Abstract
Herein, we compared the association intensity of estimated glomerular filtration rate (eGFR) equations using creatinine (Cr) or cystatin C (CysC) with hyperphosphatemia and secondary hyperparathyroidism occurrence, which reflect the physiological changes occurring during chronic kidney disease (CKD) progression. This study included 639 patients treated between January 2019 and February 2022. The patients were divided into low- and high-difference groups based on the median value of the difference between the Cr-based eGFR (eGFRCr) and CysC-based eGFR (eGFRCysC). Sociodemographic and laboratory factors underlying a high difference between eGFRCr and eGFRCysC were analyzed. The association intensity of eGFRCr, eGFRCysC and both Cr- and CysC-based eGFR (eGFRCr-CysC) was compared using the area under the receiver operating characteristic curve (AuROC) values for hyperphosphatemia and hyperparathyroidism occurrence in the overall cohort and the low- and high-difference groups. Age > 70 years and CKD grade 3 based on eGFRCr were significant factors affecting the high differences. eGFRCysC and eGFRCr-CysC showed higher AuROC values than that of eGFRCr, especially in the high-difference group and in patients with CKD grade 3. Our results show that CysC should be evaluated in patients with significant factors, including age > 70 years and CKD grade 3, to accurately assess kidney function to better determine the physiological changes in CKD progression and predict prognosis accurately.
Similar content being viewed by others
Introduction
Creatinine (Cr) is the most widely used indicator to estimate kidney function, and assessing the estimated glomerular filtration rate (eGFR) using the serum Cr is recommended for assessing kidney function in the clinical field1,2. However, since serum Cr can be affected by various factors, including age, muscle mass, race, diet, drugs, and renal tubular secretion, its ability to reflect kidney function alone is limited3. Cystatin C (CysC) is constantly produced in all nucleated cells, and is excreted by the kidneys. The measurement of CysC is recommended as an alternative indicator of kidney function as it is less affected by other factors than serum Cr is4,5,6. However, CysC is affected by smoking, inflammation, adiposity, certain malignancies, and glucocorticoid use; therefore, it also has some limitations as an indicator of kidney function7,8,9.
The Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) presented equations for Cr-based eGFR (eGFRCr), CysC-based eGFR (eGFRCysC), and both Cr- and CysC-based eGFR (eGFRCr-CysC)10. Many previous studies have reported that the eGFRCr-CysC has the highest accuracy as a direct measurement of GFR (mGFR) and that it is also the most accurate index in patients with diabetes, liver cirrhosis, or solid tumors4,11,12,13. Serum Cr tends to be high in Black people regardless of their kidney function; therefore, race is considered in previous CKD-EPI equations containing serum Cr. However, in 2021, the CKD-EPI proposed new equations regardless of race, and the new eGFRCr-CysC equation showed higher accuracy with mGFR compared to those of previous equations10.
Hyperphosphatemia and secondary hyperparathyroidism are common clinical features of chronic kidney disease (CKD)14. As CKD gradually progresses to an advanced stage, the prevalence of hyperphosphatemia and secondary hyperparathyroidism is increased via various mechanisms, including a decrease in renal phosphorus excretion and increase in bone resorption. In other words, hyperphosphatemia and secondary hyperparathyroidism are considered to be physiological changes following CKD progression, and are associated with cardiovascular complications and mortality in CKD15,16,17.
Many studies have reported on the accuracy of the three eGFR equations (eGFRCr, eGFRCysC, and eGFRCr-CysC) and mGFR. However, the association of each of the eGFR equations for the occurrence of hyperphosphatemia and secondary hyperparathyroidism has not yet been elucidated. Therefore, the present study compared the association intensity of eGFRCr, eGFRCysC, and eGFRCr-CysC with the occurrence of hyperphosphatemia and hyperparathyroidism with hyperphosphatemia in patients with CKD, especially in patients with a high difference between the eGFRCr and eGFRCysC.
Results
Comparison of baseline characteristics between the low- and high-difference groups
Patients were divided into low- and high-difference groups based on the median value of the difference between the eGFRCr and eGFRCysC (|eGFRCr-eGFRCysC|: < 6.353 ml/min/1.73 m2 and ≥ 6.353 ml/min/1.73 m2, respectively). Table 1 shows the baseline characteristics of the low- and high-difference groups. The proportion of patients aged > 70 years with CKD grade 3 based on eGFRCr was significantly higher in the high-difference group than in the low-difference group. The proportion of males and the prevalence of hypertension (HTN) were lower in the high-difference group compared with those in the low-difference group, with marginal statistical significance (P = 0.059 and P = 0.061, respectively). The high-difference group had significantly higher eGFRCr and lower eGFRCysC values than those of the low-difference group. The eGFRCr-CysC showed no significant difference between the two groups. Most laboratory test findings, including the urine protein/creatinine ratio (PCR), showed no significant difference between the two groups, except for serum Cr and intact parathyroid hormone (PTH) levels.
Distribution of the differences between eGFR Cr and eGFR CysC and scatter plot of eGFR Cr and eGFR CysC
Figure 1 shows the distribution of the differences between eGFRCr and eGFRCysC. The median value of the difference between eGFRCr and eGFRCysC was 6.353 ml/min/1.73 m2, and the highest number of patients (n = 58) had an eGFRCr and eGFRCysC difference of 3–4 ml/min/1.73 m2. Figure 2 shows the scatter plot of eGFRCr and eGFRCysC in the overall cohort and in the low- and high-difference groups. All three groups showed a positive correlation between eGFRCr and eGFRCysC. The low-difference group had the highest correlation coefficient. In the scatter plot of the high-difference group; eGFRCr was greater than eGFRCysC in most patients (307/319, 96.2%).
Scatter plot and correlation analysis of eGFRCr and eGFRCysC. Panels (A), (B), and (C) show the scatter plots of the overall cohort, low-difference group, and high-difference group. The Spearman’s correlation coefficient is noted on each scatter plot. eGFR, estimated glomerular filtration rate; eGFRCr, eGFR based on creatinine; eGFRCysC, eGFR based on cystatin C.
Factors responsible for the high difference between eGFR Cr and eGFR CysC
Table 2 shows the results of a logistic regression analysis conducted to identify factors in baseline characteristics that affect the occurrence of high differences in |eGFRCr-eGFRCysC|. Factors that showed statistically significant differences at baseline between the two groups were analyzed. CKD grade 3 based on the eGFRCr and age > 70 years were significant factors in the univariate analysis, with odds ratios (OR) of 3.179 (P < 0.001) and 2.011 (P < 0.001), respectively. CKD grade 3 and age > 70 years were also observed as independent factors for a high difference in |eGFRCr-eGFRCysC|, with multivariate ORs of 3.191 (P < 0001) and 2.048 (P < 0.001), respectively.
Comparison of AuROC values of eGFR equations for hyperphosphatemia and hyperparathyroidism with hyperphosphatemia
Figure 3 shows the receiver operating characteristic (ROC) curves and area under the ROC curve (AuROC) values of the eGFRCr, eGFRCysC, and eGFRCr-CysC for hyperphosphatemia occurrence in the overall cohort and in the low- and high-difference groups. In the overall cohort, the AuROC values of eGFRCysC and eGFRCr-CysC were slightly higher than that of eGFRCr (0.682 for eGFRCysC and 0.683 for eGFRCr-CysC vs. 0.663 for eGFRCr). In the low-difference group, the AuROC values of eGFRCysC and eGFRCr-CysC showed no significant difference from eGFRCr. However, in the high-difference group, the AuROC values of eGFRCysC and eGFRCr-CysC were higher than that of eGFRCr, and the increase was more prominent in the high-difference group than in the overall cohort (0.655 for eGFRCysC and 0.644 for eGFRCr-CysC vs. 0.615 for eGFRCr).
ROC curves and AuROC values of each eGFR equation for hyperphosphatemia. Panels (A), (B), and (C) show the ROC curves for each eGFR equation for the overall cohort, low-difference group, and high-difference group. The AuROC values of the eGFR equations are noted in each panel. AuROC, area under the ROC curve; eGFR, estimated glomerular filtration rate; eGFRCr, eGFR based on creatinine; eGFRCysC, eGFR based on cystatin C; eGFRCr-CysC, eGFR based on both creatinine and cystatin C; ROC, receiver operating characteristic.
Figure 4 shows the ROC curves and AuROC values of eGFRCr, eGFRCysC, and eGFRCr-CysC for hyperparathyroidism with hyperphosphatemia occurrence in the overall cohort and low- and high-difference groups. In the overall cohort and low-difference group, the AuROC values of eGFRCysC and eGFRCr-CysC were not markedly different from that of eGFRCr. However, in the high-difference group, the AuROC values of eGFRCysC and eGFRCr-CysC were slightly higher than that of eGFRCr (0.677 for eGFRCysC and 0.675 for eGFRCr-CysC vs. 0.658 for eGFRCr).
ROC curves and AuROC values of each eGFR equation for hyperparathyroidism with hyperphosphatemia. Panels (A), (B), and (C) show the ROC curves for each eGFR equation for the overall cohort, low-difference group, and high-difference group. The AuROC values of the eGFR equations are noted in each panel. AuROC, area under the ROC curve; eGFR, estimated glomerular filtration rate; eGFRCr, eGFR based on creatinine; eGFRCysC, eGFR based on cystatin C; eGFRCr-CysC, eGFR based on both creatinine and cystatin C; ROC, receiver operating characteristic.
Comparison of AuROC values of the eGFR equations for hyperphosphatemia in the subpopulation with CKD grade 3
Table 3 shows the AuROC values of eGFRCr, eGFRCysC, and eGFRCr-CysC in the total, low- and high-difference groups for hyperphosphatemia occurrence in patients with CKD grade 3 based on eGFRCr. Similar to the results of the entire cohort (includes CKD grades 3 and 4), eGFRCysC and eGFRCr-CysC showed higher AuROC values than that of eGFRCr for hyperphosphatemia in the overall and high-difference group. Moreover, the increase in AuROC values of eGFRCysC and eGFRCr-CysC was more pronounced in patients with CKD grade 3 (0.719 for eGFRCr-CysC vs. 0.676 for eGFRCr) compared with those of the entire cohort (0.683 for eGFRCr-CysC vs. 0.663 for eGFRCr).
Discussion
In this study, eGFRCysC and eGFRCr-CysC were better indicators of hyperphosphatemia than eGFRCr was, both in the overall cohort and in the high-difference group. Similarly, eGFRCysC and eGFRCr-CysC were better indicators of hyperparathyroidism with hyperphosphatemia than eGFRCr was in the high-difference group. These findings were more pronounced in patients with CKD grade 3 based on the eGFRCr.
In the present study, age > 70 years and CKD grade 3 (low CKD grade) based on the eGFRCr were independent factors for a high difference in |eGFRCr-eGFRCysC|. Male sex, history of HTN, and intact PTH levels showed statistically significant differences between the low- and high-difference groups, but these factors were not observed as significant factors in the binary logistic regression analysis. Sarcopenia increases with age, and the serum Cr level, which is affected by muscle mass, is lower in older people; therefore, the difference between eGFRCr and eGFRCysC may be high in these groups18. Previous studies reported a positive correlation between age and eGFR difference19,20,21, consistent with the results of the present study. Whether urine PCR affects the eGFR difference is unclear; however, the degree of proteinuria tends to be low in the high-difference group22,23,24. In the present study, the urine PCR was also low in the high difference group, although the difference was not statistically significant. CysC is known to be associated with inflammation25; however, no significant difference was observed in C-reactive protein (CRP) levels between the low- and high-difference groups in the present study. This may be due to a low level of acute inflammation, as the participants in the present study were stable outpatients.
As CKD progresses, urinary phosphate excretion decreases, which causes hyperphosphatemia followed by secondary hyperparathyroidism through interrelated mechanisms26,27. In addition, the skeleton plays a very important role in the phosphorus balance. As CKD progresses, bone resorption increases and outpaces bone formation, which in turn increases the release of phosphorus into the blood. For this reason, we excluded patients taking phosphate-binding agents, calcitriol, and cinacalcet from our analysis, as these could significantly affect phosphorus and intact PTH levels. In any case, it is well-known that hyperphosphatemia and hyperparathyroidism are physiologic changes accompanying CKD progression28. The eGFRCysC and eGFRCr-CysC were identified as more useful indicators associated with hyperphosphatemia and hyperparathyroidism with hyperphosphatemia than eGFRCr was in the overall cohort, especially in the high-difference group. This suggests that measuring and evaluating CysC in addition to Cr is necessary to accurately diagnose the patient’s current condition and predict prognosis in the clinical field in patients with risk factors, such as CKD grade 3 or old age.
Interestingly, the average serum Cr level was lower and the proportion of CKD grade 3 based on eGFRCr was higher in the high-difference group. As CKD progresses, tubular Cr secretion increases, which is known to result in underestimation of kidney function29. However, tubular CysC secretion rarely occurs as it is freely filtered in the glomerulus and is mostly absorbed and degraded in the proximal tubule30. Therefore, the risk of underestimating the GFR by CysC may be relatively lower compared with that by Cr. In patients with CKD grade 3 based on the eGFRCr, the AuROC values of eGFRCysC and eGFRCr-CysC improved more markedly compared with that of eGFRCr. This suggests that evaluating CKD grade 3 using only eGFRCr is likely to result in a significant underestimation of kidney function.
Patients with eGFR difference > 15 ml/min/1.73 m2 were excluded from the present study according to a previous report which stated that a > 15 ml/min/1.73 m2 difference between eGFRCr and eGFRCysC is probably linked to a disproportionate effect of non-GFR factors by one of the two biomarkers (Cr and CysC)24,31. Although many previous studies have shown that eGFRCr-CysC has the highest accuracy with mGFR, it is necessary to keep in mind that the eGFRCr-CysC equation was developed in a relatively healthy population with an average age of 47 years and an mGFR of 68 ml/min/1.73 m2. This study is significant in that it reveals for the first time the usefulness of eGFRCr-CysC in relation to hyperphosphatemia and hyperparathyroidism, which are physiological changes following the progression of advanced CKD.
Our study has several limitations. First, because this was a retrospective, cross-sectional study, we could not analyze the patients’ future clinical course. Second, we used inorganic phosphorus and PTH levels as indicators of physiological changes in CKD, however, the change in fibroblast growth factor 23 (FGF-23) level precedes hyperphosphatemia and is observed concurrently with PTH level elevation32,33. Although FGF-23 may better reflect the physiological changes in CKD, it is not yet routinely measured in clinical practice. Nevertheless, it is significant that we analyzed a large population of over 600 patients, and this is the first study to compare Cr and CysC levels in association with hyperphosphatemia and secondary hyperparathyroidism with hyperphosphatemia.
In conclusion, patients aged > 70 years and with a low CKD grade according to the eGFRCr could be at high risk for a high difference in the eGFRCr and eGFRCysC. Compared with that of eGFRCr, the eGFRCysC and eGFRCr-CysC showed a stronger association with the physiological changes of CKD progression (hyperphosphatemia and hyperparathyroidism), and the effect was particularly evident in the high-difference group and low CKD grade subpopulation. Although the usefulness of CysC is well known, in actual clinical practice many physicians still evaluate kidney function based only on Cr. Evaluating kidney function by including CysC is necessary to accurately evaluate patient kidney function and predict prognosis, at least in selected patients (such as the elderly or those with CKD grade 3) based on factors related to the high-difference of |eGFRCr-eGFRCysC|.
Methods
Study design
This was a single-center retrospective cross-sectional study. We used outpatient clinic data from the Clinical Data Warehouse system of Konyang University Hospital between January 2019 and February 2022. We collected the data of adult patients (≥ 19 years old) diagnosed with CKD grade 3 or 4 as defined by the Kidney Disease: Improving Global Outcomes guideline (eGFRCr categories of grade 3: 30–59 ml/min/1.73 m2, grade 4: 15–29 ml/min/1.73 m2)34. Serum Cr, CysC, inorganic phosphorus, calcium, and PTH levels were tested on the same day (n = 835).
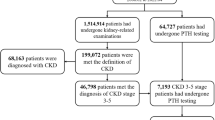
We excluded patients taking phosphate-binding agents (sevelamer, lanthanum, calcium-based phosphate binder) or PTH-lowering agents (calcitriol, cinacalcet) (n = 56), those with a history of cancer (n = 9), and those with a difference between eGFRCr and eGFRCysC > 15 ml/min/1.73 m2 (n = 131). Therefore, 639 patients were enrolled in this study and divided into low- and high-difference groups based on the median value of |eGFRCr-eGFRCysC|: < 6.353 ml/min/1.73 m2 and ≥ 6.353 ml/min/1.73 m2, respectively (Fig. 5).
Study design. Of the 835 patients with CKD grades 3 and 4 based on the eGFRCr, 196 were excluded according to the exclusion criteria, and 639 were finally included. Patients were divided into two groups based on a median |eGFRCr-eGFRCysC| of 6.353 ml/min/1.73 m2. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; eGFRCr, eGFR based on creatinine; eGFRCysC, eGFR based on cystatin C; PTH, parathyroid hormone.
This study was performed in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of Konyang University Hospital (KYUH 2022-10-003). The need to obtain informed patient consent was waived by the Institutional Review Board of Konyang University Hospital (KYUH 2022-10-003) because the patient data were extracted in an anonymized form.
Data collection
Baseline sociodemographic characteristics, including sex; age; history of HTN (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medications), diabetes mellitus (HbA1c ≥ 6.5%, fasting glucose level ≥ 126 mg/dl, or oral hypoglycemic agent or insulin administration), and cancer (patients diagnosed with the 10th Revision code of the International Classification of Diseases: C16, C18, C19, C22, C34, and C50), were obtained. We further collected information on the serum hemoglobin, albumin, CRP, uric acid levels, and urine PCR.
Calculations of eGFR Cr , eGFR CysC, and eGFR Cr-CysC
Serum Cr and CysC levels were measured using a Beckman Coulter AU5800. Serum Cr levels were measured with the isotope dilution mass spectroscopy-traceable Jaffe method using picric acid. Serum CysC levels were measured by turbidity analysis. The CKD-EPI eGFR equations, the 2021 CKD-EPI creatinine equation (eGFRCr), 2012 CKD-EPI cystatin C equation (eGFRCysC), and 2021 CKD-EPI creatinine-cystatin C equation (eGFRCr-CysC) were as follows: eGFRCr = 142 × min (SCr/κ, 1)a × max (SCr/κ, 1)−1.200 × 0.9938age × (1.012 if female), eGFRCysC = 133 × min (SCys/0.8, 1)−0.499 × max (SCys/0.8, 1)−1.328 × 0.996age × (0.932 if female), and eGFRCr-CysC = 135 × min (SCr/κ, 1)b × max (SCr/κ, 1)−0.544 × min (SCys/0.8, 1)−0.323 × max(SCys/0.8, 1)−0.778 × 0.9961age × (0.963 if female). SCr is the serum Cr level; SCys is the serum CysC level; κ is 0.7 for females and 0.9 for males; a is − 0.241 for females and − 0.302 for males, and b = − 0.219 for females and − 0.144 for males3,4,10.
Measurements and definition of hyperphosphatemia and hyperparathyroidism
Serum inorganic phosphorus levels were measured using a photometric ultraviolet test with molybdate using a Beckman Coulter AU5800. Serum PTH levels were measured by two-site immunoenzymatic assay using a Beckman Coulter DxI. Hyperphosphatemia was defined as a serum inorganic phosphorus level > 4.6 mg/dl, while hyperparathyroidism was defined as a serum PTH level > 65 pg/ml35. The AuROC values of the eGFR equations for the occurrence of hyperphosphatemia and hyperparathyroidism with hyperphosphatemia were analyzed.
Statistical analyses
The baseline sociodemographic characteristics and laboratory findings were compared between the low- and high-difference groups. Continuous variables are expressed as the mean and standard deviation and were compared using the Student’s t-test. Categorical variables are expressed as numbers (percentages) and were compared using the Chi-square or Fisher’s exact test, as appropriate. Since both eGFRCr and eGFRCysC showed a non-normal distribution, Spearman’s correlation analysis was performed in the overall cohort and low- and high-difference groups to analyze the correlation between eGFRCr and eGFRCysC. Multivariate binary logistic regression analysis was performed to delineate the relationship between the factors and the high difference in |eGFRCr-eGFRCysC|. We compared the AuROC values between eGFRCr, eGFRCysC, and eGFRCr-CysC to analyze the association intensity related to the occurrence of hyperphosphatemia and hyperparathyroidism with hyperphosphatemia in the overall cohort and low- and high-difference groups. A subgroup analysis was performed for patients with CKD grade 3 based on the eGFRCr, and the AuROC values of the three eGFR equations were compared. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA).
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
Levin, A. & Stevens, P. E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 85, 49–61 (2014).
Pathologists, C. O. A. Current status of reporting estimated glomerular filtration rate (eGFR). See https://www.cap.org/apps/docs/committees/chemistry/current_status_of_reporting_eGFR.pdf (last checked 15 June 2011) (2010).
Levey, A. S., Stevens, L. A. & Schmid, C. H. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 20–29 (2012).
McMahon, G. M. & Waikar, S. S. Biomarkers in nephrology: Core curriculum 2013. Am. J. Kidney Dis. 62, 165–178 (2013).
Tenstad, O., Roald, A. B., Grubb, A. & Aukland, K. Renal handling of radiolabelled human cystatin C in the rat. Scand. J. Clin. Lab. Invest. 56, 409–414 (1996).
Stevens, L. A. et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 75, 652–660 (2009).
Kwon, W. S., Kim, T. S., Nahm, C. H., Moon, Y. & Kim, J. J. Aberrant cystatin-C expression in blood from patients with breast cancer is a suitable marker for monitoring tumor burden. Oncol. Lett. 16, 5583–5590 (2018).
Zhang, X. et al. Clinical significance of detection of cathepsin X and cystatin C in the sera of patients with lung cancer. Zhongguo Fei Ai Za Zhi 16, 411–416 (2013).
Inker, L. A. et al. New Creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 385, 1737–1749 (2021).
Zou, L. X. et al. Comparison of bias and accuracy using cystatin C and creatinine in CKD-EPI equations for GFR estimation. Eur. J. Intern. Med. 80, 29–34 (2020).
Cheuiche, A. V., Queiroz, M., Azeredo-da-Silva, A. L. F. & Silveiro, S. P. Performance of cystatin C-based equations for estimation of glomerular filtration rate in diabetes patients: A prisma-compliant systematic review and meta-analysis. Sci. Rep. 9, 1418 (2019).
Costa, E. S. V. T. et al. A prospective cross-sectional study estimated glomerular filtration rate from creatinine and cystatin C in adults with solid tumors. Kidney Int. 101, 607–614 (2022).
Sprague, S. M., Martin, K. J. & Coyne, D. W. Phosphate balance and CKD-mineral bone disease. Kidney Int. Rep. 6, 2049–2058 (2021).
Zhou, C., Shi, Z., Ouyang, N. & Ruan, X. Hyperphosphatemia and cardiovascular disease. Front. Cell Dev. Biol. 9, 644363 (2021).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Eddington, H. et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 2251–2257 (2010).
Walston, J. D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 24, 623–627 (2012).
Kim, H. et al. The difference between cystatin C- and creatinine-based eGFR is associated with adverse cardiovascular outcome in patients with chronic kidney disease. Atherosclerosis 335, 53–61 (2021).
Kang, E. et al. Discrepant glomerular filtration rate trends from creatinine and cystatin C in patients with chronic kidney disease: Results from the KNOW-CKD cohort. BMC Nephrol. 21, 280 (2020).
Pinsino, A. et al. The difference between cystatin C- and creatinine-based assessment of kidney function in acute heart failure. ESC Heart Failure 9, 3139–3148 (2022).
Chen, D. C. et al. Association of intraindividual difference in estimated glomerular filtration rate by creatinine vs cystatin C and end-stage kidney disease and mortality. JAMA Netw. Open 5, e2148940 (2022).
Potok, O. A. et al. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: A cohort analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). Am. J. Kidney Dis. 76, 765–774 (2020).
Chen, D. C., Potok, O. A., Rifkin, D. & Estrella, M. M. Advantages, limitations, and clinical considerations in using cystatin C to estimate GFR. Kidney360 3, 1807–1814 (2022).
Shlipak, M. G. et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 369, 932–943 (2013).
Lim, E. et al. Effects of education on low-phosphate diet and phosphate binder intake to control serum phosphate among maintenance hemodialysis patients: A randomized controlled trial. Kidney Res. Clin. Pract. 37, 69–76 (2018).
Cunningham, J., Locatelli, F. & Rodriguez, M. Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options. Clin. J. Am. Soc. Nephrol. 6, 913–921 (2011).
Hruska, K. A., Mathew, S., Lund, R., Qiu, P. & Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 74, 148–157. https://doi.org/10.1038/ki.2008.130 (2008).
Zhang, X. et al. Tubular secretion of creatinine and kidney function: an observational study. BMC Nephrol. 21, 108 (2020).
Lee, H. S. et al. Comparison of glomerular filtration rates calculated by different serum cystatin C-based equations in patients with chronic kidney disease. Kidney Res. Clin. Pract. 33, 45–51 (2014).
An, J. N. et al. Serum cystatin C to creatinine ratio is associated with sarcopenia in non-dialysis-dependent chronic kidney disease. Kidney Res. Clin. Pract. 41, 580–590 (2022).
Jüppner, H. Phosphate and FGF-23. Kidney Int. 79, S24–S27. https://doi.org/10.1038/ki.2011.27 (2011).
Oh, K.-H. et al. The KNOW-CKD Study: What we have learned about chronic kidney diseases. Kidney Res. Clin. Pract. 39, 121–135 (2020).
Group K. D. I. G. O. C.-M. W. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 76, S1–S130 (2009).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Acknowledgements
This study was supported by the Konyang University Myunggok Research Fund (2018-05 and 2021-02). This study is an investigator-led project with no specific participation by funders.
Author information
Authors and Affiliations
Contributions
B.M.: participated in designing the study, collecting, analyzing, interpreting data, and writing the paper. S.R.Y.: participated in analyzing the data. S.H.Y.: participated in collecting the data. J.D.K.: participated in designing the study. W.J.H.: participated in analyzing and interpreting the data. W.M.H.: participated in designing the study, analyzing, and interpreting data. Y.P.: participated in designing the study, analyzing and interpreting data, and writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Min, B., Yun, SR., Yoon, SH. et al. Comparison of the association intensity of creatinine and cystatin C with hyperphosphatemia and hyperparathyroidism in patients with chronic kidney disease. Sci Rep 13, 3855 (2023). https://doi.org/10.1038/s41598-023-31048-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31048-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.