Abstract
Telomeres, the nucleotide sequences that protect the ends of eukaryotic chromosomes, shorten with each cell division and telomere loss may be influenced by environmental factors. Telomere length (TL) decreases with age in several species, but little is known about the sources of genetic and environmental variation in the change in TL (∆TL) in wild animals. In this study, we tracked changes in TL throughout the natural lifespan (from a few months to almost 9 years) of free-living house sparrows (Passer domesticus) in two different island populations. TL was measured in nestlings and subsequently up to four times during their lifetime. TL generally decreased with age (senescence), but we also observed instances of telomere lengthening within individuals. We found some evidence for selective disappearance of individuals with shorter telomeres through life. Early-life TL positively predicted later-life TL, but the within-individual repeatability in TL was low (9.2%). Using genetic pedigrees, we found a moderate heritability of ∆TL (h2 = 0.21), which was higher than the heritabilities of early-life TL (h2 = 0.14) and later-life TL measurements (h2 = 0.15). Cohort effects explained considerable proportions of variation in early-life TL (60%), later-life TL (53%), and ∆TL (37%), which suggests persistent impacts of the early-life environment on lifelong telomere dynamics. Individual changes in TL were independent of early-life TL. Finally, there was weak evidence for population differences in ∆TL that may be linked to ecological differences in habitat types. Combined, our results show that individual telomere biology is highly dynamic and influenced by both genetic and environmental variation in natural conditions.
Similar content being viewed by others
Introduction
Telomeres are short DNA repeats that protect the ends of linear chromosomes1. Telomere length (TL) decreases due to incomplete end replication during cell division2, and telomere loss can be accelerated by oxidative stress (3,4,5, but see6). When telomeres become critically short, replicative cell senescence may be induced7. Consequently, telomeres are implicated in organismal senescence8 and TL is considered a hallmark of ageing9. However, TL or the change in TL (∆TL) is often found to be independent of chronological age10, but may be influenced by several environmental factors and experiences11,12,13. For instance, telomeres may shorten in response to efforts associated with reproduction14,15, growth16, or harsh abiotic conditions in free-living populations17. Consequently, TL may be a causal mediator of effects of growth and early-life conditions on later-life senescence18,19, and TL may predict fitness components such as survival and reproductive success in wild animals20,21.
TL of somatic cells decreases over lifetimes in many vertebrate species22, but there are exceptions to this pattern across vertebrates23,24,25. Some studies suggest that most telomere loss occurs during early life22,26,27,28. There are also indications that stress experienced early in life, such as that associated with changes in the tempo of growth, has delayed consequences for later-life telomere shortening18 and oxidative stress29. However, many studies have been restricted to the use of single cross-sectional TL measurements, particularly in early-life studies, which can be influenced by selective loss of phenotypes at later ages30. Early-life TL has been shown to correlate with TL later in life in some species31,32. However, there are also studies suggesting that telomere shortening rates are greater in individuals with initially longer telomeres27,33,34,35 perhaps because longer telomeres present a larger target for oxidative damage36,37,38,39. Such an effect may shape the observed associations between early-life TL, stress exposure and fitness19,40,41.
Heritability estimates of TL vary greatly across species and populations30,42,43,44. However, if TL is only measured in adults, it is not clear whether the estimated heritability reflects additive genetic effects (VA) of TL itself versus VA of individual susceptibility to telomere shortening during their lifetime prior to TL measurement, or both. Indeed, little is known about the heritability of telomere shortening rates. Hjelmborg et al.45 estimated a heritability of telomere shortening of h2 = 0.28 in adult human twins (n = 652), which was smaller than their estimated heritability for adult TL (h2 = 0.64) and TL heritabilities reported in other human studies (e.g.46). In contrast, Bauch et al.47 found a low heritability of telomere shortening rates (h2 = 0.09) during the first month of life in western jackdaws (Coloeus monedula, n = 474), but a high heritability of early-life TL (h2 = 0.74).
In this longitudinal study, we use 24 years of blood sampling (n = 3061) from two insular house sparrow (Passer domesticus) populations to track changes in individual TL throughout natural lifespans. First, we investigate how TL changes with age within individuals from the nestling stage to 9 years of age. In a previous cross-sectional study in these house sparrow populations, we found some evidence for a negative association between TL and age among 5–14 days old nestlings (n = 266243). In other house sparrow populations, and with a smaller (cross-sectional) sample size, we did not detect trends in TL with age among 5–17 days old nestlings (n = 56648). Second, we examine within-individual consistency in TL. Third, we decompose genetic and environmental contributions to variation in ∆TL, early-life and later-life TL. Fourth, we test whether early-life TL is associated with changes in TL. That is, whether individuals with initially longer telomeres also experience more TL shortening. Finally, we explore factors affecting ∆TL through life in the two populations. These populations differ in the values of several key life-history traits49 and we have previously found differences in the associations between environmental conditions and early-life TL of these two populations50. Furthermore, both TL and ∆TL may be sex-specific in some species51 and TL may be negatively associated with body size in house sparrows43,48 and in other species16. We therefore also test for differences between the two populations in ∆TL and for effects of sex and body size on ∆TL.
Methods
Study system
This study involved two unmanipulated island populations of house sparrows in an archipelago in northern Norway that are part of a metapopulation study (see map in50). Birds were monitored on Hestmannøy (66° 33′ N, 12° 50′ E) from 1994 to 2020 and on Træna (66° 30′ N, 12°05′ E) from 2004 to 2020. The house sparrow is a small, globally distributed passerine that lives naturally in close association with human habitation, and human activities provide the natural basis of existence for house sparrows52. The average population generation time and lifespan in populations similar to those in this study is about 2 years53, with a maximum recorded lifespan for this species of 20 years in the wild54. On Hestmannøy, the sparrows live mainly on dairy farms, and on Træna they live mainly in gardens in a small village. Nests in cavities inside buildings and other human-made structures (mainly on Hestmannøy) or in nest boxes (on both islands) were visited regularly during the breeding season (May–August) from 1994 to 2013 to ring fledglings with a unique color-ring combination at around 10 days of age (5–14 days). Nestling tarsometatarsus (tarsus) was measured using calipers to nearest 0.01 mm. We estimated age-standardized nestling tarsus length as the residuals of a linear regression of tarsus length on age and age squared separately for each sex and population. Juveniles and adults were captured using mist nets mainly during summer and autumn (May to October) from 1994 to 2020. A small blood sample (25 μL) was collected from all nestlings and recaptured juveniles and adults via venipuncture of the brachial vein. Blood was stored in 96% ethanol at room temperature in the field and at −20 °C in the laboratory prior to DNA extraction as described in Pepke et al.48. The study was carried out with permits from the Norwegian Animal Research Authority (FOTS id 11904) and the Ringing Centre at Stavanger Museum, Norway.
Telomere length measurements
Relative erythrocyte telomere lengths (TL) were measured using the real-time quantitative polymerase chain reaction (qPCR) amplification method55,56 as described in Pepke et al.48. DNA was extracted from blood using the ReliaPrep Large Volume HT gDNA Isolation System (Promega). DNA concentration was measured using a FLUROostar Omega scanner (BMG Labtech) and diluted with dH2O to yield 1.67 ng/mL, corresponding to 10 ng of DNA per well, and stored at −78 °C. All samples had a 260/280 absorbance ratio of 1.8–2.2 and DNA concentration > 15 ng μl−1. Telomeric sequence was measured relative to the amount of the non-variable gene GAPDH and a reference sample. Primers, qPCR assay setup and thermal profiles are described in detail in Pepke et al.48. Assays were prepared with the Absolute blue qPCR SYBR green Low Rox master mix (ThermoFisher scientific). A two-fold serial dilution was included on all plates to make a standard curve. Samples were randomized across qPCR plates and run in triplicates, and details of these qPCR runs and efficiencies (all plates within 100 ± 10%, mean telomere and GAPDH assay efficiencies were 97.5 ± 3.9% and 97.6 ± 4.2%, respectively) are given in Pepke et al.43. All reactions were carried out by the same person (MLP). Average reference sample cycle thresholds across all plates were 10.54 ± 0.03 SD and 21.53 ± 0.02 SD for telomere and GAPDH assays, respectively. DNA re-extractions followed by runs on different plates revealed highly correlated TL measurements (R2 = 0.75, see details in Pepke et al.43). Data were analyzed using qBASE57 while controlling for differences in amplification efficiency between plates and inter-run variation.
Early-life TL was obtained for 2746 nestlings (n = 2110 from Hestmannøy and n = 636 from Træna) aged 5–14 days old (see Table S1 in the Supporting Information for sample size details). In addition, 228 (n = 195 from Hestmannøy and n = 33 from Træna) individuals were blood sampled at least once as juveniles and/or adults, providing 315 additional later-life TL measurements. 223 of these individuals had also been sampled as nestlings. The longitudinal data set consists of 226 individuals that were sampled at least twice (165 individuals were sampled twice, 44 sampled thrice, 11 sampled four times and 6 sampled five times), with the number of TL samples taken ranging from 2 to 5 (mean 2.4 ± 0.7) samples per individual (536 samples in total). The time interval between first and last TL measurements ranged from 15 days and up to 3245 days (9 years, Fig. S1). The total number of TL samples was 3061 from 2751 individuals.
Pedigree information
Molecular sex determination and microsatellite pedigree construction for these populations are described in Jensen et al.53 and Billing et al.58. House sparrows are socially monogamous, but extra-pair paternity occurs at rates of 14–18% in wild populations59,60. We assigned dummy parents to nestlings with one or two missing parents (n = 64), assuming that nestlings within the same clutch were full siblings and thus had the same (dummy) parents. The dummy parents (n = 45) were included in the pedigree as founders. The pedigree was ordered using MasterBayes61 and pruned to only contain informative individuals from the longitudinal TL data set using functions in MCMCglmm62. The pruned pedigree included 750 individuals (472 maternities and 484 paternities).
Changes in telomere length with age
We investigated the relationship between TL and age (in days) using within-subject centering63,64. This approach allows us to discriminate between effects of age on TL due to selective disappearance at the population level from those due to within-individual TL shortening. For each individual we calculated ∆age by subtracting the individual’s mean age from each sampled age (in days), either with or without log10-transformation of age. First, we investigated relationships between TL and age including all TL measurements (n = 2977 measurements of n = 2667 individuals, excluding 84 individuals with missing sex information), thus, individuals with only one TL measurement (n = 2441) had ∆age = 0. We constructed linear mixed-effect models (LMMs) using the lme4 package65 with log10-transformed TL as the response variable. Mean age (among-individual effect) and ∆age (within-individual effect) were included as fixed effects covariates. Similar models were fitted with age log10-transformed to linearize models. Models including ∆age squared were fitted to account for effects such as a decelerating rate of TL shortening with age. Sex and population identity were included as fixed factors, with individual identity and year included as random intercepts in all models. The five resulting candidate models were compared using Akaike’s information criterion corrected for small sample sizes (AICc66). Furthermore, we compared the within- and between-individual effects by including the effect of age instead of ∆age, in which case the effect of mean age represents the difference between the within- and between-subject effects. If the within-individual slope is e.g. more negative than the between-individual slope, this suggests that individuals with short telomeres are more likely to disappear from the population. Models were validated visually using diagnostic plots and all model parameters are reported from models refitted with restricted maximum likelihood (REML). All analyses were performed in R v. 4.2.067.
We have previously found no associations between nestling TL and survival in these sparrow populations, which showed high (presumably extrinsic) juvenile mortality50. Effects of selective disappearance of individuals with short TL and/or higher telomere shortening rates could therefore be masked by the majority of individuals having only one early-life TL measurement. We therefore compared relationships between TL and age within and among individuals with multiple TL measurements (n = 536 measurements of 226 individuals) using the same procedure described above. The within-individual age effect will be the same in the two approaches, but excluding individuals with only one (early-life) TL measurement (all of which were only sampled as nestlings) allows us to investigate whether selective disappearance may act on TL later in life, as expected if age-dependent TL predicts remaining lifespan68.
Repeatability of telomere length
We used all longitudinal samples (n = 536 from 226 individuals) to estimate adjusted individual repeatability69 in TL over the lifespan. We used the rptR package70 to fit a model of variation in log10-transformed TL including sex, population identity and log10-transformed age (in days) as fixed effects, and year and individual identity as random intercepts. Uncertainty in the estimate was estimated using parametric bootstrap to simulate new data and refit the model for a total of 1000 bootstrap replicates.
Correlation between early- and later-life telomere length
We tested if the first TL measurement predicted the value of the next subsequently sampled (second) TL measurement (response variable, n = 226) using a LMM (lme4) with first TL, the elapsed time in days between the two measurements (∆time), sex and population identity as fixed effects, and year as a random intercept. We tested whether including the first TL measurement improved the model using AICc.
In the Supporting Information, we also test for consistent and constant lifelong telomere elongation within individuals with at least three TL measurements (n = 61), using the variance-comparison method by Simons et al.71 to identify individuals that show increases in TL above what may be expected from measurement error.
Correlation between the change in telomere length and early-life telomere length
The first TL measurement (‘baseline’ TL) is not statistically independent from the difference between the first and last TL measurement (total ΔTL, n = 226) within an individual. Therefore, instead of using ΔTL, we followed Verhulst et al.33 in calculating the statistic D by subtracting from ΔTL the change that is expected as a result of this regression-to-the-mean effect, which is estimated from the correlation between the first and last TL measurements. D was multiplied by −1 so that a negative value indicates telomere shortening. We then tested if baseline TL was associated with D using a LMM with D as the response variable and the first TL, the time in days between the first and last TL measurements (∆time), population identity, and sex as fixed effects. Year was included as random intercept. We then tested whether including the first TL measurement improved the model by using AICc.
Heritabilities of telomere length and the change in telomere length
We constructed univariate Bayesian “animal models”72 with either the first early-life TL (n = 223) or the last (later-life) TL measurements of an individual as response variables (n = 226). Sex, population identity and age of TL measurement (continuous number of days) were included as fixed effects. TL was log10-transformed and fitted with a Gaussian error distribution using MCMCglmm62,73.
We then calculated the difference between the first and last TL measurements (total ΔTL, where negative ΔTL values indicate telomere shortening and positive values indicate lengthening) A third univariate animal model was thus constructed with ΔTL as the response variable (n = 226) and ∆time (number of days between the two TL measurements, to account for differences in sampling time intervals), sex and population identity as fixed effects.
For all three models, we estimated variance components for additive genetic effects (‘animal’, VA), brood identity (VB), and hatch year (VY). Heritabilities were calculated as the proportion of phenotypic variance (VP) explained by additive genetic variance: \({h}^{2}=\frac{{V}_{A}}{{V}_{A}+{{V}_{B}+V}_{Y}+{V}_{R}}\), where VR is the residual variance. We used inverse-Wishart priors for the random effects and residual variance73. The MCMC chain was run for 1,000,000 iterations, sampling every 300 iterations after a burn-in of 10% (100,000 iterations). All autocorrelation values were < 0.05 and effective sample sizes were > 2500. Mixing and stationarity was checked visually and using Heidelberger and Welch's convergence test74. Estimates are reported as posterior modes with lower and upper 95% highest posterior density intervals (HPD).
Factors affecting the change in telomere length
We examined factors affecting the difference between all consecutive pairs of TL measurements (i.e. ΔTL from first to second TL measurement, ΔTL from second to third TL measurement etc., n = 301 ΔTL estimates in total) within individuals with multiple TL measurements (n = 220 individuals, excluding 6 individuals with missing tarsus length measurements) using LMMs (lme4). We fitted sets of candidate models with ΔTL as response variable. As fixed effects, we included combinations of ∆time, age-standardized nestling tarsus length, population identity and sex. As random effects, we included individual identity (to account for multiple ΔTL measurements for individuals with > 2 TL samples, n = 61) and hatch year in all models. Brood identity (n = 174 broods) explained a negligible amount of variance and was excluded to reduce model complexity and facilitate model convergence. Candidate models were then compared using AICc.
Results
Changes in telomere length with age
We observed both decreases, stasis, and increases in measured TL with age (Fig. 1 and Fig. S2). After within-subject centering of age, the best model describing the relationship between TL and age including all individuals showed a negative effect of ∆agelog (β∆age_log = −0.019 ± 0.007, CI = [−0.032, −0.006], n = 2977, ∆AICc = 1.8 compared to the second-best model, Table 1 and Table S2) and a negative effect of mean age (βmean_age_log = −0.030 ± 0.006, CI = [−0.043, −0.017]). There was no evidence that these two slopes were different (β = −0.011 ± 0.009, CI = [−0.028, 0.007]), suggesting that the decrease in TL with age could be attributed to within-individual telomere shortening.
(a) Changes in individual telomere length with age in days since hatching in 2667 house sparrows (2977 measurements) sampled across 24 years. Males are shown in black and females in grey. 226 birds were measured at least twice during their lifetime and measurements on the same individual are connected by lines. The oldest sampled individual was 9 years. (b) Only individuals with multiple telomere length measurements are shown here for clarity (226 individuals with 536 measurements).
When only including individuals with longitudinal (multiple) TL measurements (Fig. 1b) the composition of the best model was identical to the above, but the among individual effect of mean age was now uncertain and close to zero (βmean_age_log = 0.009 ± 0.016, CI = [−0.021, 0.040]), ∆AICc = 1.3, Table 1 and Table S3). We thus found some evidence for a difference between the within- and among-individual effects (β = 0.025 ± 0.017, CI = [−0.008, 0.058], with a CI overlapping zero), which indicates selective disappearance of birds with short TL and/or faster telomere attrition rates through life.
Repeatability of telomere length
The adjusted repeatability of log10-transformed TL was found to be 0.092 ± 0.049 (CI = [0.000, 0.194]), which means that 9.2% of the variation in longitudinal TL measurements was explained by within-individual consistency.
Correlation between early- and later-life telomere length
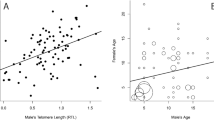
The average follow-up time (∆time) from the first to the second sampled TL measurement was 269 ± 370 SD days. Including the first TL measurement improved the model explaining variation in the second (subsequent) TL measurement (∆AICc = 4.9). There was a positive association between the first and second TL measurements (βlog10(first TL) = 0.234 ± 0.089, CI = [0.062, 0.408], Table 2 and Fig. 2). This means that individuals with long early-life TL also had long later-life TL, but with considerable individual variation (Fig. 2).
First telomere length mainly measured in nestlings plotted against the second telomere length measured in later-life (juvenile or adult) in house sparrows (n = 226). The regression line (black) reflects the estimate from Table 2 and 95% confidence intervals are shown in grey.
Correlation between the change in telomere length and early-life telomere length
The average follow-up time (∆time) from the first to the last TL measurement was 345 ± 406 SD days. After correcting for regression-to-the-mean effects, including the first TL measurement in the model describing variation in D (i.e. corrected ∆TL) did not improve the model (∆AICc = −1.7) and therefore there was no evidence for a dependency of telomere attrition on early-life TL (βfirst TL = 0.052 ± 0.077, CI = [−0.099, 0.201]).
Heritabilities of telomere length and the change in telomere length
The three univariate animal models (Table 3) revealed additive genetic variances (VA) for early-life TL (VA = 0.0182, HPD = [0.0131, 0.0235]), later-life TL (VA = 0.0205, HPD = [0.0150, 0.0272]), and ∆TL (VA = 0.0531, HPD = [0.0343, 0.0838]). The heritabilities were similar for early-life TL (h2 = 0.1358, HPD = [0.0804, 0.2090]) and later-life TL (h2 = 0.1542, HPD = [0.0917, 0.2268]), but somewhat higher for ∆TL (h2 = 0.2121, HPD = [0.1194, 0.3153]). The main source of variation in all three TL traits was hatch year, explaining 60% of the variation in early-life TL, 53% in later-life TL, and 37% in ∆TL. Brood effects explained 14–16% of the variation in each TL trait.
Factors affecting the change in telomere length
The model describing variation in all ΔTL measurements that only included the intercept was almost indistinguishable (∆AICc = 0.2) from the highest ranked model (Table S4). The highest ranked model included population identity, which indicated a tendency towards higher ΔTL in the Hestmannøy population (βpopulation[Hestmannøy] = 0.105 ± 0.072, CI = [−0.034, 0.248]), but with a CI overlapping zero. That is, individuals in the Hestmannøy population may tend to experience less telomere shortening over time than individuals from the Træna population (Fig. 3).
The change in telomere length (∆TL) across all measures of TL changes (n = 310 in total, n = 276 from Hestmannøy and n = 34 from Træna) within 226 individuals in two house sparrow populations. Negative ∆TL values indicate telomere shortening and positive ∆TL values indicate telomere lengthening. Red bars show 95% confidence intervals based on the t-distribution around the sample means.
Discussion
In accordance with many, but not all, studies on animals22,75,76 we found evidence for age-related telomere shortening within house sparrows (Fig. 1 and Table 1), as expected given the somatic costs associated with biological ageing and cumulative stress experiences (e.g.15,17). In common with other non-mammalian vertebrates, birds have nucleated erythrocytes. Therefore, TLs derived from whole blood samples are mainly measured in erythrocytes, which are normally produced in the bone marrow77. Compared to other tetrapods, avian erythrocytes have a relatively short lifespan of 1 month in vivo (28 days in house sparrows78) with ~ 3% being replaced each day79. Thus, we may expect to observe changes in blood TL in house sparrows over periods within days or weeks, while other less proliferative tissues may experience substantially less TL attrition80.
The low within-individual repeatability in TL measurements observed in this study (9.2%) was similar to other qPCR studies with large sample sizes26,81,82,83 and reflects the consistency in TL within individuals over the lifespan. Some studies using the TRF method have reported higher repeatabilities than qPCR studies (in different species, e.g.47,68,84), which may in part be attributed to shorter follow-up times and higher measurement error of qPCR, which will decrease repeatability85. We may expect low TL repeatability when including early-life stages when telomere shortening rates are expected to be most variable (85, but see22). Nevertheless, the first TL measurement predicted subsequent TL measurements within individuals, with individuals with a short early-life TL having a short TL later in life (Fig. 2). This suggests that the negative effects of growth48, environmental stressors50 and inbreeding86 on early-life TL previously described in these populations may have lasting effects on TL later in life19,84. Recent studies have found a positive genetic correlation close to 1 between TL measurements within individuals, suggesting that the same genes are involved in controlling TL at different ages32,42,47. However, our sample size was smaller than previous studies and we lack the sufficient power to estimate such genetic correlations with high precision and accuracy (e.g.87).
The heritability estimate for ∆TL (h2 = 0.21) was higher than that reported for ∆TL in western jackdaws (h2 = 0.0947), but in our study the follow-up times across all TL measurements (∆time) were much longer and more variable (25 days in Bauch et al.47 vs. 343 ± 410 SD days in this study). Correspondingly, heritability of TL shortening in humans was found to be even higher (h2 = 0.2845) in a study that had even longer follow-up times (on average 12 years). We found a considerable effect of hatch year, which explained 37% of the variance in ∆TL (vs. 4% in47). This may reflect annual environmental variation experienced by different cohorts in early-life such as weather conditions and competition43,50 and suggests that there are persistent impacts of the early-life environment on TL shortening later in life19.
The heritability estimates for early-life TL and later-life TL were of similar magnitude (h2 ~ 0.15, Table 3), but much smaller than in the jackdaw and human studies45,47. We previously estimated the early-life TL heritability for a much larger sample of nestlings from the same populations to be smaller (h2 = 0.04, n = 266243). However, the sample in the present study only included individuals surviving until the time of the second TL measurement (as juveniles or adults). This may bias the TL heritability estimates if individuals are not missing at random with respect to the trait of interest88; e.g. if mortality (and hence missingness) depends on TL21, the distributional properties of the sampled individuals may differ from the whole population and lead to biased inferences. It is tempting to suggest that the lower heritability estimates of TL compared to ∆TL reflects a closer association between fitness and TL or the environmental conditions that TL reflects (e.g.89). Indeed, TL may be unlikely to become critically short in house sparrows48, and the early-life environment has strong influences on both TL and ∆TL, as shown in this study.
We have previously found some evidence for a negative association between early-life TL and annual reproductive success in house sparrows50. We speculated that telomere shortening later in life depended upon early-life TL to explain this pattern. However, in this study we found no evidence that early-life TL was associated with telomere shortening rates (when correcting for regression-to-the-mean—see “Methods”). Thus, individuals with short early-life TL may indeed exhibit a faster life-history involving a higher reproductive output and lower somatic maintenance50,90,91. In this study, we found weak evidence for selective disappearance of individuals (that survived fledgling and/or juvenile stages) with short telomeres (or faster telomere attrition rates), which has been observed in longitudinal studies in several species of wild birds and mammals27,32,68,82,83,92. Thus, TL measured in adulthood, or telomere attrition rate40, but not early-life TL50, may reveal the expected relationships between telomere dynamics and mortality21, but future studies are needed that more comprehensively investigate the associations between TL, ∆TL and fitness components.
House sparrows on the island of Træna tended to experience greater telomere shortening (i.e. more negative values of ∆TL) than those on Hestmannøy (Fig. 3), but the evidence for this effect was weak as the more parsimonious intercept model was almost indistinguishable from the highest ranked model (Table S4). Individuals experiencing more stressful conditions, such as harsh abiotic conditions, competition, parasite infection, anthropogenic effects and/or poor diet, have been shown to exhibit increased rates of telomere shortening in several species17. We have previously shown that early-life TL in nestling sparrows on Træna was more negatively affected by higher conspecific population densities than in the Hestmannøy population50. In line with this, we now find that the Træna population overall tends to exhibit higher rates of telomere shortening. However, further studies on multiple populations are required to disentangle the specific (environmental) effects shaping such population differences93. Contrasting intraspecific TL dynamics have also been found in different populations of European roe deer (Capreolus capreolus) whose habitats differ in food availability94, in great tits (Parus major) living in urban or rural environments where diet composition differs95, in American redstarts (Setophaga ruticilla) overwintering in different non-breeding habitat types that also vary in food availability96, in pied flycatchers (Ficedula hypoleuca) breeding in different habitats across Europe97, and in populations of spotted snow skinks (Niveoscincus ocellatus98), common lizards (Zootoca vivipara99) and moose (Alces alces100) experiencing different thermal environments.
Our study highlights the plastic nature of telomere length, which may both shorten and lengthen with time within individuals (e.g.101,102,103). Telomere lengthening has been thought to represent measurement error104, but recent studies have produced evidence that telomere lengthening occurs in several species25,26,36,71,83,105,106,107,108. Telomeres may lengthen due to the activity of the enzyme telomerase109 and other mechanisms (e.g.110,111). We identified one individual (a female from the Hestmannøy population) that showed significant consistent telomere elongation throughout life at a greater rate than might have been expected by measurement error (see Supporting Information). Recent studies on house sparrows in other populations have also showed instances of telomere lengthening within some individuals90,112 and that house sparrows may experience rather transient TL shortening in response to stressors113. However, TL also generally declined with age in another long-term study of house sparrows22,90.
Somatic telomerase activity has been detected in tissues of some species, including birds114, but is generally thought to be repressed in large bodied and long-lived species as a mechanism of tumor suppression115,116,117. However, little is known about the energetic costs of TL maintenance16,118,119, and telomerase activity and telomere maintenance are not well-known within house sparrows. For instance, cycloastrogenol (TA‐65120), which activates telomerase and lengthens telomeres in blood in mice (Mus musculus121), humans (Homo sapiens122), zebra finches (Taenopygia guttata123), and tree swallows (Tachycineta bicolor124) was found to shorten telomeres in blood in house sparrow fledglings125. Experimental manipulations of TL or telomerase activity123,126,127 may be necessary to further our understanding of the causal role of telomere dynamics in shaping organismal life-histories119.
In conclusion, we found evidence of general telomere shortening with age within individuals, but also several instances of apparent telomere lengthening and at least one case of consistent lengthening through life in wild house sparrows. Early-life TL predicted later-life TL, but the change in TL was independent of early-life TL. There was a moderate heritability of ∆TL, which was higher than the heritability of TL, but most of the variation in both ∆TL and TL was explained by cohort effects. Furthermore, we found indications of population differences in ∆TL that may be linked to habitat differences. Combined, our study indicates that telomere dynamics are influenced by both genetic and environmental variation, and that TL may be more phenotypically flexible within individuals than previously anticipated.
Data availability
Data is available on the Open Science Framework (OSF) https://doi.org/10.17605/OSF.IO/4CJ3S.
References
Blackburn, E. H. Structure and function of telomeres. Nature 350, 569–573. https://doi.org/10.1038/350569a0 (1991).
Levy, M. Z., Allsopp, R. C., Futcher, A. B., Greider, C. W. & Harley, C. B. Telomere end-replication problem and cell aging. J. Mol. Biol. 225, 951–960. https://doi.org/10.1016/0022-2836(92)90096-3 (1992).
von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 27, 339–344 (2002).
Reichert, S. & Stier, A. Does oxidative stress shorten telomeres in vivo? A review. Biol. Lett. 13, 20170463. https://doi.org/10.1098/rsbl.2017.0463 (2017).
Armstrong, E. & Boonekamp, J. Does oxidative stress shorten telomeres in vivo?. Ageing Res. Rev. https://doi.org/10.1016/j.arr.2023.101854 (2023).
Boonekamp, J. J., Bauch, C., Mulder, E. & Verhulst, S. Does oxidative stress shorten telomeres?. Biol. Let. 13, 20170164. https://doi.org/10.1098/rsbl.2017.0164 (2017).
Greider, C. W. Telomeres and senescence: The history, the experiment, the future. Curr. Biol. 8, R178–R181. https://doi.org/10.1016/S0960-9822(98)70105-8 (1998).
Aubert, G. & Lansdorp, P. M. Telomeres and aging. Physiol. Rev. 88, 557–579. https://doi.org/10.1152/physrev.00026.2007 (2008).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039 (2013).
Vaiserman, A. & Krasnienkov, D. Telomere length as a marker of biological age: State-of-the-art, open issues, and future perspectives. Front. Genet. 11, 630186–630186. https://doi.org/10.3389/fgene.2020.630186 (2021).
Monaghan, P. Organismal stress, telomeres and life histories. J. Exp. Biol. 217, 57–66. https://doi.org/10.1242/jeb.090043 (2014).
Bateson, M. & Poirier, C. Can biomarkers of biological age be used to assess cumulative lifetime experience?. Anim. Welf. 28, 41–56. https://doi.org/10.7120/09627286.28.1.041 (2019).
Renieri, E., Vakonaki, E., Karzi, V., Fragkiadaki, P. & Tsatsakis, A.M. Chapter 26—Telomere length: Associations with nutrients and xenobiotics. in Toxicological Risk Assessment and Multi-System Health Impacts from Exposure (ed. Tsatsakis, A.M.). 295–306 (Academic Press, 2021).
Sudyka, J. Does reproduction shorten telomeres? Towards integrating individual quality with life-history strategies in telomere biology. BioEssays 41, e1900095. https://doi.org/10.1002/bies.201900095 (2019).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 101, 17312. https://doi.org/10.1073/pnas.0407162101 (2004).
Monaghan, P. & Ozanne, S. E. Somatic growth and telomere dynamics in vertebrates: Relationships, mechanisms and consequences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160446. https://doi.org/10.1098/rstb.2016.0446 (2018).
Chatelain, M., Drobniak, S. M. & Szulkin, M. The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecol. Lett. 23, 381–398. https://doi.org/10.1111/ele.13426 (2020).
Salmón, P., Millet, C., Selman, C. & Monaghan, P. Growth acceleration results in faster telomere shortening later in life. Proc. R. Soc. B Biol. Sci. 288, 20211118. https://doi.org/10.1098/rspb.2021.1118 (2021).
Marasco, V., Smith, S. & Angelier, F. How does early-life adversity shape telomere dynamics during adulthood? Problems and paradigms. BioEssays 44, 2100184. https://doi.org/10.1002/bies.202100184 (2022).
Eastwood, J. R. et al. Early-life telomere length predicts lifespan and lifetime reproductive success in a wild bird. Mol. Ecol. 28, 1127–1137. https://doi.org/10.1111/mec.15002 (2019).
Wilbourn, R. V. et al. The relationship between telomere length and mortality risk in non-model vertebrate systems: A meta-analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160447. https://doi.org/10.1098/rstb.2016.0447 (2018).
Remot, F. et al. Decline in telomere length with increasing age across non-human vertebrates: A meta-analysis. Mol. Ecol. https://doi.org/10.1111/mec.16145 (2021).
Tricola, G. M. et al. The rate of telomere loss is related to maximum lifespan in birds. Philos. Trans. R. Soc. B Biol. Sci. 373, 20160445. https://doi.org/10.1098/rstb.2016.0445 (2018).
Sauer, D. J., Heidinger, B. J., Kittilson, J. D., Lackmann, A. R. & Clark, M. E. No evidence of physiological declines with age in an extremely long-lived fish. Sci. Rep. 11, 9065. https://doi.org/10.1038/s41598-021-88626-5 (2021).
Vernasco, B. J. et al. Longitudinal dynamics and behavioural correlates of telomeres in male wire-tailed manakins. Funct. Ecol. https://doi.org/10.1111/1365-2435.13715 (2020).
Spurgin, L. G. et al. Spatio-temporal variation in lifelong telomere dynamics in a long-term ecological study. J. Anim. Ecol. 87, 187–198. https://doi.org/10.1111/1365-2656.12741 (2018).
Salomons, H. M. et al. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B Biol. Sci. 276, 3157–3165. https://doi.org/10.1098/rspb.2009.0517 (2009).
Frenck, R. W., Blackburn, E. H. & Shannon, K. M. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. 95, 5607. https://doi.org/10.1073/pnas.95.10.5607 (1998).
Alonso-Alvarez, C., Bertrand, S., Faivre, B. & Sorci, G. Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct. Ecol. 21, 873–879. https://doi.org/10.1111/j.1365-2435.2007.01300.x (2007).
Dugdale, H. L. & Richardson, D. S. Heritability of telomere variation: It is all about the environment!. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160450. https://doi.org/10.1098/rstb.2016.0450 (2018).
Martens, D. S. et al. Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. EBioMedicine https://doi.org/10.1016/j.ebiom.2020.103164 (2021).
Froy, H. et al. Heritable variation in telomere length predicts mortality in Soay sheep. Proc. Natl. Acad. Sci. 118, e2020563118. https://doi.org/10.1073/pnas.2020563118 (2021).
Verhulst, S., Aviv, A., Benetos, A., Berenson, G. S. & Kark, J. D. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for “regression to the mean”. Eur. J. Epidemiol. 28, 859–866. https://doi.org/10.1007/s10654-013-9845-4 (2013).
Atema, E., van Noordwijk, A. J. & Verhulst, S. Telomere dynamics in relation to experimentally increased locomotion costs and fitness in great tits. Mol. Ecol. https://doi.org/10.1111/mec.16162 (2021).
Nordfjäll, K. et al. The individual blood cell telomere attrition rate is telomere length dependent. Plos Genet 5, e1000375. https://doi.org/10.1371/journal.pgen.1000375 (2009).
Grasman, J., Salomons, H. M. & Verhulst, S. Stochastic modeling of length-dependent telomere shortening in Corvus monedula. J. Theor. Biol. 282, 1–6. https://doi.org/10.1016/j.jtbi.2011.04.026 (2011).
op den Buijs, J., van den Bosch, P.P.J., Musters, M.W.J.M. & van Riel, N.A.W.,. Mathematical modeling confirms the length-dependency of telomere shortening. Mech. Ageing Dev. 125, 437–444. https://doi.org/10.1016/j.mad.2004.03.007 (2004).
Fairlie, J. & Harrington, L. Enforced telomere elongation increases the sensitivity of human tumour cells to ionizing radiation. DNA Repair 25, 54–59. https://doi.org/10.1016/j.dnarep.2014.11.005 (2015).
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365. https://doi.org/10.1038/ncb2466 (2012).
Wood, E. M. & Young, A. J. Telomere attrition predicts reduced survival in a wild social bird, but short telomeres do not. Mol. Ecol. 28, 3669–3680. https://doi.org/10.1111/mec.15181 (2019).
Boonekamp, J. J., Mulder, G. A., Salomons, H. M., Dijkstra, C. & Verhulst, S. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. Biol. Sci. 281, 20133287. https://doi.org/10.1098/rspb.2013.3287 (2014).
Vedder, O. et al. Telomere length is heritable and genetically correlated with lifespan in a wild bird. Mol. Ecol. https://doi.org/10.1111/mec.15807) (2021).
Pepke, M. L. et al. Genetic architecture and heritability of early-life telomere length in a wild passerine. Mol. Ecol. 31, 6360–6381. https://doi.org/10.1111/mec.16288 (2022).
Chik, H. Y. J., Sparks, A. M., Schroeder, J. & Dugdale, H. L. A meta-analysis on the heritability of vertebrate telomere length. J. Evol. Biol. 35, 1283–1295. https://doi.org/10.1111/jeb.14071 (2022).
Hjelmborg, J. B. et al. The heritability of leucocyte telomere length dynamics. J. Med. Genet. 52, 297. https://doi.org/10.1136/jmedgenet-2014-102736 (2015).
Broer, L. et al. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet. 21, 1163–1168. https://doi.org/10.1038/ejhg.2012.303 (2013).
Bauch, C., Boonekamp, J. J., Korsten, P., Mulder, E. & Verhulst, S. High heritability of telomere length and low heritability of telomere shortening in wild birds. Mol. Ecol. https://doi.org/10.1111/mec.16183 (2021).
Pepke, M. L. et al. Artificial size selection experiment reveals telomere length dynamics and fitness consequences in a wild passerine. Mol. Ecol. 31, 6224–6238. https://doi.org/10.1111/mec.16340 (2022).
Araya-Ajoy, Y. G. et al. Variation in generation time reveals density regulation as an important driver of pace-of-life in a bird metapopulation. Ecol. Lett. 24, 2077–2087. https://doi.org/10.1111/ele.13835 (2021).
Pepke, M. L. et al. Causes and consequences of variation in early-life telomere length in a bird metapopulation. Ecol. Evol. 12, e9144. https://doi.org/10.1002/ece3.9144 (2022).
Remot, F. et al. No sex differences in adult telomere length across vertebrates: A meta-analysis. R. Soc. Open Sci. 7, 200548. https://doi.org/10.1098/rsos.200548 (2020).
Anderson, T.R. Biology of the Ubiquitous House Sparrow: From Genes to Populations. 547 (Oxford University Press, 2006).
Jensen, H., Steinsland, I., Ringsby, T. H. & Sæther, B. E. Evolutionary dynamics of a sexual ornament in the house sparrow (Passer domesticus): The role of indirect selection within and between sexes. Evolution 62, 1275–1293. https://doi.org/10.1111/j.1558-5646.2008.00395.x (2008).
Fransson, T., Jansson, L., Kolehmainen, T., Kroon, C. & Wenninger, T. EURING List of Longevity Records for European Birds (2017).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res. https://doi.org/10.1093/nar/30.10.e47 (2002).
Criscuolo, F. et al. Real-time quantitative PCR assay for measurement of avian telomeres. J. Avian Biol. 40, 342–347. https://doi.org/10.1111/j.1600-048X.2008.04623.x (2009).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19. https://doi.org/10.1186/gb-2007-8-2-r19 (2007).
Billing, A. M. et al. Evidence of inbreeding depression but not inbreeding avoidance in a natural house sparrow population. Mol. Ecol. 21, 1487–1499. https://doi.org/10.1111/j.1365-294X.2012.05490.x (2012).
Ockendon, N., Griffith, S. C. & Burke, T. Extrapair paternity in an insular population of house sparrows after the experimental introduction of individuals from the mainland. Behav. Ecol. 20, 305–312. https://doi.org/10.1093/beheco/arp006 (2009).
Hsu, Y.-H., Schroeder, J., Winney, I., Burke, T. & Nakagawa, S. Costly infidelity: Low lifetime fitness of extra-pair offspring in a passerine bird. Evolution 68, 2873–2884. https://doi.org/10.1111/evo.12475 (2014).
Hadfield, J. D., Richardson, D. S. & Burke, T. Towards unbiased parentage assignment: Combining genetic, behavioural and spatial data in a Bayesian framework. Mol. Ecol. 15, 3715–3730. https://doi.org/10.1111/j.1365-294X.2006.03050.x (2006).
Hadfield, J. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 1, 1–22. https://doi.org/10.18637/jss.v033.i02 (2010).
van de Pol, M. & Verhulst, S. Age-dependent traits: A new statistical model to separate within- and between-individual effects. Am. Nat. 167, 766–773. https://doi.org/10.1086/503331 (2006).
van de Pol, M. & Wright, J. A simple method for distinguishing within-versus between-subject effects using mixed models. Anim. Behav. 77, 753 (2009).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
Hurvich, C. M. & Tsai, C.-L. Regression and time series model selection in small samples. Biometrika 76, 297–307. https://doi.org/10.1093/biomet/76.2.297 (1989).
R Core Team. R: A Language and Environment for Statistical Computing. 4.2.0 Ed. (R Foundation for Statistical Computing, 2022).
Bichet, C. et al. Telomere length is repeatable, shortens with age and reproductive success, and predicts remaining lifespan in a long-lived seabird. Mol. Ecol. 29, 429–441. https://doi.org/10.1111/mec.15331 (2020).
Nakagawa, S. & Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. 85, 935–956. https://doi.org/10.1111/j.1469-185X.2010.00141.x (2010).
Stoffel, M. A., Nakagawa, S. & Schielzeth, H. rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. https://doi.org/10.1111/2041-210X.12797 (2017).
Simons, M. J. P., Stulp, G. & Nakagawa, S. A statistical approach to distinguish telomere elongation from error in longitudinal datasets. Biogerontology 15, 99–103. https://doi.org/10.1007/s10522-013-9471-2 (2014).
Kruuk, L. E. B. Estimating genetic parameters in natural populations using the “animal model”. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 359, 873–890. https://doi.org/10.1098/rstb.2003.1437 (2004).
Hadfield, J. MCMCglmm Course Notes. http://cran.r-project.org/web/packages/MCMCglmm/vignettes/CourseNotes.pdf (2019).
Heidelberger, P. & Welch, P. D. Simulation run length control in the presence of an initial transient. Oper. Res. 31, 1109–1144. https://doi.org/10.1287/opre.31.6.1109 (1983).
Dantzer, B. & Fletcher, Q. E. Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp. Gerontol. 71, 38–47 (2015).
Louzon, M., Coeurdassier, M., Gimbert, F., Pauget, B. & de Vaufleury, A. Telomere dynamic in humans and animals: Review and perspectives in environmental toxicology. Environ. Int. 131, 105025. https://doi.org/10.1016/j.envint.2019.105025 (2019).
Stier, A., Reichert, S., Criscuolo, F. & Bize, P. Red blood cells open promising avenues for longitudinal studies of ageing in laboratory, non-model and wild animals. Exp. Gerontol. 71, 118–134. https://doi.org/10.1016/j.exger.2015.09.001 (2015).
Gillooly, J. F., Hayward, A., Hou, C. & Burleigh, J. G. Explaining differences in the lifespan and replicative capacity of cells: A general model and comparative analysis of vertebrates. Proc. R. Soc. B Biol. Sci. 279, 3976–3980. https://doi.org/10.1098/rspb.2012.1129 (2012).
Glomski, C. A. & Pica, A. The Avian Erythrocyte: Its Phylogenetic Odyssey (CRC Press, 2016).
Daniali, L. et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 4, 1597. https://doi.org/10.1038/ncomms2602 (2013).
Sparks, A. M. et al. Telomere heritability and parental age at conception effects in a wild avian population. Mol. Ecol. https://doi.org/10.1111/mec.15804 (2021).
Fairlie, J. et al. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 15, 140–148. https://doi.org/10.1111/acel.12417 (2016).
van Lieshout, S. H. J. et al. Individual variation in early-life telomere length and survival in a wild mammal. Mol. Ecol. 28, 4152–4165. https://doi.org/10.1111/mec.15212 (2019).
Benetos, A. et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621. https://doi.org/10.1111/acel.12086 (2013).
Kärkkäinen, T., Briga, M., Laaksonen, T. & Stier, A. Within-individual repeatability in telomere length: A meta-analysis in nonmammalian vertebrates. Mol. Ecol. https://doi.org/10.1111/mec.16155 (2021).
Pepke, M. L. et al. Inbreeding is associated with shorter early-life telomere length in a wild passerine. Conserv. Genet. 23, 639–651. https://doi.org/10.1007/s10592-022-01441-x (2022).
Sodini, S. M., Kemper, K. E., Wray, N. R. & Trzaskowski, M. Comparison of genotypic and phenotypic correlations: Cheverud’s conjecture in humans. Genetics 209, 941. https://doi.org/10.1534/genetics.117.300630 (2018).
Steinsland, I., Larsen, C. T., Roulin, A. & Jensen, H. Quantitative genetic modeling and inference in the presence of nonignorable missing data. Evolution 68, 1735–1747. https://doi.org/10.1111/evo.12380 (2014).
Mousseau, T. A. & Roff, D. A. Natural selection and the heritability of fitness components. Heredity 59, 181–197. https://doi.org/10.1038/hdy.1987.113 (1987).
Heidinger, B. J., Kucera, A. C., Kittilson, J. D. & Westneat, D. F. Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Proc. R. Soc. B Biol. Sci. 288, 20210560. https://doi.org/10.1098/rspb.2021.0560 (2021).
Giraudeau, M., Angelier, F. & Sepp, T. Do telomeres influence pace-of-life-strategies in response to environmental conditions over a lifetime and between generations?. BioEssays 41, 1800162. https://doi.org/10.1002/bies.201800162 (2019).
Salmón, P., Nilsson, J. F., Watson, H., Bensch, S. & Isaksson, C. Selective disappearance of great tits with short telomeres in urban areas. Proc. R. Soc. B Biol. Sci. 284, 20171349. https://doi.org/10.1098/rspb.2017.1349 (2017).
Burraco, P., Lucas, P. M. & Salmón, P. Telomeres in a spatial context: a tool for understanding ageing pattern variation in wild populations. Ecography 2022, e05565. https://doi.org/10.1111/ecog.05565 (2022).
Wilbourn, R. V. et al. Age-dependent associations between telomere length and environmental conditions in roe deer. Biol. Lett. 13, 20170434. https://doi.org/10.1098/rsbl.2017.0434 (2017).
Salmón, P., Nilsson, J. F., Nord, A., Bensch, S. & Isaksson, C. Urban environment shortens telomere length in nestling great tits, Parus major. Biol. Lett. 12, 20160155. https://doi.org/10.1098/rsbl.2016.0155 (2016).
Angelier, F., Vleck, C. M., Holberton, R. L. & Marra, P. P. Telomere length, non-breeding habitat and return rate in male American redstarts. Funct. Ecol. 27, 342–350. https://doi.org/10.1111/1365-2435.12041 (2013).
Kärkkäinen, T. et al. Population differences in the length and early-life dynamics of telomeres among European pied flycatchers. Mol. Ecol. https://doi.org/10.1111/mec.16312 (2021).
Fitzpatrick, L. J., Olsson, M., Pauliny, A., While, G. M. & Wapstra, E. Individual telomere dynamics and their links to life history in a viviparous lizard. Proc. R. Soc. B Biol. Sci. 288, 20210271. https://doi.org/10.1098/rspb.2021.0271 (2021).
Dupoué, A. et al. Lizards from warm and declining populations are born with extremely short telomeres. Proc. Natl. Acad. Sci. 119, e2201371119. https://doi.org/10.1073/pnas.2201371119 (2022).
Fohringer, C. et al. Large mammal telomere length variation across ecoregions. BMC Ecol. Evolut. 22, 105. https://doi.org/10.1186/s12862-022-02050-5 (2022).
Hares, M. C. et al. Telomere dynamics in wild banded mongooses: Evaluating longitudinal and quasi-longitudinal markers of senescence. Exp. Gerontol. 107, 67–73. https://doi.org/10.1016/j.exger.2017.09.021 (2018).
Criscuolo, F., Pillay, N., Zahn, S. & Schradin, C. Seasonal variation in telomere dynamics in African striped mice. Oecologia 194, 609–620. https://doi.org/10.1007/s00442-020-04801-x (2020).
Svenson, U. et al. Blood cell telomere length is a dynamic feature. PLoS ONE 6, e21485. https://doi.org/10.1371/journal.pone.0021485 (2011).
Steenstrup, T., Hjelmborg, J. V. B., Kark, J. D., Christensen, K. & Aviv, A. The telomere lengthening conundrum—Artifact or biology?. Nucleic Acids Res. 41, e131–e131 (2013).
Bateson, M. & Nettle, D. The telomere lengthening conundrum—It could be biology. Aging Cell 16, 312–319. https://doi.org/10.1111/acel.12555 (2017).
Brown, T. J. et al. Causes and consequences of telomere lengthening in a wild vertebrate population. Mol. Ecol. https://doi.org/10.1111/mec.16059 (2021).
McLennan, D. et al. Telomere elongation during early development is independent of environmental temperatures in Atlantic salmon. J Exp. Biol. 221, 178616. https://doi.org/10.1242/jeb.178616 (2018).
Hoelzl, F. et al. Telomeres are elongated in older individuals in a hibernating rodent, the edible dormouse (Glis glis). Sci. Rep. 6, 36856. https://doi.org/10.1038/srep36856 (2016).
Shay, J. W. & Wright, W. E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 20, 299–309. https://doi.org/10.1038/s41576-019-0099-1 (2019).
Cesare, A. J. & Reddel, R. R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 11, 319. https://doi.org/10.1038/nrg2763 (2010).
Lanna, A. et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat. Cell Biol. https://doi.org/10.1038/s41556-022-00991-z (2022).
Bennett, S. et al. Evidence of paternal effects on telomere length increases in early life. Front. Genet. 13, 1–9. https://doi.org/10.3389/fgene.2022.880455 (2022).
Young, R. C. et al. Stressors interact across generations to influence offspring telomeres and survival. Proc. R. Soc. B Biol. Sci. 289, 20220868. https://doi.org/10.1098/rspb.2022.0868 (2022).
Haussmann, M. F., Winkler, D. W., Huntington, C. E., Nisbet, I. C. & Vleck, C. M. Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp. Gerontol. 42, 610–618. https://doi.org/10.1016/j.exger.2007.03.004 (2007).
Pepke, M. L. & Eisenberg, D. T. A. On the comparative biology of mammalian telomeres: Telomere length co-evolves with body mass, lifespan and cancer risk. Mol. Ecol. 31, 6286–6296. https://doi.org/10.1111/mec.15870 (2022).
Tian, X. et al. Evolution of telomere maintenance and tumour suppressor mechanisms across mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160443. https://doi.org/10.1098/rstb.2016.0443 (2018).
Pepke, M. L., Ringsby, T. H. & Eisenberg, D. T. A. The evolution of early‐life telomere length, pace‐of‐life and telomere‐chromosome length dynamics in birds. Mol. Ecol. 00, 1–15, https://doi.org/10.1111/mec.16907 (2023).
Eisenberg, D. T. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am. J. Hum. Biol. 23, 149–167. https://doi.org/10.1002/ajhb.21127 (2011).
Smith, S., Hoelzl, F., Zahn, S. & Criscuolo, F. Telomerase activity in ecological studies: What are its consequences for individual physiology and is there evidence for effects and trade-offs in wild populations. Mol. Ecol. https://doi.org/10.1111/mec.16233 (2021).
Yu, Y., Zhou, L., Yang, Y. & Liu, Y. Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp. Ther. Med. 16, 2175–2182. https://doi.org/10.3892/etm.2018.6501 (2018).
de Jesus, B. B. et al. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10, 604–621. https://doi.org/10.1111/j.1474-9726.2011.00700.x (2011).
Salvador, L. et al. A natural product telomerase activator lengthens telomeres in humans: A randomized, double blind, and placebo controlled study. Rejuvenation Res. 19, 478–484. https://doi.org/10.1089/rej.2015.1793 (2016).
Reichert, S. et al. Experimental increase in telomere length leads to faster feather regeneration. Exp. Gerontol. 52, 36–38. https://doi.org/10.1016/j.exger.2014.01.019 (2014).
Wolf, S. E., Stansberry, K. R., Content, K. R. & Rosvall, K. A. A putative telomerase activator has tissue-specific effects on telomere length in a developing songbird. J. Avian Biol. https://doi.org/10.1111/jav.02639 (2021).
Vangorder-Braid, J. T. et al. TA-65 does not increase telomere length during post-natal development in house sparrow chicks (Passer domesticus). J. Exp. Zool. Part A Ecol. Integr. Physiol. https://doi.org/10.1002/jez.2449 (2021).
Criscuolo, F., Smith, S., Zahn, S., Heidinger, B. J. & Haussmann, M. F. Experimental manipulation of telomere length: Does it reveal a corner-stone role for telomerase in the natural variability of individual fitness?. Philos. Trans. R Soc. Lond. B Biol. Sci. 373, 20160440. https://doi.org/10.1098/rstb.2016.0440 (2018).
Muñoz-Lorente, M. A., Cano-Martin, A. C. & Blasco, M. A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 10, 4723. https://doi.org/10.1038/s41467-019-12664-x (2019).
Acknowledgements
We thank all field workers, the island inhabitants, and laboratory technician Randi Røsbak for their contributions. This study was partly funded by the Research Council of Norway (274930) and through its Centres of Excellence scheme (223257).
Funding
Open access funding provided by Norwegian University of Science and Technology.
Author information
Authors and Affiliations
Contributions
M.L.P. measured telomeres, analyzed the data, and wrote the manuscript with contributions from all authors. T.H.R., H.J. and B.-E.S. designed the study system. W.B. and P.M. advised telomere measurements. T.K., H.J., J.W. and Y.G.A.-A. advised statistical analyses. T.H.R., H.J., T.K., P.S.R., Y.G.A.-A. and M.L.P. contributed to the fieldwork.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pepke, M.L., Kvalnes, T., Wright, J. et al. Longitudinal telomere dynamics within natural lifespans of a wild bird. Sci Rep 13, 4272 (2023). https://doi.org/10.1038/s41598-023-31435-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31435-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.