Abstract
The striped hyena (Hyaena hyaena) is considered “Near Threatened” globally and “Vulnerable” in the Middle East. In Israel, the species has experienced extreme population fluctuations owing to poisoning campaigns during the British Mandate (1918–1948) which were also further exacerbated by the Israeli authorities in the mid-twentieth century. We collated data from the archives of the Israel Nature and Parks Authority for the past 47 years to elucidate the temporal and geographic trends of this species. During this period we found a 68% increase in population and the estimated density is at present 2.1 individuals/100km2. This is significantly higher than all previous estimates for Israel. It appears that the major factors contributing to their phenomenal increase in number are the increase in prey availability because of the intensification of human development, preying on Bedouin livestock, the extinction of the leopard (Panthera pardus nimr), and the hunting of wild boars (Sus scorfa) and other agricultural pests in some parts of the country. Reasons should also be sought in increasing people's awareness as well as in advanced technological capabilities that have allowed an improved observation and reporting system. Future studies need to understand the effects of the large concentrations of striped hyenas on the spatial distribution and temporal activity of other sympatric wildlife to ensure the continued persistence of the wildlife guilds in the Israeli nature.
Similar content being viewed by others
Introduction
The striped hyena (Hyaena hyaena; here on hyena) is a large-bodied, facultative nocturnal scavenger1. Across most of its range the species occurs mostly in open habitats in arid to semi-arid environments2.
The hyena is considered to be globally “Near Threatened” by the International Union for Conservation of Nature (IUCN) Red List, and is reported with a decreasing global population trend3. However, regionally for the Middle East it is classified as “Vulnerable”1. From the conservation point of view the species is described as one that “experiences ongoing deliberate and incidental persecution coupled with a decrease in its prey base such that it may come close to meeting a continuing decline of 10% over the next three generations3.” The main causes of this projection is the abhorrence of this species among humans, including a plethora of superstitious beliefs, e.g., as grave robbers4, and/or damage to agriculture and livestock5,6,7. Therefore it is not surprising that various types of systemic and institutional action plans were implemented to reduce hyena population.
In Israel, the species has experienced extremes population declines owing to either targeted or accidental poisonings, and it especially susceptible to this because of their scavenging behavior8,9,10. Historically it was widespread throughout the country11 and in the nineteenth century was also very common12,13,14. However, it has been driven to the verge of extinction at the turn of the twentieth century15,16. It was the consequence of two temporal and geographically extensive incidents of poising occurred in Israel—the first during the British Mandate when between 1918 and 1948 the authorities placed strychnine poisoned donkey carcasses in order to control Golden Jackals (Canis aureus) to try and prevent rabies, but in the process also killed off a large number of hyenas3. And the second when Israeli authorities in the 1950s and 1960s assorted to extensive poisoning campaigns, resulting in many species either going regionally extinct or with greatly reduced populations8,9.
In the 1970s the estimated population consisted of ca. 130 individuals, i.e., the density for Israel with an area of 22,145 km2, was 0.06 hyenas/100 km217,18. In the 1990s the estimated population size in the Negev Desert was estimated to be > 0.016/km219. At the beginning of the twenty-first century, according to the Israeli Red Book the population size was estimated as ca. 200 individuals, i.e., 0.09/100 km220. However, Hadad21, estimated the population at ca. 400 individuals (0.18/100 km2), but then revised the number to ca. 1000 (0.45/100 km2;16). Further, the species is persecuted indiscriminately in the West Bank22,23, wherein it is killed for its body parts which are considered to have medicinal values, or is killed because of a range of superstitious beliefs connected to the underworld. Hence, the Striped Hyena populations both in Israel and the West Bank in the second half of the twentieth century were reported in relatively low numbers16,18,21.
Owing to the uncertainty of the status of the hyenas in Israel, and the different levels of human and ecological pressures that they experience across the length of the country, and based on a 47-year time series data, we developed a temporal and geographic model of the population trend of the species in Israel. We hypothesized that the termination of targeted persecution by the authorities in the first half of the twentieth century and the rapid urbanization of central and northern Israel would be conducive to reviving local hyena populations. Allowing that this is now the largest carnivore in the country that interacts frequently with humans, it is of conservation concern that the human attitudes towards this large carnivore remain positive.
Methods
Data collection
The data for estimating the population trend of the hyena in Israel and the West Bank were obtained from the Scientific Data Department of the Israel Nature and Parks Authority (INPA). The INPA was created in 1998 by merging two independent organizations—The National Parks Authority and The Nature Reserves Authority (NRA; https://en.parks.org.il/). The NRA was established in 1964 as the official arm of the Israeli Government to enforce wildlife protection laws. During the years 1975—2022, i.e., a total of 47 years, the INPA has detailed records of 5,860 observations of hyena (Fig. 1), which we have used in the present analyses. Initially, i.e., during 1975–2008, the observations recorded were those submitted by INPA rangers and citizens. From 2008, the INPA introduced the application from smartphone “Cyber Ranger” to report observation directly from the field.
Data processing
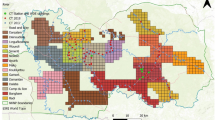
All observations were assigned geographical coordinates (Fig. 2A). However, the hyenas are not individually marked, and we were unable to control for the effect of reporting the same individual multiple times in a year. Thus, in order to balance the concentration of observations from urbanized areas with open areas (with low human activity) and at the same time to minimize the probability of population overestimation, we combined observations into time-spatial groups.
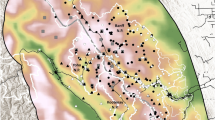
Map of Israel and the West Bank (A) with red dots marking the locations of the striped hyena (Hyaena hyaena) over 47 years of research, and 1255 analytical squares (blue); (B) analytical squares in which they were found at least once in 47 years; (C) the research area in which the density was precisely determined. The background is from Google Earth.
In the first step, we divided Israel and the West Bank into 5/5 km squares (Fig. 2A). As a result, we got 1255 analytical surfaces (the coordinates of the center of each square are shown in Online Appendix B). Next, separately for each of the 47 years, we assigned each observation to a given analytical surface assuming that two or more adults reported within a year and at a distance of not more than 1000 m, are sightings of the same individual (Fig. 2B). Using this approach, we believe that we have to some degree controlled the effect of randomness of observation, which is common in citizen science collected data.
Statistical analysis
To evaluate the trend analysis we used generalized additive mixed models GAMM where the number of individuals in the given grid cells (ygr) was considered as an additive effect of year and analytical surface (grid cells). In the modeling framework we used ‘year’ as the fixed factor (f(year)) and the id of grid cells as the random factor. The model was developed with Poisson distribution. The analysis was based on the ptrend procedure implemented in the poptrend library (for theoretical background of the analysis see Knape24).
We also used generalized additive mixed models GAMM25 to analyze not only in time (temporal) but also in space (geographical) population trends. Namely, the annual rate of population change was modeled as a function of the latitude and longitude of a given grid cell. In this way, apart from determining the population trend, we were able to indicate on a specific map for the variability of the population trend (positive/negative) in the whole of Israel. As the response variable we used the logarithm of the number of individuals of a given grid cell “g” in year “r”. This variable was modeled with a Poisson-Gamma distribution (pg). The ‘Year’ variable was added to the modeling framework as a fixed factor (f(year)) and as a continuous variable in interactive relation with the longitude and latitude (s(longitude, latitude, year)). Furthermore, the id of the grid cells (id_gc) was used as the random factor. The model was considered in two variants: spline s and linear l; such that the equation was:
where ygr—of number of individuals of a given grid cells g in year r, pg—Poisson-Gamma distribution, s—spline, gc—id of grid cell.
The differences between the two models were tested using likelihood ratio tests.
Furthermore, we also calculated annual population growth rate (λ) which is an exponential model and ensures the estimate of statistical growth of the population per year26.
where ny2—number of individuals in year 2, while ny1 means number of individuals in the year preceding ny2.
Based on the next GAMM we also estimated the species density. Response variable were expressed as the quotient of the population size in a given grid cell in year and the population trend obtained from the model described above. This variable was implemented in GAMM with Poisson-Gamma distribution where as predictor interactive and additive effect longitude and latitude and year were used. Based on this model we created predictive maps of the spatial density distribution.
Institutional review board statement
The study did not require ethical approval and was conducted in the framework of the Israel Nature & Parks Authority.
Ethics
The data is from the archives of the Scientific Data Department of the Israel Nature and Parks Authority, and is used under their jurisdiction. No animals were handled in the study.
Results
During the 47-year study, we recorded a total of 5,860 individuals in 723 grid cells out of 1255 that cover all of Israel and the West Bank. The mean value of individual per year was 131.2 (95% CL 77.63–184.99) while, per grid cell was 8.71 (95% CL 4.41–10.02). The fewest individuals were observed in 1976 and 1995 (1 and 3, respectively), while the most individuals were recorded in 2022 (703). We found a linear increasing trend in the number of reported individuals over the past 47 years (r = 0.68, p < 0.001, Fig. 1). All observations were assigned geographical coordinates (Fig. 2A) and illustrated that most observations were near urban agglomerations.
Trend population modeling based on GAMM with "smooth" fit (R2 = 0.294) showed that in Israel between 1975 and 2022 the hyena population increased on average by 68% (95% CL 10–161%). However, on a scale of 47 years, we identified a period of population decline between 1984 and 1990, in which the population decreased by − 16% (95% CL − 27 to − 3.8%) and in 2005–2022 the population increased by 156% (95% CL 87–245%; Figs. 3A, 4). The loglinear fit (Fig. 3B) also showed an increased population trend, but the model with the "smooth" fit was better (LSR test, chi-square = 34.76, P < 0.0001) than the linear. The annual population growth rate (λ) in the exponential model for the entire period = 1.53 (95% CL 0.96–2.10), indicating that on average the population increase by 53% per year.
Population trends of the striped hyena (Hyaena hyaena) in Israel and the West Bank based on GAMM with smooth (A) and loglinear fits (B). Error bars show the 95% confidence limits around the yearly estimates; black, red and green lines show smooth-fitted to the yearly point estimates, blue area represents 95% CL for the smooth fit.
The estimated density for the whole country is currently 2.1 individuals/100km2 (95% CL 0.3–6.2), but we found a large spatial variance (Fig. 5). However, the predicted density in the controlled study area (Fig. 2C) was 0.22 per/km2 (95% CL 0.20–0.25) and was slightly higher than the density for the whole country and lower than the real density found during field studies in this area (0.34 individuals/km2). However, the differences between real and predicted density were not statistically significant (chi-square = 0.018, p = 0.89).
Discussion
The hyena, despite being elusive, cryptic, nocturnal, and solitary1,27,28,29 and mainly found in semi-arid and arid regions18,30; in Israel, appears to have overcome most environmental restrictions and at present occupies all habitats31,32. They also appear to have overcome their fear of humans and have become human commensals33,34,35. We discovered that most observations were near urban agglomerations. We assume that this may be due to two reasons—first, increased hyena activity in specific areas owing to access to anthropogenic food base33. Extensive areas for human habitation and agriculture is accompanied by and large quantities of garbage dumps, resulting in not only the commensal carnivore populations increasing greatly36 but also an increase in body size, which was also reported for hyenas33. Furthermore, establishing of feeding stations (restaurants) for scavengers has allowed access to food for carnivores, including hyenas37. The second reason for the high activity of hyenas near urban agglomerations may be due from a methodological artifact where higher human activity in reporting observed animals in urban areas than in open areas.
Ilani17 reported that in Israel hyenas were either non-existent or extremely rare for a 10–25 km wide area along the Mediterranean coastline from Rafiah in the Gaza strip to Rosh Hanikra on the border with Lebanon; and also inland in the Jezreel Valley and the lower Galilee regions. The species also existed in low numbers in the Upper Galilee and the Golan Heights, and from the Gilboa Mountains and south along the rift valley, the numbers were small but stable.
In most Middle East countries, the status of the hyena ranges from “Vulnerable” to “Critically Endangered”4,38,39,40,41, including “Endangered” in Israel20. However, one should be very careful in changing this status to higher, e.g., based on the population growth trends we obtained. The population numbers are mainly dependent on habitat fragmentation of optimal habitats40,41 and human persecution in the form of poisoning4,8,9, illegal wildlife trade23,28, retaliatory killing39, collection of sexual organs for traditional medicines42, and a result from the disproportionately large rate of mortality as road kills10,28,42. Climate change also appears to influence hyena populations, but the influence on the Israeli population remains unknown43.
So the only solutionis similar to Ilani17, a country-wide monitoring survey to elucidate the actual regional densities and reassess the species status and how the authorities can ensure the continued persistence of a viable population in Israel. It is necessary because, despite our use of a complex analytical procedure and independent evaluation of our estimation, there is still the risk of under- or overestimating the population size. This study is based on archival data, albeit correct to the present because it is based on observations and not regular surveys. The assumption that two or more hyena sightings within a year at a distance of < 1000 m belong to the same individual may be potentially misleading given that hyenas exhibit a complex social structure44. However, this approach is the only solution to ensure that the population is estimated correctly. We came to this conclusion during a heuristic review of the data. The points lying < 1000 m apart is the supposed area of a single individual. Furthermore, as described before, the degree of interaction between individuals44 despite all may vary between seasons, something that has yet to be described for this species. Thus, no clear evidence exists that hyena sightings < 1000 m away belong to two or more individuals.
Next, the assignment post-hoc of observations to the analytical square and the predictive procedure also can generate random distortions that cannot be avoided in this kind of citizen science, especially since the data comes from a period of almost 50 years. Over the years, there has been a change in people's awareness. Advanced technological capabilities have allowed an improved observation (e.g., smartphone applications) and reporting system that has dramatically increased the number of reports filed. However, this also resulted in the same individuals being reported over the years with no ability to discern between them.
However, no matter the level of underestimation, there is no doubt that the hyena population is increasing in Israel. This is indirectly ground-proofed in the number of road-kills10 and other studies in specific regions16,18,19,21,30,31,32. The extensive fieldwork and ground-proofing of almost 4-decades supports this conclusion in the Judea region in central Israel10,15,16,21.
As mentioned earlier, the benign behavior of the hyenas and the fact that they do not endanger humans has resulted in some regions they are now tolerated to the point where they have become human commensals33,34,35. We suspect, this has also led to a lower perception of risk by the hyena, but it requires further study. We speculate that the rapid population growth is the consequence of intensification of human development—whether as the building of new towns and cities and expanding old ones, introducing new roads and infrastructure, and increasing the acreage of agricultural and silvicultural areas45. Furthermore, agricultural and industrial intensification produces much organic waste in the desert regions, which the hyenas exploit. Also, in the semi-arid regions, where the Bedouins allow their livestock to forage in the winters, this results in an abundance of domestic prey readily available for the hyenas46. The extinction of the leopard (Panthera pardus nimr)47,48,49 essentially leaves the hyena as the largest of the Carnivora in the country with minimal intra-specific competition53.
Similar to Shamon et al.32, we also found the highest density of hyenas in the Carmel Mountains in northern Israel. We assume this is probably a result of the increase in prey availability of “agricultural pests” in the region and the subsequent human-wildlife conflict50. Hunters were contracted to kill as many wild boars (Sus scorfa) and golden Jackal (Canis aureus) as possible and whose carcasses were not removed16. This rich resource allowed foraging carnivores, including hyenas, to establish substantial populations within a relatively small area quickly. However, unlike Panda et al.51 we are unable to elucidate the competitive inter- or intra-specific interactions with other carnivores.
Our results show that Israel’s hyena density is 2.1 individuals/100 km2. In central Kenya, the estimated minimum density was 0.3 adults/100 km22, while estimates for neighboring Lebanon were 0.11/100 km24. Several studies have also been conducted on striped hyena densities in central India (0.45/100 km2;52) and Gir National Park in Gujarat in western India (0.7/100 km2;53). However, the highest density was observed in Kumbhakgarh (6.5/100 km2) and at Esrana (3.7/100 km2;54), as well as at Ranthambore Tiger Reserve (5.5/100 km2), and the Sawai Mansingh Wildlife Sanctuary (12.0/100 km2;35).
In conclusion, we present the most recent evaluation of the hyena population in Israel and the West Bank and demonstrate that the species appears to have recovered dramatically from previous poisoning episodes, despite it being persecuted in certain regions by specific ethnic groups6,23,27,38,55, that it has rebounded impressively6,38,40. The hyenas have learned to exploit human waste and are not afraid of humans. However, the coexistence between the wild hyena population and humans must be treated with caution56. Their proximity to human habitation and lack of fear of humans can result in undesired conflicts in the future, similar to that occurring in the Mount Carmel region with wild boars50. The relevant authorities must indulge in education programs explaining the dangers of contact with wildlife and feeding them to ensure that public health is not susceptible and that the hyena populations are monitored for expansion and human exploitation. The authorities must try to nullify the anthropogenic influence on the hyena’s high densities and evaluate the environment’s actual carrying capacity for this large carnivore. The ramifications of these studies can also be projected to other areas of the species distribution57 and help the species persist in the wild across its range.
Data availability
The data is available directly from the author, Reuven Yosef, ryosef60@gmail.com.
References
Kruuk, H. Feeding and social behaviour of the striped hyaena (Hyaena vulgaris). East Afr. Wildl. J. 14, 91–111 (1976).
Wanger A.P. Behavioral ecology of the striped hyena (Hyaena hyaena) . Diss. Montana State University-Bozeman, College of Letters & Science (2006).
AbiSaid, M. & Dloniak, S.M.D. Hyaena hyaena. The IUCN Red List of Threatened Species 2015: e.T10274A45195080 (2015).
Abi-Said, M. R. & Abi-Said, D. M. Distribution of the striped hyena (Hyaena hyaena syriaca Matius, 1882) (Carnivora: Hyaenidae) in urban and rural areas of Lebanon. Zool. Middle East 42, 3–1 (2007).
Al Younis, J. S. Hyaenas in Eastern Jordan. IUCN Hyaena Spec. Group Newsl. 6, 2–3 (1993).
Qarqaz, M. A., Abu Baker, M. A. & Amr, Z. S. Status and ecology of the Striped Hyena, Hyaena hyaena, in Jordan. Zool. Middle East 33, 87–92 (2004).
Bhandari, S., Youlatos, D., Thapamagar, T. & Bhusal, D. R. Shrinking striped hyena (Hyaena hyaena Linnaeus, 1758) distribution in Nepal. Eur. J. Wildl. Res. 67, 1–4 (2021).
Mendelssohn, H. Mass destruction of bird-life by secondary poisoning from insecticides and rodenticides. Atlantic Nat. 17, 247–248 (1962).
Mendelssohn, H. The impact of pesticides on bird life in Israel. Bull. Int. Council Bird Preserv. 11, 75–104 (1972).
Hadad, E., Kosicki, J. Z. & Yosef R. Spatial modeling of road collisions of striped hyena (Hyaena hyaena) in Israel. Ecol. Res.
Ilani, G. Hyaenas in Israel. Israel Land Nat. 10–18 (1975).
Tristram, H. B. The land of Israel: A journal of travels in palestine. Society for Promoting Christian Knowledge, London. 657 pp (1866).
Schmitz, E. J. Wird Palästina wieder jüdisch werden?. Das Heilige Land 54, 92–95 (1910).
Schmitz, E. J. Kampf mit einem Leoparden. Das Heilige Land 56, 23–27 (1912).
Hadad, E. The persecution of the striped hyaena by humans. Teva HaDvarim 258, 68–78 (2017).
Hadad, E. 2021. Israel a heaven and a haven for striped hyaenas. Teva HaDvarim 310, 52–63 (2021).
Ilani, G. Zoogeographic survey of carnivores in Israel (Golan, Judea and Samaria, Sinai). Pp. 84—94 Unpublished Internal report, Israel Nature Reserves Authority (1979).
Skinner, J. D. & Ilani, G. The striped hyaena Hyaena hyaena of the Judean and Negev Deserts and a comparison with the brown hyaena H. brunnea. Israel J. Zool. 28, 229–232 (2013).
van Aarde, R. J., Skinner, J. D., Knight, M. H. & Skinner, D. C. Range use by a striped hyaena (Hyaena hyaena) in the Negev desert. J. Zool. 216, 575–577 (1988).
Dolev, A. & Pervolutzky, A. Endangered species in Israel. Red list of threatened species. Pp. 257 Hyaena hyaena (Linnaeus 1758). Nature & Parks Authority and SPNI, Keter Publishers, Jerusalem (2002).
Hadad, E. Striped hyaenas in Israel. Teva HaDvarim 232, 3–14 (2015).
Albada, I. M. Primary survey of the striped hyena, Hyaena hyaena, (Linnaeus, 1758) (Carnivora:Hyaenidae) status in the West Bank Governorates, Palestine. Glob. Scholast. Res. J. Mulitidiscip. 1, 39–44 (2015).
Handal, E. N., Qumsiehm, G. H., Hammash, S. Y. & Qumsiyeh, M. B. Status and conservation of the striped hyena (Hyaena hyaena) in the occupied Palestinian Territories (West Bank). Jordan J. Nat. History 6, 11–18 (2019).
Knape, J. Decomposing trends in Swedish bird populations using generalized additive mixed models. J. Appl. Ecol. 53, 1852–1861 (2016).
Wood, S. N. Generalized additive models: An introduction with R 2nd edn. (Chapman & Hall/CRC, 2017).
Mills L.S. Conservation of wildlife populations. Demography, genetics, and management. Second Edition. Wiley–Blackwell, Oxford (2013).
Rieger, I. Hyaena hyaena. Mammalian Species 150, 1–5. The American Society of Mammalogists (1981).
Hofer, H. & Mills, M.G.L. Worldwide distribution of Hyaenas. In: M.G.L. Mills and H. Hofer (eds), Hyaenas. Status survey and conservation action plan, pp. 39–63. IUCN/SSC Hyaena Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK (1998a).
Hofer, H. & Mills, M.G.L. Population size, threats and conservation status of hyaenas. In: M.G.L. Mills and H. Hofer (eds) Hyaenas. Status Survey and Conservation Action Plan, pp. 64–79. IUCN/SSC Hyaena Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK (1998b).
Bar-Ziv, E., Picardi, S., Kaplan, A., Avgar, T. & Berger-Tal, O. Sex differences dictate the movement patterns of Striped Hyenas, Hyaena hyaena, in a human-dominated landscape. Front. Ecol. Evol. 10, 897132 (2022).
Shamon, H., Sorek, M., Dan, H. & Shapira, I. A large carnivore in a rapidly changing environment: occurrence and density of the striped hyaena hyaena hyaena in Israel. The 54th Israel Zoological Society Conference, Tel Aviv University, Ramat Aviv (2017).
Shamoon, H. & Shapira, I. Limiting factors of Striped Hyaena, Hyaena hyaena, distribution and densities across climatic and geographical gradients (Mammalia: Carnivora). Zool. Middle East 65, 189–200 (2019).
Yom-Tov, Y. Body sizes of carnivores commensal with humans increased over past 50 years. Funct. Ecol. 17, 323–327 (2003).
Monchot, H. & Mashkour, M. Hyenas around the city (Kashan, Iran). J. Taphon. 8, 17–32 (2010).
Panda, D. et al. High striped hyena density suggests coexistence with humans in an agricultural landscape Rajasthan. PLoS ONE 17, e0266832 (2022).
Mendelssohn, H. & Yom-Tov, Y. A report of birds and mammals which have increased their distribution and abundance in Israel due to human activity. Isr. J. Zool. 45, 35–47 (1998).
Meretsky, V. J. & Mannan, R. W. Supplemental feeding regimes for Egyptian vultures in the Negev Desert Israel. J. Wildl. Manag. 63, 107–115 (1999).
Mallon, D. & Budd, K. (eds). Regional red list status of carnivores in the Arabian Peninsula. Cambridge, UK and Gland Switzerland: IUCN, and Sharjah, UAE: Environment and Protected Areas Authority vi+49pp (2011).
Jaffa, N. A. B. K-P. S. The Arabian striped Hyena (Hyaena hyaena sultana Pocock, 1934) (Carnivora: Hyaenidae) in the Kingdom of Saudi Arabia. Gazelle Palestin. Biol. Bull. 185, 1–39 (2020).
Cogal, M., Ilemin, Y. & Sozen, M. Status and distribution of the Striped Hyaena, Hyaena hyaena, in Turkey: An updated assessment (Carnivora: Mammalia). Turk. J. Zool. 45, 131–141 (2021).
Almasieh, K., Mohammadi, A. & Alvandi, R. Identifying core habitats and corridors of a near threatened carnivore, striped hyaena (Hyaena hyaena) in southwestern Iran. Sci. Rep. 12, 3425 (2022).
Tourani, M., Moqanaki, E. M. & Kiabi, B. H. Vulnerability of striped hyaenas, Hyaena hyaena, in a human-dominated landscape of Central Iran. Zool. Middle East 56, 133–136 (2012).
Bhandari, S. et al. Climate change threatens striped hyena (Hyaena hyaena) distribution in Nepal. Mammal Res. 67, 433–443 (2022).
Tichon, J., Gilchrist, J. S., Rotem, R., Ward, P. & Spiegel, O. Social interactions in striped hyena inferred from camera trap data: Is it more social than previously thought?. Curr. Zool. 66, 345–353 (2020).
Dean, W. R. J., Seymour, C. L., Joseph, G. S. & Foord, S. H. A review of the impacts of roads on wildlife in semi-arid regions. Diversity 11, 81 (2019).
Landau, Y., Abu-Rabiya, A., Avlegon, A. & Abu-Siam, S. Seasonl grazing of livestock by Beduin in KKL forests: Developments from 2009 to 2014. Forests 15, 30–39 (2015).
Jacobson, A. P. et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 4, e1974 (2016).
Lazagabaster, I. A. et al. Changes in the large carnivore community structure of the Judean Desert in connection to Holocene human settlement dynamics. Sci. Rep. 11, 3548 (2021).
Lazagabaster, I. A. et al. Cave paleozoology in the Judean Desert: assembling records of Holocene mammal communities. J. Quarten. Sci. 37, 651–663 (2022).
Davidson, A., Malkinson, D. & Shanas, U. Wild boar foraging and risk perception—variation among urban, natural, and agricultural areas. J. Mammal. 20, 1–11 (2022).
Panda, D. et al. Competitive interactions with dominant carnivores affect carrion acquisition of striped hyena in a semi-arid landscape of Rajasthan India. Mammal Res. https://doi.org/10.1007/s13364-022-00663-1 (2022).
Mandal, D., Basak, K., Mishra, R. P., Kaul, R. & Mondal, K. Staus of leopard Panthera pardus and striped hyena Hyaena hyaena and their prey in Achanakmar Tiger Reserve, Central Inida. J. Zool. Stud. 4, 34–41 (2017).
Alam, M. S., Khan, J. A. & Pathak, B. J. Striped hyena (Hyaena hyaena) status and factors affecting its distribution in the Gir National Park and Sanctuary India. Folia Zool. 64, 32–39 (2015).
Singh, P., Gopalaswamy, A. M. & Karanth, K. U. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J. Mammol. 91, 1152–1159 (2010).
Handal, E. N., Amr, Z. S., Basha, W. S. & Qumsiyeh, M. B. Illegal trade in wildlife vertebrate species in the West Bank Palestine. J. Asia-Pac. Biodivers. 14, 636–639 (2021).
Kumbhojkar, S., Yosef, R., Benedetti, Y. & Morelli, F. Human-leopard (Panthera pardus fusca) co-existence in Jhalana Forest Reserve India. Sustainability 11, 3912 (2019).
Bhandari, S., Bhusal, D. R., Psaralexi, M. & Sgardelis, S. Habitat preference indicators for striped hyena (Hyaena hyaena) in Nepal. Glob. Ecol. Conserv. 27, e01619 (2021).
Author information
Authors and Affiliations
Contributions
E.H.—conceptualization, implementation; J.Z.K.—GIS, data analysis; R.Y., J.Z.K., E.H.—quality control, writing paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hadad, E., Kosicki, J.Z. & Yosef, R. Population trends of striped hyena (Hyaena hyaena) in Israel for the past five decades. Sci Rep 13, 3982 (2023). https://doi.org/10.1038/s41598-023-31137-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31137-2
This article is cited by
-
Thanatological behavior in striped hyena (Hyaena hyaena)
acta ethologica (2024)
-
Borrelia persica infection in wild carnivores in Israel: molecular characterization and new potential reservoirs
Parasites & Vectors (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.