patients with high CRP [model 4: HR (95% CI) = 2.21 (1.24–3.94), P = 0.007] had a shorter OS than patients with low CRP, patients with high CAR [model 4: HR (95% CI) = 2.56 (1.46–4.47), P = 0.001] had a shorter OS than those with low CAR, and patients with high LCR [model 4: HR (95% CI) = 2.43 (1.47–4.02), P = 0.001] had a shorter OS than patients with low LCR (Table 2).
Multivariate survival analysis of the IR index in women with breast cancer indicated that patients with high LHR [model 4: HR (95% CI) = 2.40 (1.25–4.61), P = 0.008] had a shorter OS than those with low LHR and patients with high TG/ HDL-c [model 4: HR (95% CI) = 3.51 (1.08–11.36), P = 0.036] had a shorter OS than those with low TG/HDL-c. However, TyG [model 4: HR (95% CI) = 1.42 (0.73–2.78), P = 0.302] and TC/HDL-c [model 4: HR (95% CI) = 1.40 (0.85–2.30), P = 0.185] were not significant survival predictors in women with breast cancer (Table 2).
Survival analysis stratified by different BMI groups
We analyzed the prognostic value of systemic inflammatory markers and IR markers in different BMI subgroups. In the BMI < 24 kg/m2 subgroup, we observed that all markers did not show significant prognostic value (All P > 0.05). In the BMI ≥ 24 kg/m2 subgroup, patients with high CRP [Adjusted HR (95% CI) = 2.39 (1.00–5.71), P = 0.049], high CAR [Adjusted HR (95% CI) = 2.85 (1.23–6.60), P = 0.014], high LCR [Adjusted HR (95% CI) = 4.32 (2.06–9.06), P < 0.001], high TyG [Adjusted HR (95% CI) = 2.87 (1.20–6.85), P = 0.017], or high LHR [Adjusted HR (95% CI) = 2.91 (1.20–7.06), P = 0.018] predicted worse prognoses, while TG/HDL-c and TC/HDL-c did not show significant prognostic value (All P > 0.05) (Table 3).
Combined analysis of prognostic systemic inflammatory indicators and IR index
We performed a combined survival analysis with the prognostic systemic inflammatory index and IR index. In all patients, CAR combined with LHR or LCR combined with LHR predicted a longer OS in women with breast cancer. The prognosis of patients in the low CAR and high LHR or high CAR and low LHR group [Adjusted HR (95% CI) = 2.21 (1.27–3.87), P = 0.005] and the high CAR and high LHR group [Adjusted HR (95% CI) = 3.91 (1.56–9.81), P = 0.004] was worse than in patients in the low CAR and low LHR groups. The prognosis of patients in the high LCR and high LHR or low LCR and low LHR group [Adjusted HR (95% CI) = 2.30 (1.36–3.87), P = 0.002] and the low LCR and high LHR group [Adjusted HR (95% CI) = 3.89 (1.65–9.21), P = 0.002] was worse than in patients in the high LCR and low LHR groups. However, no prognostic information was generated by the other combinations. In addition, when we performed a combined survival analysis in different BMI subgroups, we only observed a significant survival difference in the combined analysis of LCR and LHR in the high BMI subgroup. The prognosis of patients in the high LCR and high LHR or low LCR and low LHR group [Adjusted HR (95% CI) = 3.61 (1.69–7.69), P = 0.001] and the low LCR and high LHR group [Adjusted HR (95% CI) = 7.79 (2.42–25.11), P = 0.001] was worse than that of the patients in the low LCR and low LHR groups (Table 4).
Discussion
In this study, we found that the levels of inflammation (CRP, CAR, and LCR) and IR (TyG, LHR, TG/HDL-c, and TC/HDL-c) in breast cancer patients with BMI ≥ 24 kg/m2 were significantly higher than those in patients with BMI < 24 kg/m2. In other words, inflammation and IR levels in overweight or obese patients are high. The expression of adipocytokines in human adipose tissue and their corresponding circulating concentrations are influenced by human fat mass. In obese patients, there was also a positive correlation between adipocyte TNF-α expression and plasma TNF-α concentration with BMI40. Plasma IL-6 and CRP concentrations were also positively correlated with BMI23,41. Obesity is a common cause of chronic inflammation, and white adipose tissue (WAT) in obese patients is infiltrated by immune cells, including macrophages and lymphocytes, at the systemic and tissue level. WAT inflammation is associated with increased circulating levels of CRP and IL-616. Obesity is a well-established risk factor for IR and type II diabetes. With the rising prevalence of obesity, an increasing number of patients at cancer diagnosis are overweight or obese and have impaired glycemic control. Obesity and excess adipose tissue lead to increased production of free fatty acids, leptin, and cytokines, and these metabolic abnormalities are associated with decreased physical activity and increased triglycerides, leading to hyperinsulinemia and IR42.
We also examined the relationship between systemic inflammation and IR and breast cancer survival. Studies have shown an increased risk of breast cancer in obese postmenopausal women, and it has been hypothesized that circulating estrogen levels may be elevated in obese postmenopausal women43. Therefore, considering these potential interference factors, we separated patients into different age groups and adjusted the survival analyses to reduce the interference caused by estrogen levels in different age groups. We found that elevated systemic inflammatory markers (CRP, CAR, and LCR) were all significantly associated with reduced OS in breast cancer patients. Similarly, we observed a significant association between increased IR markers (LHR and TG/ HDL-c) and decreased OS in breast cancer patients.
Pierce et al. analyzed the prognostic value of inflammatory markers in women with stage 0 to IIIA breast cancer in a multicenter, prospective, cohort study and found that CRP was associated with poor prognosis in women with breast cancer compared with the highest and lowest tertiles [HR 2.27; 95% CI 1.27–4.08]26. Gunter et al. and Albuquerque et al. also found that CRP levels were positively associated with breast cancer risk27,28. Zhou et al. used propensity score matching to estimate the prognostic role of CAR in non-metastatic breast cancer patients and found that elevated CAR levels were associated with increased age, postmenopausal status, and a higher risk of recurrence or death in breast cancer patients. Elevated CAR was an independent risk factor for long-term prognosis, predicting decreased disease-free survival [HR 2.225; P = 0.024] and OS [HR 9.189; P = 0.003] of breast cancer patients29. Chen et al. found that preoperative CAR could be an important independent prognostic marker for HER2-negative, luminal breast cancer, and elevated CAR was associated with poorer disease-free survival and cancer-specific survival30. In this study, for the first time, we found that LCR could be used as an independent prognostic marker in breast cancer patients. Previous studies reported that LCR is associated with poor prognosis in other tumors, such as colorectal cancer33, gastric cancer32, and hepatocellular carcinoma44. As for IR prognostic indicators, previous studies have shown that LHR is associated with poor prognosis in colorectal cancer45,46 and gastric cancer47. Dai et al. analyzed the relationship between TG/ HDL-c and prognosis in triple-negative breast cancer patients and found that patients with high TG/ HDL-c was associated with poor OS [HR: 1.935; 95% CI 1.032–3.629]48. Similar results showed that TG/ HDL-c was associated with poor prognosis in other cancers, including in endometrial cancer49 and gastric cancer50.
We observed markers of inflammation and IR in different BMI subgroups and found that LCR could predict survival in different BMI subgroups. And CRP, CAR, TyG, LHR predict the prognosis of patients within the high BMI subgroup. The results of the combined survival analyses showed that the inflammatory insulin combination of LCR&LHR and CAR&LHR could differentiate the prognosis of breast cancer patients. Especially, LCR&LHR could also significantly differentiate the prognosis of patients in the high BMI subgroup. Furthermore, the observation that breast WAT inflammation predicts a poorer clinical course in breast cancer patients is consistent with earlier reports showing that TNF-α , IL-1beta, IL-6 and CRP promote tumor growth in a mouse model of obesity and elevated levels of IL-6 and CRP were associated with the development and progression with female breast cancer16. Inflammation and IR are closely related. The IR state that develops with increased obesity is associated with activation of inflammatory responses in different organ sites, including adipose tissue, liver, and skeletal muscle, which increases secretion and systemic levels of proinflammatory cytokines51. Some adipocytokines help regulate insulin action and are associated with IR syndromes52. Leptin interferes with insulin signaling, and in type II diabetes, plasma leptin levels correlate with the degree of IR, a relationship independent of BMI and body fat mass53,54. Thus, the IR syndrome is associated with hyperleptinemia and hyperinsulinemia55, which allows endocrine hyperactivity of these proteins at target sites, including mammary epithelial tissue and vascular endothelial cells. Adipose tissue TNF-α expression was also positively correlated with plasma insulin concentrations56, and increased adipocyte secretion of TNF-α was associated with decreased insulin sensitivity in obese individuals41. In abdominal obesity, high circulating TNF-α levels are associated with hyperinsulinemia and IR57. IR is also associated with human adipose tissue-derived IL-641. Adipose tissue has biological activities that regulate appetite, inflammation, insulin sensitivity, fat metabolism, and energy balance58. Excessive adipose tissue will lead to the production of inflammatory cytokines and the upregulation of nuclear factor-κB, leading to increased nitric oxide and reactive oxygen species, resulting in IR, excess glucose, and increased free fatty acid, thereby further spreading inflammation59.
Our study has several strengths. First, this is a prospective, cohort study of women with breast cancer based on a multi-medical center trial to analyze the prognosis of different systemic inflammation and IR markers. Second, our study analyzed the inflammation and IR levels in different BMI subgroups and examined high-inflammation and high-IR status in overweight or obese female breast cancer patients to identify the best markers of inflammation and IR. Our study also has some limitations. First, we only collected a fasting blood sample and thus, cross-sectional data. Longitudinal data is needed for a patient's observation of inflammation and IR. Second, although we consider the effect of hormonal levels in patients and make prognostic adjustments for different ages, we still need to collect relevant data. Third, different pathological types of breast cancer may cause heterogeneity, and the results of more pathological types need to be included. Fourth, our IR-related metric is only a surrogate metric, and we cannot deny its simplicity and feasibility, but the assessment of patients' IR status still needs to be done.
Conclusion
In conclusion, our data showed that higher CRP, CAR, LCR, LHR, and TG/ HDL-c were associated with increased risk in women with breast cancer. Elevated BMI showed the higher inflammation and IR levels in women with breast cancer. The combination of CAR and LHR or LCR and LHR could significantly predict the prognosis of women with breast cancer, while the combination of LCR and LHR can significantly predict prognosis in those patients with overweight or obese patients.
Materials and methods
Study population
The data collected in this study from women with breast cancer were obtained from a prospective, multi-medical center-based cancer population study in China between 2013 and 2021. The hospitals included Fujian Cancer Hospital, Bethune First Hospital of Jilin University, Zhejiang Cancer Hospital, First Affiliated Hospital of Sun Yat-Sen University, Chongqing Daping Hospital, and Chongqing Third People's Hospital. The inclusion criteria for this study were: 1. Female patients aged not less than 18 years; 2. Pathologically diagnosed with breast cancer; and 3. Clearly conscious and able to communicate autonomously. There were no strict exclusion criteria. The current study complied with the Declaration of Helsinki, was approved by the Human Research Committees at the various medical centers, and all participants provided informed consent.
Anthropometric and laboratory measurements
At the start of the study, participants' demographic information, medical and family history, and quality of life assessment were collected through questionnaires administered by trained investigators. All research centers, which participated in your study, had the same standards of biomarkers laboratory testing. Baseline clinical characteristics collected from patients included age, body mass index (BMI), comorbidities (diabetes, yes/no; hypertension, yes/no; and coronary heart disease, yes/no), tumor-related information (family history of cancer, tumor stage, surgery, yes/no; radiation therapy, yes/no; chemotherapy, yes/no; immunotherapy, yes/no; and tumor metastasis, yes/no), Karnofsky performance status (KPS), triceps skinfold thickness (TSF), and laboratory test indicators [C-reactive protein (CRP), fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), and high-density lipoprotein cholesterol (HDL-c)].
The patient's body measurements were obtained by clinicians or nurses, height and weight were measured while the patients were wearing light hospital gowns and socks, and TSF was obtained by taking the average of three measurements with a skinfold caliper. BMI was defined as the ratio of weight (kg) to height squared (m2). Blood samples from patients were collected for analysis in the laboratory within 48 h prior to admission after patients had fasted for at least 8 h prior to sample collection. The index of CAR and LCR were calculated by: CRP/albumin and Lymphocyte/CRP, respectively. The TyG index was defined as Ln [TC (mg/dl) * FBG (mg/dl)]/2. The ratios LDL-c/HDL-c (LHR), TG/HDL-c, and TC/HDL-c were defined as: LDL-c/HDL-c, TG/HDL-c, and TC/HDL-c, respectively.
Outcomes
Overall survival (OS), representing the study endpoint, was calculated from the date of diagnosis of cancer until death or last follow-up. Follow-up of patients was completed by follow-up staff.
Statistical analyses
Data are shown as percentages, mean ± standard deviation, or median ± interquartile interval. Baseline characteristics of obese and nonobese populations were compared using the chi-square test and Fisher's exact test for categorical variables and a t-test for continuous normal distribution variables (Wilcoxon test for non-parametric variables). Cutoff values were generated by largest selected rank statistical analysis method for continuous data (see Supplementary Fig. S1 online).
The prognostic AUC curves were performed to selcet the optimal inflammation index and IR index. The survival curves were calculated using the Kaplan–Meier method, and the level of significance was assessed using the log-rank test. Associations between prognostic factors and OS were examined using multivariable Cox proportional hazards regression models, and results were reported as hazard ratios (HRs) and 95% confidence intervals (95% CIs). We assessed confounding covariates by adding each covariate sequentially to the base model. Model 0: unadjusted; model 1: adjusted for BMI; model 2: adjusted for age, tumor stage, and BMI; model 3: adjusted for age, tumor stage, BMI, KPS, surgery, chemotherapy, radiotherapy, immunotherapy, family history of cancer, tumor metastasis, diabetes, hypertension, and coronary heart disease.; model 4: adjusted for age, tumor stage, BMI, KPS, surgery, chemotherapy, radiotherapy, immunotherapy, family history of cancer, tumor metastasis, diabetes, hypertension, coronary heart disease, and TSF.
All P values were two-sided. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using the R software version 4.1.1.
Ethics approval
This study followed the Helsinki declaration. All participants signed an informed consent form, and this study was approved by the Institutional Review Board of each hospital (Registration number: ChiCTR1800020329).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 (2016).
Lee, D. Y. et al. Impact of systemic inflammation on the relationship between insulin resistance and all-cause and cancer-related mortality. Metabolism 81, 52–62 (2018).
Korniluk, A., Koper, O., Kemona, H. & Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 186, 57–62 (2017).
Argiles, J. M., Lopez-Soriano, F. J. & Busquets, S. Counteracting inflammation: A promising therapy in cachexia. Crit. Rev. Oncog. 17, 253–262 (2012).
Fearon, K. C. et al. Pancreatic cancer as a model: Inflammatory mediators, acute-phase response, and cancer cachexia. World J. Surg. 23, 584–588 (1999).
Argiles, J. M. & Lopez-Soriano, F. J. Insulin and cancer (Review). Int. J. Oncol. 18, 683–687 (2001).
Tayek, J. A. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J. Am. Coll. Nutr. 11, 445–456 (1992).
Glicksman, A. S. & Rawson, R. W. Diabetes and altered carbohydrate metabolism in patients with cancer. Cancer 9, 1127–1134 (1956).
Dev, R., Bruera, E. & Dalal, S. Insulin resistance and body composition in cancer patients. Ann. Oncol. 29, ii18–ii26 (2018).
Fonseca, G., Farkas, J., Dora, E., von Haehling, S. & Lainscak, M. Cancer cachexia and related metabolic dysfunction. Int. J. Mol. Sci. 21, 2321 (2020).
Formiguera, X. & Canton, A. Obesity: Epidemiology and clinical aspects. Best Pract. Res. Clin. Gastroenterol. 18, 1125–1146 (2004).
Finucane, M. M. et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 377, 557–567 (2011).
Duggan, C. et al. Associations of insulin resistance and adiponectin with mortality in women with breast cancer. J. Clin. Oncol. 29, 32–39 (2011).
Majed, B., Moreau, T., Asselain, B., Curie Institute Breast Cancer, G. Overweight, obesity and breast cancer prognosis: Optimal body size indicator cut-points. Breast Cancer Res. Treat. 115, 193–203 (2009).
Iyengar, N. M., Gucalp, A., Dannenberg, A. J. & Hudis, C. A. Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J. Clin. Oncol. 34, 4270–4276 (2016).
van Kruijsdijk, R. C., van der Wall, E. & Visseren, F. L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 18, 2569–2578 (2009).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004).
Sartipy, P. & Loskutoff, D. J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 100, 7265–7270 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Cancello, R. et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54, 2277–2286 (2005).
Wang, Z. & Nakayama, T. Inflammation, a link between obesity and cardiovascular disease. Mediat. Inflamm. 2010, 535918 (2010).
Amin, M. N. et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab. Syndr. 13, 1213–1224 (2019).
Guo, Q. et al. IGF-I CA19 repeat polymorphisms and cancer risk: A meta-analysis. Int. J. Clin. Exp. Med. 8, 20596–20602 (2015).
Leung, K. C., Doyle, N., Ballesteros, M., Waters, M. J. & Ho, K. K. Insulin regulation of human hepatic growth hormone receptors: Divergent effects on biosynthesis and surface translocation. J. Clin. Endocrinol. Metab. 85, 4712–4720 (2000).
Pierce, B. L. et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 27, 3437–3444 (2009).
Albuquerque, K. V. et al. Pre-treatment serum levels of tumour markers in metastatic breast cancer: A prospective assessment of their role in predicting response to therapy and survival. Eur. J. Surg. Oncol. 21, 504–509 (1995).
Gunter, M. J. et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J. Natl. Cancer Inst. 107, djv169 (2015).
Zhou, L. et al. A retrospective propensity score matched study of the preoperative C-reactive protein to albumin ratio and prognosis in patients with resectable non-metastatic breast cancer. Med. Sci. Monit. 25, 4342–4352 (2019).
Chen, F. et al. Prognostic significance of neutrophil-to-lymphocyte ratio and C-reactive protein/albumin ratio in luminal breast cancers with HER2-negativity. Front. Oncol. 12, 845935 (2022).
Zhang, X. et al. The nutrition-inflammation marker enhances prognostic value to ECOG performance status in overweight or obese patients with cancer. JPEN J. Parenter Enter. Nutr. 47, 109–119 (2022).
Okugawa, Y. et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin. Nutr. 39, 1209–1217 (2020).
Okugawa, Y. et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg. 272, 342–351 (2020).
Goodwin, P. J. et al. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J. Clin. Oncol. 20, 42–51 (2002).
Pasanisi, P. et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int. J. Cancer. 119, 236–238 (2006).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214-223 (1979).
Fritz, J. et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int. J. Epidemiol. 49, 193–204 (2020).
Kheirollahi, A. et al. Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis. 19, 235 (2020).
Zhou, M. et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of beta cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 15, 104 (2016).
Rose, D. P., Komninou, D. & Stephenson, G. D. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes. Rev. 5, 153–165 (2004).
Kern, P. A., Ranganathan, S., Li, C., Wood, L. & Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 280, E745-751 (2001).
Gallagher, E. J. & LeRoith, D. Insulin, insulin resistance, obesity, and cancer. Curr. Diabetes Rep. 10, 93–100 (2010).
Lorincz, A. M. & Sukumar, S. Molecular links between obesity and breast cancer. Endocr. Relat. Cancer. 13, 279–292 (2006).
Ni, H. H. et al. Combining pre- and postoperative lymphocyte-C-Reactive protein ratios can better predict hepatocellular carcinoma prognosis after partial hepatectomy. J. Inflamm. Res. 15, 2229–2241 (2022).
Liu, Y. L., Qian, H. X., Qin, L., Zhou, X. J. & Zhang, B. Serum LDL-C and LDL-C/HDL-C ratio are positively correlated to lymph node stages in males with colorectal cancer. Hepatogastroenterology. 58, 383–387 (2011).
Notarnicola, M. et al. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology 68, 371–374 (2005).
Ma, M. Z., Yuan, S. Q., Chen, Y. M. & Zhou, Z. W. Preoperative apolipoprotein B/apolipoprotein A1 ratio: A novel prognostic factor for gastric cancer. Onco Targets Ther. 11, 2169–2176 (2018).
Dai, D. et al. Pretreatment TG/HDL-C ratio is superior to triacylglycerol level as an independent prognostic factor for the survival of triple negative breast cancer patients. J. Cancer. 7, 1747–1754 (2016).
Luo, Y. Z. et al. Pretreatment triglycerides-to-high density lipoprotein cholesterol ratio in postmenopausal women with endometrial cancer. Kaohsiung J. Med. Sci. 35, 303–309 (2019).
Sun, H. et al. Triglyceride-to-high density lipoprotein cholesterol ratio predicts clinical outcomes in patients with gastric cancer. J. Cancer. 10, 6829–6836 (2019).
McNelis, J. C. & Olefsky, J. M. Macrophages, immunity, and metabolic disease. Immunity 41, 36–48 (2014).
Matsuzawa, Y., Funahashi, T. & Nakamura, T. Molecular mechanism of metabolic syndrome X: Contribution of adipocytokines adipocyte-derived bioactive substances. Ann. N. Y. Acad. Sci. 892, 146–154 (1999).
Fischer, S. et al. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 39, 105–110 (2002).
Wauters, M. et al. Leptin levels in type 2 diabetes: Associations with measures of insulin resistance and insulin secretion. Horm. Metab. Res. 35, 92–96 (2003).
Leyva, F. et al. Hyperleptinemia as a component of a metabolic syndrome of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 18, 928–933 (1998).
Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L. & Spiegelman, B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 (1995).
Aldhahi, W. & Hamdy, O. Adipokines, inflammation, and the endothelium in diabetes. Curr. Diabates Rep. 3, 293–298 (2003).
Ibrahim, M. M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 11, 11–18 (2010).
Sonnenberg, G. E., Krakower, G. R. & Kissebah, A. H. A novel pathway to the manifestations of metabolic syndrome. Obes. Res. 12, 180–186 (2004).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing. We are grateful to all the participants who have been part of the project and to the many members of the study teams at different study centers who have enabled this research.
Funding
This work was supported by the National Key Research and Development Program [Grant No. 2017YFC1309200, 2022YFC2009600] and the Beijing Municipal Science and Technology Commission [Grant No. SCW2018-06].
Author information
Authors and Affiliations
Contributions
R.G.T. wrote the manuscript. R.G.T., X.H.L., and H.C.L. analyzed and interpreted the patient data, R.G.T., X.H.L., H.C.L., and S.H.P. made substantial contributions to the conception, design, and intellectual content of the studies. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruan, GT., Xie, HL., Hu, CL. et al. Comprehensive prognostic effects of systemic inflammation and Insulin resistance in women with breast cancer with different BMI: a prospective multicenter cohort. Sci Rep 13, 4303 (2023). https://doi.org/10.1038/s41598-023-31450-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31450-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.