Abstract
Precipitation and deposition of asphaltene are considered as catastrophic issues facing the petroleum industry. Asphaltene deposition mainly occurs at variety places such as formation pore spaces, pumps, pipelines, wellbore, wellhead, tubing, surface facilities and safety valves causing operational problems, production deficiencies and enormous economic losses. This work aims to study the effect of series of synthesized aryl ionic liquids (ILs) containing different alkyl chains, named as R8-IL, R10-IL, R12-IL, and R14-IL, on the onset precipitation point of asphaltene in crude oil. R8-IL, R10-IL, R12-IL, and R14-IL were synthesized with high yields (the yield varied between 82 and 88%) and characterized via different tools of analysis (FTIR, 1H NMR, and Elemental Analysis). Their Thermal Gravimetric Analysis (TGA) was investigated and showed a reasonable degree of stability. It was found that R8-IL (short alkyl chain) has the highest stability, while R14-IL (long alkyl chain) is the lowest one. Quantum chemical calculations were conducted to study the reactivity and geometry of their electronic structures. Moreover, surface and interfacial tension of them were studied. It was found that the efficiency of the surface active parameters increased by increasing the length of the alkyl chain. The ILs were evaluated to delay the onset precipitation point of asphaltene using to different methods; the kinematic viscosity and the refractive index. Results from the two methods showed delaying of onset precipitation after the addition of the prepared ILs. The asphaltene aggregates was dispersed due to the π–π* interactions and hydrogen bonds formation with the ILs.
Similar content being viewed by others
Introduction
Crude oil still plays a significant role in the energy domain, although researchers are seeking different sources of energy because of high demand1,2. The use of primary and secondary oil recovery techniques leaves more than 30% of the oil unrecovered inside the pores of the reservoirs. Asphaltene is the heaviest and most aromatic component of crude oil; it is critical to overall aspects in upstream or downstream operations because of its nature to coordinate and form clusters3. The viscosity of crude oil is strongly affected by asphaltene; consequently, all areas of resource exploitation are affected, including flow assurance, low distillate, and emulsion stability, resulting in wettability and phase separation problems. According to its solubility, asphaltene is insoluble in short alkane chains and completely soluble in aromatics, e.g., benzene, toluene, and xylene (BTX)4. Different asphaltene inhibition treatments have been improved to enhance the properties of crude oil: carbon rejection technologies; solvent deasphalting (SDA); mild cracking solvent deasphalting (MCSD); and the aquathermolysis method. The aquathermolysis method has been reported as the most effective technique for viscosity reduction in heavy crude oil, increasing saturates and aromatics while decreasing resin and asphaltene. Additionally, it requires a severe amount of energy and causes environmental hazards5,6. As a matter of fact, resin in crude oil serves as asphaltene inhibitor because of its functional groups and alkyl chains have the ability to link between the asphaltene and nonpolar medium7,8. Many synthesized chemicals that have similar structure to resins can enhance the asphaltene stabilization in the system. Most of the reported chemicals that have been used as potential asphaltene dispersants include oxazolidines6, n-aryl amino alcohol9, benzoic acid, phthalic acid, and salicylic acid10. All these chemicals are toxic compounds that may cause many environmental hazards. From this point, ionic liquids (ILs) as a new eco-friendly class of chemicals were suggested by researchers11,12. ILs have attracted significant interest in a wide variety of industrial applications because of their distinctive characteristics and great compatibility with environmental issues13. Negligible vapor pressure, recyclability, high thermal stability, non-corrosive, high surface activity, and slightly lower toxicity are all appropriate properties for ILs to be considered as environmentally preferable and better sustainable the conventional surfactant14,15,16,17,18. The ILs properties owning to poor coordination combination between cations and anions which make possible alterations in the chemical structures subsequently, they can perform better in different applications19,20; enhanced oil recovery21,22,23, scale removal, catalysis, capturing of CO224, solvent extraction25, electrochemistry, natural gas purification26, desulphurization, crude oil dissolution and IFT reduction27,28. ILs were reported by Liu et al.29 for the first time in asphaltene dissolution, and it was noticed that the most effective ILs contained conjugated aromatic cations and anions with strong hydrogen bond acceptors. Meanwhile, there was another work reported by Boukherissa et al. on the usage of boronic ILs (1-propyl boronic acid-3- alkylimidazolium bromide) in asphaltene dispersion30. They predicted that the boronic acid moiety would reduce asphaltene aggregation and improve interactions between asphaltenes and ionic liquids. Also, acidic IL (3-(2-carboxybenzoyl)-1-methyl-1H-imida zol-3-ium chloride) was reported to prevent flocculation of asphaltenes31. Ghanem et.al. reported the effect of alkylated imidazoleum sulfonate ILs as effective asphaltene dispersants7. Protic ILs can cause the dissolution of asphaltenes via cation interactions and charge transfer to form complexes with asphaltene molecules. They all concluded that the electrostatic interaction and hydrogen bond formation promoted avoiding asphaltene accumulation32.
This work aims to synthesize and study the effect of some aryl imidazolium ionic liquids containing different alkyl chains, named R8-IL, R10-IL, R12-IL, and R14-IL, on the onset precipitation point of asphaltene in crude oil. They were synthesized with high yields, (the yield varied between 82 and 88%), and characterized via different tools of analysis (FTIR, 1H NMR, and Elemental Analysis). In addition, surface tension study was conducted to evaluate the activity of the prepared ionic liquids. The crude oil was characterized according to standard methods. The prepared ionic liquids were evaluated as asphaltene dispersant using the kinematic viscosity and the refractive index technique.
Experimental work
Materials
1H-Imidazole(≥ 99%), 1-Bromo Octane (≥ 99%), 1-bomodecane(≥ 98%), 1-bromododecane (≥ 99%), 1-bomotetradecane (≥ 99%), 1-(chloromethyl)-4-methylbenzenepotassium hydroxide (≥ 98%), heptanes (≥ 97%), benzene (≥ 98%), n-hexane (≥ 99%), aluminum oxide (neutral) and chloroform (≥ 98%) were supplied by Biochem. All the used chemicals and solvents were of analytical grade and were used with no further purifuication. The used heavy crude oil in this study was received from the Egyptian Petroleum Company. Different physical characteristics and the SARA analysis are tabulated in Table 1. Most of the physico-chemical properties and SARA test of the crude oil were conducted depending on standards of the American Society for Testing and Material (ASTM). Results indicate that the crude oil has a low API degree, a high value of kinematic viscosity, in addition to a high content of asphaltene and a low content of saturates.
Methodology
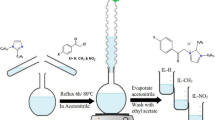
Synthesis of R8-IL, R10-IL, R12-IL, and R14-IL was conducted where, a series of four alkyl imidazole were obtained by adding a known portion of 1H-imidazole (0.1 mol) and potassium hydroxide (KOH) in acetonitrile (50 ml) with continuous stirring. 1-bromo octane, 1-bomo decane,1-bromo dodecane and 1-bomo tetradecane (0.1 mol) was added dropwise to the previous mixture for 3 h untill the formation of a white precipitate from KCl. After filtration process, the filtrate was concentrated under vaccum and different alkyl imidazole ionic liquids were obtained11. The four 1-alkyl imidazole derivatives were refluxed with 1-(chloromethyl)-4-methylbenzene in acetonitrile for six hours to yield 1-alkyl-3-(4-methylbenzyl)-H-imidazol-3-ium chloride derivetives26, as shown in Fig. 1. The TLC technique was used to confirm the purity of the prepared ionic liquids. These compounds showed good solubility in varities of polar solvents33.
Characterization of the prepared ILs
The prepared ILs were characterized using different tools of analysis. Elemental analysis was determined using elementar analyzer, in which an excess of oxygen is used to burn the sample. FTIR spectra of the prepared ILs were analysed in transmittance mode using KBr pellets in the range of 4000–400 cm-1 by the Nicolet IS-10 Spectrometer-ThermoFisher, USA. The FTIR spectrometer was equipped with a standard fast recovery deuterated triglycine sulfate detector (DTGS). 1H-NMR spectra of the prepared ionic liquids were investigated using BRUKER NMR Spectroscopy, (Germany) using DMSO as a solvent at frequency equal to 400 MHz. Thermal Analyzer SDT Q500 V20.10 with heating rate of 10 ºC/min was used to determin the thermal gravimetric analysis of the prepared ionic liquid. Surface and Interfacial tension of the ionic liquids were investigated at 298 K by Du-Nouy tensiometer depending on a platinum ring. The apparatus was firstly calibrated using deionized water, which recorded about 72 ± 0.5 m/Nm34. After that solutions containing a series concenterations of ILs (0.001–0.00001 mol/L) were prepared and measured and different parameters were calculated.
Asphaltene isolation from crude oil
In this work the asphaltene was isolated from heavy crude oil come from Egyptian oilfield exactly as reported in IP-143 as a standared test method. Aknown portion of crude oil (10 g) was added to 300 ml n-heptane and refluxed for an hour. The refluxed solution was left in dark place until cooling. After that the solution was poured on an ashless filter paper to separate the filterate that contains asphaltene, inorganic and waxy substances. The filter paper was folded many times and entered to a soxhelet to be cleaned with hot n-heptane from any impurities or waxy components. After that the asphaltene was isolated from any inorganic substances via disolvation in hot toluene35. At the end, the toluene was evaporated and the asphatene was weighted to determine the asphaltene content according to the following equation.
where M is the asphaltene mass and G is the crude oil mass.
Asphaltene onset precipitation
The evaluation of the onset precipitation point of asphaltene is often conducted by the viscometric method, in which the crude oil is treated with a series of successive volumes of asphaltene precipitant like n-heptane36. For each run, series of samples were prepared to cover the precipitant concentration range of 0–100 vol percent from the oriinal crude oil, in the absence and presense of the prepared ionic liquids. The concentration range of the added dispersants was of 500–2000 ppm. After that the mixtures were settled for a period of time till equilibrium. The kinematic viscosities of the prepared mixtures are determined using cannon fensky viscometer at 40 °C. A relation between the the kinematic viscosity and the concentration of the precipant are being drawn to detect the onset precipitation point of asphaltene. The previous steps are repeated after the addition of the dispersants to be evaluated7. The refractive index method can be used to confirm the obtained results from the viscometric method. This method uses the asphaltene itself to design a model oil with n-heptane/toluene mixture (Heptol mixture)37.
Results and discussion
Characterization of the ILs
Table 2 contains the molecular structures of the prepared ionic liquids. The molecular weight of R8-IL, R10-IL, R12-IL, and R14-IL is 320.90, 348.95, 377.01, and 405.06, respectively, while the yield varied between 82 and 88%. The reaction yield was calculated according to the following equation:
\({\text{Yield}}\;\% = \left( {{\text{Actual}}\;{\text{ yield}}/{\text{Theoretical}}\;{\text{yield}}} \right) \times {1}00.\)
Elemental analysis
The elements content of the prepared ILs provides information about the role of the organic molecules. The molecular formula can be confirmed by comparing the theoretical data with the experimental ones.The data in Table 3 showed that the calculated C, H, N, O, and S values are compatible with the recorded values.
FT-IR spectra of the prepared ILs
The FT-IR spectra of R8-IL, R10-IL, R12-IL, and R14-IL are shown in Figure 2, and the values of the characteristic peaks are reported in Table 4. The asymmetric stretching vibrational mode belongs to the hydrogen bonded H2O molecules was recorded as a broad band around 3408–3420 cm−138. The peaks that appeared between 3131 and 3073 cm−1 are attributed to aromatic C–H, and related to the vibrational motion inside the imidazole ring. Aliphatic C–H stretching peaks appeared in the range of 2956 and 2852 cm−1, where the peaks that were observed around 2956 and 2925 cm−1 are attributed to the asymetric stretching of the terminal methyl group and the methylene unit in the alkyl chain of the prepared ionic liquids, respectively. In addition, the symmetric stretching peaks of CH3 and CH2 appeared around 2872 and 2852 cm−1, respectively. The symmetric and asymmetric vibrational modes are usually assigned between 2954 and 2870 cm−1 which become more intense peaks by increasing the alkyl chain length by more than six CH2 groups39. The peaks that appeared between 1561 and 1517 cm−1 are attributed to C–C vibration inside the imidazole ring. The appeared peaks between 1466 and 1456 cm−1 are belonged to C=C aromatic. Stretching vibration modes that resorted to C–N in the imidazole ring appeared at 1377 cm−1. Another stretching vibrational peaks appeared at 1157–1156 cm−1 are attributed to C−H in plane.
1H-NMR of the prepared ILs
The proton NMR spectra for R8-IL, R10-IL, R12-IL, and R14-IL were studied and showed approximately nearby chemical shifts, as shown in Figures S1, S2, S3, and S4 in the supplementary materials. The adjacent chemical shifts of each ionic liquid can be concluded as follows:
For R8-IL
δ 9.29 (1H, s), 7.81 (2H, d), 7.79 (2H, d), 7.69 (2H, s), 5.42 (2H, s), 4.16 (2H, t), 3.37 (3H, s), 2.51 (2H, t), 1.79 (8H, m), 1.24 (2H, s), 0.86 (3H, t).
For R10-IL
δ 9.19 (1H, s), 7.78 (2H, d), 7.70 (2H, d), 7.47 (2H,s), 5.43 (2H, s), 4.17 (2H, t), 3.38 (3H, s), 2.51 (2H, t), 1.79 (12H, m), 1.25 (2H, s), 0.86 (3H, t).
For R12-IL
δ 9.20 (1H, s), 7.80 (2H, d), 7.69 (2H, d), 7.46 (2H, s), 5.43 (2H, s), 4.16 (2H, t), 3.39 (3H, s), 2.51 (2H, t), 1.79 (16H, m), 1.24 (2H, s), 0.86 (3H, t).
For R14-IL
δ 9.50 (1H, s), 7.85 (2H, d), 7.67 (2H, d), 7.34 (2H, s), 5.41 (2H, s), 4.17 (2H, t), 3.41 (3H, s), 2.28 (2H, t), 1.76 (20H, m), 1.24 (2H, s), 0.88 (3H, t).
From the above data, the distinct chemical shifts at δ 9.2 (1H, s) displayed the characteristic peaks of the imidazolium protons and δ 5.4 (2H, s) are attributed to the benzylic protons of alkyl imidazole, demonstrating the effective incorporation of imidazolium group.
Thermal gravimetric analysis (TGA)
Thermal gravimetric analysis thermograms of R8-IL, R10-IL, R12-IL, and R14-IL are shown in Fig. 3, and their main measuring values are reported in Table 5. It is obvious that the prepared compounds are thermally stable and R8-IL is the most thermaly stable compound, followed by R10-IL, R12-IL, and R14-IL. This may refered to the variation in the length of the alkyl chains that were connected to imidazolium rings of the synthetic compounds40. In breif, it is obvious that the maximum thermal stability was associated with the shortest alkyl chain connected to the imidazolium ring40,41.
Quantum chemical parameters
The quantum chemical calculations were conducted via the Gaussian trial package Hartree–Fock method to study the electronic structures (EHOMO & ELUMO), the energy gap (ΔE), and the molecular geometry of the prepred ILs, as shown in Fig. 4. The quantum calculations were conducted using the basis set 6-31G. As shown in Table 6, increasing the length of the alkyl chain of R8-IL to R14-IL is directly proportional to the values of EHOMO , ELUMO, and softness (σ), while it is inversely proportional to ΔE and dipole moment (µ) values. Figure 4 shows the ability of the prepared ILs to act as electron donors to asphaltene compounds. The dispersion reactivity of the ionic liquid towards asphaltene aggregates is determined by ΔE, where the efficiency of the prepared ILs is increased by decreasing the ΔE of each compound. Therefore, R14-IL > R12-IL > R10-IL > R8-IL in reactivity which lowers the needed energy to move electrons from HOMO to LUMO. This is because the smaller the energy gap (ΔE), the easier the absorption between the ionic liquid and the surface of the asphaltenes, which is in turn better for the dispersion efficiency of the ionic liquid. R10-IL recorded a lower ionization energy (I) so, it indicates the highest dispersion potential against asphaltene molecules. R14-IL possesses the highest dipole moment (µ), while R8-IL has the lowest value, as shown in Fig. 5. Dipole moment is releated to the molecule’s global polarity, so the compound with a higher dipole moment value shows more reactivity. Softness is another term that demonstrate the reactivity of the compounds, where the soft compounds indicate more reactivity than the hard molecule’s, therefore R14-IL > R12-IL > R10-IL > R8-IL in reactivity .
Surface tension measurements of the prepared ILs
The values of critical micelle concentration (CMC) and the surface tension at CMC of each IL are presented in Table 7, In addition to the interfacial tension (IFT), and the area per molecule at the surface (Amin). All these data were obtained from Fig. 6, in which the surface tension is ploted against minus the logarithm of the concentrations of the prepared ionic liquids (of R8-IL, R10-IL, R12-IL, and R14-IL).
Surface parameters of the prepared imidazolium-based ionic liquids, such as surface tension (γcmc), interfacial tension (IFT), Critical Micelle Concentration (CMC), surface excess (max), and minimum surface area per molecule (Amin) were calculated using surface tensiometry and are listed in Table 7.
Surface tension (γ) and critical micelle concentration
At 25 °C, Fig. 6 shows the variation of surface tension against -log concentration of the prepred ILs. At the same concentration, the length of the attached alkyl chain of the prepared ILs has a significant effect on the surface active parameters. Where, increasing the length of the alkyl chain to 12 methylene groups reduces the surface tension dramatically. While the IL that has a short alkyl chain (R8-IL) displayed a reduced dropping in the surdface tension. This is due to the longer alkyl chain has a greater proclivity to be adsorbed at the air–water interface42. Due to the variations in polarity between the hydrophobic alkyl chainand the aqueous medium, the repulsion between the two is amplified by increasing the amount of the repeated methylene groups. At increasing the concentrations of the ILs, the surface tension curve values essentially remain constant, revealing the critical micelle concentration values for each IL. The data in Table 7 revealed that the interfacial tension of the ionic liquids under consideration was decreased with increasing the length of the alkyl chain. The values of critical micelle concentration were clearly reduced when the length of the hydrophobic chain was increased. Because of the long alkyl chains (> 12 methylene groups) have the tendency to be coiled, the CMC value can be easily negatively affected with no additional discernible effects.
The efficiency (PC20) and the effectiveness (πcmc)
Table 7 shows the efficacy values of the prepared ionic liquids. It is clear that increasing the length of the hydrophobic alkyl chain, increasing the efficiency. This is due to the adsorption efficiency at surfaces is directly proportional to the number of methylene groups in the hydrophobic alkyl chain. The effectiveness (πcmc) of the surface tension is determinmed by the surface tension of the IL solution at critical micelle concentration (γcmc). R12-IL has the most efficient surface active parameters as it has the lowest γcmc. Adsorption effectivness has a significant role in influencing the characteristics of surfactants, including foaming, wetting, and emulsification43.
Maximum surface excess (Γmax)
The values of Γmax of the prepared ionic liquid is decreased by increasing the number of methylene groups as shown in Table 7. It is known that the activity of surface active agent increases by decreasing the surface tension44. One of the most important uses of surface active compounds as a crucial field of chemistry in many applications involves pumping the compound to the interface to generate and adsorbed layer45. The data revealed that R12-IL has the large area per molecule at the surface, indicating that molecules with long alkyl chains are flexible and less tightly pace at the air–water interface46.
Thermodynamics of energy adsorption
Data from Table 7 revealed that the prepared ILs have negative values of standard free energy for both micellization and adsorption, indicating that both happened spontaneously. This is due to the presence of repulsion forces between the polar solvent and the hydrophobic alkyl chains. The adsorption process is more preferred than micellization because the adsorption energy is lower than the micellization energy of each ionic liquid.
The prepared ionic liquids showed high negative values of free energy of adsorption, which encourages their utilization in many important applications such as asphaltene dispersion and inhibition, corrosion inhibition, and antimicrobial utility.
The larger values of ΔGoads than those of ΔGomic indicate that the adsorption process at the solution/air interface is more preferable than the micellization process in the bulk of the solution47,48.
Evaluation of the synthesized dispersants
In this work, the synthesized ionic liquids R8-IL, R10-IL, R12-IL, and R14-IL were assessed as dispersants for asphaltene aggregates using the viscometric and the refractive index methods. We believe that our research is the first to test asphaltene dispersants utilizing dead oil using the refractive index approach.
Viscometric method
This technique is predicated on the notion that crude oil containing asphaltene is a colloidal solution in which asphaltenes are the suspended particles and the resin constituent serves as the asphaltene stabilizer. This stabilized system might become upset in the event of the introduction of a precipitant such as n-heptane that has the ability to desorp the resin preserver film from the asphaltene surfaces. Without the presence of resin, the asphaltene molecules have the ability to interact with each other to form aggregates of different sizes. The effect of the asphaltene precipitator (n-heptane) on the onset of precipitation with and without the prepared ionic liquids (R8-IL, R10-IL, R12-IL, and R14-IL) is shown in Fig. 7. The kinematic viscosity of the titrant mixture (asphaltenic crude oil and n-heptane) decreased while the concentration of n-heptane increased until it reached a deviation point. After that, a small increase in one point was observed, followed by a great decrease in the viscosity of the titrant mixture. This point of deviation is known as the asphaltene onset precipitation49. This point was observed in the blank crude oil after adding 6 ml of n-heptane. This volume was increased to 10, 12, 16, and 18 ml after using 1000 ppm of each R8-IL, R10-IL, R12-IL, and R14-IL, respectively. Table 8 contains the needed concentration of n-heptane to detect the onset precipitation point of asphaltene after using different concentrations of the preapared dispersants. Increasing the concentration of the ionic liquids positively affects the onset of asphaltene precipitation. It is obvious that the onset precipitation point of asphaltene was delayed while the length of the attached alkyl chain to the dispersant was increased. However R14-IL contains 14 carbon atoms and has the longest alkyl chain, R12-IL is more effective as an asphaltene dispersant. This is attributed to the coiling effect of the extra-long alkyl chain in R14-IL50.
Refractive index method
Precipitation of asphaltenes decreases the polarity properties of the mixture and consequently the value of the refractive index. Increasing the percentage of the precipitant (n-heptane) in the Heptol mixture reduces the refractive index of the mixture in a linear trend as shown in Fig. 8. This behavior also appeared for the asphaltene/heptol mixture until the asphaltene onset precipitation was detected. After reaching this point, the polarity of the remaining asphaltene/heptol mixture decreased, and this may be attributed to the reduced amount of suspended asphaltene molecules. Therfore, the values of refractive index was deviated from its linear trend. The deviation appeared at low concentration of n-heptane as shown in Fig. 8 at point (a) without dispersant, while at point (b) the onset precipitation of asphaltene appeared at nearly 60% of the precipitant after using the ionic liquid as a dispersant.
In order for ionic liquids to stabilize the asphaltene in the media, two key processes must take place: first, the adsorption of the ionic liquid polar head on the surface of asphaltene molecules due to the acid-base interaction between the ionic liquid and the asphaltene molecules, and second, the formation of a stable non-polar alkyl layer around the asphaltene molecules32. Consequently, ionic liquid can operate as a bridge between polar asphaltene molecules and the non-polar medium of the crude oil. In general, asphaltene inhibitors are characterized by their high aromaticity and polarity. Therefore, the alkylated ionic liquids seem to be more superior than conventional aromatic inhibitors.
Conclusion
Four different ionic liquids, named R8-IL, R10-IL, R12-IL, and R14-IL, were synthesized and well characterized using different spectroscopic methods such as elemental analysis, FT-IR, and proton NMR. Moreover, the thermal stability of prepared ILs was measured and showed a high level of thermal stability. In addition, the surface tension was measured and showed good surface active poperities. A quantum study of the ionic liquids was conducted to investigate the quantum parameters such as EHOMO and ELUMO, the energy gap, and the geometry optimization of the electronic structure. It was found that the reactivity of the prepared ILs increased with the number of the attached methylene groups.
The prepared ILs were tested to disperse the asphaltene agglomerates using the viscosity and the refractive index techniques. With regard to the all ionic liquids, the strength of dispersion was increased with increasing the concentration of the prepared ionic liquids up to 2000 ppm. This suggests that increasing the amount of the ionic liquid, increases its adsorption on the surface of asphaltene micelles. R12-IL showed its high value of inhibition at only 1000 ppm, so the optimum concentration is 1000 ppm.
The dispersion behavior has a regular tendency, with increasing the number of the attached methylene groups increasing the efficiency of dispersion. Because of the coiling effect of the extra-long alkyl chains, this trend loses its relevan as the length of the alkyl chain exceeds 12 carbon atoms.
Data availability
The data that support the findings in the present study are available from the corresponding author upon request.
References
Ellabban, O., Abu-Rub, H. & Blaabjerg, F. Renewable energy resources: current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 39, 748–764 (2014).
El-Nagar, R. A., Ghanem, A. A., Nessim, M. I. Capture of CO2 from Natural Gas Using Ionic Liquids. Shale Gas—New Aspects and Technologies; IntechOpen: London, UK, 2018. 2: p. 83–99.
Desouky, S. et al. Catalytic aquathermolysis of Egyptian heavy crude oil. Int. J. Chem. Mol. Eng. 7(8), 638–643 (2013).
Nordgård, E. L., Landsem, E. & Sjöblom, J. Langmuir films of asphaltene model compounds and their fluorescent properties. Langmuir 24(16), 8742–8751 (2008).
Lei, H. et al. Experimental investigation and application of the asphaltene precipitation envelope. Energy Fuels 29(11), 6920–6927 (2015).
Molnárné Guricza, L. and W. Schrader, New separation approach for asphaltene investigation: argentation chromatography coupled with ultrahigh-resolution mass spectrometry. Energy Fuels, 2015. 29(10): p. 6224–6230.
Ghanem, A. et al. Synthesis and characterization of imidazolium-based ionic liquids and evaluating their performance as asphaltene dispersants. Materials 15(4), 1600 (2022).
Wiehe, I. A. & Jermansen, T. G. Design of synthetic dispersants for asphaltenes. Pet. Sci. Technol. 21(3–4), 527–536 (2003).
Chávez-Miyauchi, T. E. et al. N-aryl amino-alcohols as stabilizers of asphaltenes. Fuel 110, 302–309 (2013).
Karambeigi, M. A., Nikazar, M. & Kharrat, R. Experimental evaluation of asphaltene inhibitors selection for standard and reservoir conditions. J. Petrol. Sci. Eng. 137, 74–86 (2016).
El-Nagar, R. et al. Application of asymmetric dicationic ionic liquids for oil spill remediation in sea water. Arab. J. Chem. 14(5), 103123 (2021).
Pillai, P., Maiti, M. & Mandal, A. Mini-review on recent advances in the application of surface-active ionic liquids: petroleum industry perspective. Energy Fuels 36(15), 7925–7939 (2022).
Alharthy, R. D., El-Nagar, R. A. & Ghanem, A. Laboratory experiments on the in situ upgrading of heavy crude oil using catalytic aquathermolysis by acidic ionic liquid. Materials 15(17), 5959 (2022).
Atilhan, M. & Aparicio, S. Review on chemical enhanced oil recovery: utilization of ionic liquids and deep eutectic solvents. J. Petrol. Sci. Eng. 205, 108746 (2021).
Manshad, A. K. et al. Wettability alteration and interfacial tension (IFT) reduction in enhanced oil recovery (EOR) process by ionic liquid flooding. J. Mol. Liq. 248, 153–162 (2017).
Pillai, P., Kumar, A. & Mandal, A. Mechanistic studies of enhanced oil recovery by imidazolium-based ionic liquids as novel surfactants. J. Ind. Eng. Chem. 63, 262–274 (2018).
Shojaee, S. A. et al. A new correlation for estimating thermal conductivity of pure ionic liquids. Fluid Phase Equilib. 354, 199–206 (2013).
Atef, Y., and Ghanem, A.. ionic liquids based on different chain fatty acids as green corrosion inhibitors for C-steel in produced oilfield water. In IOP Conference Series: Materials Science and Engineering. 2020. IOP Publishing.
Plechkova, N. V. & Seddon, K. R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 37(1), 123–150 (2008).
El-Nagar, R. et al. Rheological and physical properties of ionic liquids with ammonium cations as synthetic lubricants. Egypt. J. Chem. 61(2), 349–360 (2018).
Somoza, A. et al. Design and performance analysis of a formulation based on SDBS and ionic liquid for EOR in carbonate reservoirs. J. Petrol. Sci. Eng. 209, 109856 (2022).
Ghanem, A. et al. Predicting the compressibility factor of natural gas by using statistical modeling and neural network. Energies 15(5), 1807 (2022).
Pillai, P. & Mandal, A. Synthesis and characterization of surface-active ionic liquids for their potential application in enhanced oil recovery. J. Mol. Liq. 345, 117900 (2022).
Nessim, M.I., et al. Effect of ionic liquids in CO2 capture from natural gas. Life Sci J., 2013. 10(1).
Li, X. et al. Ionic liquid enhanced solvent extraction for bitumen recovery from oil sands. Energy Fuels 25(11), 5224–5231 (2011).
El-Nagar, R. et al. Investigating the efficiency of newly prepared imidazolium ionic liquids for carbon dioxide removal from natural gas. J. Mol. Liq. 237, 484–489 (2017).
Saien, J. & Hashemi, S. Long chain imidazolium ionic liquid and magnetite nanoparticle interactions at the oil/water interface. J. Petrol. Sci. Eng. 160, 363–371 (2018).
Sakthivel, S. et al. Synergistic effect of lactam, ammonium and hydroxyl ammonium based ionic liquids with and without NaCl on the surface phenomena of crude oil/water system. Fluid Phase Equilib. 398, 80–97 (2015).
Liu, Y. et al. Ionic liquids: novel solvents for petroleum asphaltenes. Chin. J. Chem. Eng. 13(4), 564 (2005).
Boukherissa, M. et al. Ionic liquids as dispersants of petroleum asphaltenes. Energy Fuels 23(5), 2557–2564 (2009).
Shadman, M. M. et al. An investigation of the effect of aromatic, anionic and nonionic inhibitors on the onset of asphaltene precipitation. J. Oil Gas Petrochem. Technol. 1(1), 17–28 (2014).
Shadman, M. et al. The effect of inhibitors on asphaltene precipitation in crude oil using the viscometric method. Energy Sources Part A: Recovery Util. Environ. Effects 34(9), 827–838 (2012).
El-hoshoudy, A., Ghanem, A. & Desouky, S. Imidazolium-based ionic liquids for asphaltene dispersion; experimental and computational studies. J. Mol. Liq. 324, 114698 (2021).
Adenaya, A., et al. Effects of Natural and Artificial Surfactants on Diffusive Boundary Dynamics and Oxygen Exchanges across the Air–Water Interface. In Oceans. 2021. MDPI.
Lashkarbolooki, M., Ayatollahi, S. & Riazi, M. Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 28(11), 6820–6829 (2014).
Sanati, A. et al. Inhibition of asphaltene precipitation using hydrophobic deep eutectic solvents and ionic liquid. J. Mol. Liq. 334, 116100 (2021).
Tavakkoli, M. et al. Indirect method: a novel technique for experimental determination of asphaltene precipitation. Energy Fuels 29(5), 2890–2900 (2015).
Brycki, B. et al. Synthesis, structure and antimicrobial properties of novel benzalkonium chloride analogues with pyridine rings. Molecules 22(1), 130 (2017).
Orrego-Ruiz, J. A. et al. Quality prediction from hydroprocessing through infrared spectroscopy (IR). Energy Fuels 26(1), 586–593 (2012).
Yu, J. et al. Synthesis and characterisation of novel nopyl-derived phosphonium ionic liquids. J. Mol. Liq. 316, 113857 (2020).
Monteiro, B. et al. Thermal stability and specific heats of coordinating ionic liquids. Thermochim. Acta 684, 178482 (2020).
Pillai, P. & Mandal, A. Wettability modification and adsorption characteristics of imidazole-based ionic liquid on carbonate rock: implications for enhanced oil recovery. Energy Fuels 33(2), 727–738 (2019).
Łuczak, J. et al. Self-organization of imidazolium ionic liquids in aqueous solution. Colloids Surf. A 329(3), 125–133 (2008).
Fu, D. et al. Micellization, surface activities and thermodynamics study of pyridinium-based ionic liquid surfactants in aqueous solution. RSC Adv. 9(49), 28799–28807 (2019).
Negm, N., Salem, M. & Zaki, M. Solubilization behaviors of nonpolar substrates using double tailed cationic surfactants. J. Dispersion Sci. Technol. 30(8), 1167–1174 (2009).
Nessim, M., Zaky, M. & Deyab, M. Three new gemini ionic liquids: synthesis, characterizations and anticorrosion applications. J. Mol. Liq. 266, 703–710 (2018).
Deyab, M., Zaky, M. & Nessim, M. Inhibition of acid corrosion of carbon steel using four imidazolium tetrafluoroborates ionic liquids. J. Mol. Liq. 229, 396–404 (2017).
More, U. et al. Effect of imidazolium-based ionic liquids on the aggregation behavior of twin-tailed cationic gemini surfactant in aqueous solution. J. Dispersion Sci. Technol. 38(3), 393–402 (2017).
Shadman, M. M., Dehaghani, A. H. S. & Badizad, M. H. How much do you know about the methods for determining onset of asphaltene precipitation?. Petroleum 3(3), 287–291 (2017).
Bavoh, C. B. et al. Assessing the impact of an ionic liquid on NaCl/KCl/polymer water-based mud (WBM) for drilling gas hydrate-bearing sediments. J. Mol. Liq. 294, 111643 (2019).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.G. and R.E.-N.; methodology, R.E.-N and A.G.; software, M.N., A.G. and R.E.-N; validation, A.G. and R.E.-N.; formal analysis, R.A.E.-N., M.M., and D.I.; investigation, A.G. and R.A.E.-N.; writing—original draft preparation, A.G. and R.E.-N; review and editing, A.G., R.E.-N., D.I. and M.N.; supervision, M.M. and M.N.; project administration, A.G.; All authors have read and agreed to submit the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Nagar, R.A., Nessim, M.I., Ismail, D.A. et al. Investigation the effect of different ionic liquids based-aryl imidazole on the onset precipitation of asphaltene. Sci Rep 13, 4054 (2023). https://doi.org/10.1038/s41598-023-31066-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31066-0
This article is cited by
-
Evaluation of ionic liquids based imidazolium salts as an environmentally friendly corrosion inhibitors for carbon steel in HCl solutions
Scientific Reports (2024)
-
Taking a look accurately at the alteration of interfacial asphaltene film exposed to the ionic surfactants as demulsifiers
Scientific Reports (2023)
-
Imidazolium-based ionic liquids as dispersants to improve the stability of asphaltene in Egyptian heavy crude oil
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.