Abstract
Prognostic effect of discontinuing renin–angiotensin–aldosterone-system-inhibitor (RAASi) for patients with heart failure (HF) after acute myocardial infarction (AMI) whose left ventricular (LV) systolic function was restored during follow-up is unknown. To investigate the outcome after discontinuing RAASi in post-AMI HF patients with restored LV ejection fraction (EF). Of 13,104 consecutive patients from the nationwide, multicenter, and prospective Korea Acute Myocardial Infarction-National Institutes of Health (KAMIR-NIH) registry, HF patients with baseline LVEF < 50% that was restored to ≥ 50% at 12-month follow-up were selected. Primary outcome was a composite of all-cause death, spontaneous MI, or rehospitalization for HF at 36-month after index procedure. Of 726 post-AMI HF patients with restored LVEF, 544 maintained RAASi (Maintain-RAASi) beyond 12-month, 108 stopped RAASi (Stop-RAASi), and 74 did not use RAASi (RAASi-Not-Used) at baseline and follow-up. Systemic hemodynamics and cardiac workloads were similar among groups at baseline and during follow-up. Stop-RAASi group showed elevated NT-proBNP than Maintain-RAASi group at 36-month. Stop-RAASi group showed significantly higher risk of primary outcome than Maintain-RAASi group (11.4% vs. 5.4%; adjusted hazard ratio [HRadjust] 2.20, 95% confidence interval [CI] 1.09–4.46, P = 0.028), mainly driven by increased risk of all-cause death. The rate of primary outcome was similar between Stop-RAASi and RAASi-Not-Used group (11.4% vs. 12.1%; HRadjust 1.18 [0.47–2.99], P = 0.725). In post-AMI HF patients with restored LV systolic function, RAASi discontinuation was associated with significantly increased risk of all-cause death, MI, or rehospitalization for HF. Maintaining RAASi will be necessary for post-AMI HF patients, even after LVEF is restored.
Similar content being viewed by others
Introduction
Renin–angiotensin–aldosterone-system-inhibitors (RAASi) are recommended as a class I indication for patients with heart failure with reduced ejection fraction (HFrEF) as part of guideline-directed medical therapy (GDMT), as they alleviate symptoms, reduce hospitalizations due to heart failure (HF), and enhance overall survival1,2,3,4. GDMT for HFrEF can improve myocardial function in up to half of patients5,6,7, and patients with restored myocardial function (heart failure with restored ejection fraction [HFresEF]) tend to have better clinical outcomes8,9,10,11.
In clinical practice, a common question among patients with HFresEF is whether RAASi should be continued even after recovery of left ventricular (LV) systolic function despite potential issues with adverse drug reaction, polypharmacy, and financial burden12. Although the net benefit of continued RAASi for HFresEF has not yet been clearly determined12, there is also limited evidence or consensus on the feasibility and safety of discontinuation of RAASi after recovery of LV systolic function1,13,14. Notably, a substantial proportion of patients with HFresEF have remaining cardiac pathologies that increase risk of future adverse events, even with continued treatment15. Furthermore, in the recent TRED-HF trial, most asymptomatic dilated cardiomyopathy (DCM) patients with HFresEF eventually relapsed after stopping treatment12.
In patients with ischemic heart disease, RAASi may further reduce the risk of future coronary events in addition to their benefits on morbidity and mortality related to HF1,12. Furthermore, unlike DCM patients in which underlying pathophysiology cannot be changed, impaired myocardial perfusion in patients with AMI can be treated by revascularization, which would help recovery of myocardial function. Therefore, the prognostic impact of continuation or discontinuation of RAASi after recovery of LV systolic function in post-AMI patients may be different from that in DCM patients. In this regard, we aimed to investigate the prognostic impact of discontinuation of RAASi in post-AMI patients with HFresEF using a large prospective registry.
Methods
Study population
Study population of the present study was derived from a nationwide, multicenter, and prospective Korean Acute Myocardial Infarction-National Institutes of Health (KAMIR-NIH) registry. The KAMIR-NIH is a dedicated prospective registry which consecutively enrolled AMI patients at 20 tertiary university hospitals eligible for primary PCI from November 2011 to December 2015 without any exclusion criteria. Detailed study protocols have been published elsewhere16.
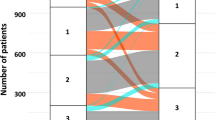
For the current analysis, we selected AMI patients with baseline LVEF < 50%, whose LV systolic function restored to ≥ 50% at 12-month follow-up echocardiographic examination. We excluded patients without echocardiography data at baseline or 12-month follow-up, without information on RAASi at 12-month follow-up, who died before 12-month follow-up echocardiography, or who were lost to follow-up after 12-month follow-up echocardiography. Of 13,104 consecutive patients from the KAMIR-NIH registry, 726 patients were selected and classified into 3 groups: (1) those who maintained RAASi treatment both at baseline and 12-month follow-up (Maintain-RAASi group); (2) stopped RAASi at 12-month follow-up, based on restored EF (Stop-RAASi group); or (3) did not use RAASi both at baseline and 12-month follow-up (RAASi-Not-Used group) (Fig. 1). Starting from the index date of 12-month follow-up echocardiography, patients were followed up to 24-month (36-month after AMI).
Study flow. Study flow of the present study is shown. From the KAMIR-NIH registry, a total of 726 HFresEF patients were selected for analysis and follow-up during 36-month after index procedure. Abbreviations: AMI, acute myocardial infarction; FU, follow-up; HFresEF, heart failure with restored ejection fraction; KAMIR-NIH, Korea Acute Myocardial Infarction-National Institutes of Health; LVEF, left ventricular ejection fraction; RAASi, renin–angiotensin–aldosterone-system-inhibitor.
The protocol of KAMIR-NIH was approved by the ethics committee (the Institutional Review Board of each participating center and Samsung Medical Center, Seoul, Korea) and was conducted according to the principles of the Declaration of Helsinki. All enrolled patients provided written informed consent. In cases of patients being unable to consent due to clinical status, a relative was informed and could provide consent on behalf of that patient.
Patient management, data collection and follow-up
Patient treatment was performed according to current standard practice. The choice of treatment strategy, type, diameter, and length of stents, the use of medications, intravascular imaging devices, thrombus aspiration, or hemodynamic support devices were left to the operator’s discretion. Unless there was an undisputed reason for discontinuing dual antiplatelet therapy, all patients were recommended to be given aspirin indefinitely plus clopidogrel or other potent antiplatelet agents, such as prasugrel or ticagrelor, for at least 1 year. Choice of P2Y12 inhibitors prescribed was left to operator’s discretion in accordance with the guidelines and patient bleeding risk.
Demographic features and cardiovascular risk factors were collected by patient interviews or review of medical records. During hospitalization, findings of coronary angiography and detailed procedural characteristics of percutaneous coronary intervention as well as information on discharge medications were collected. All patients were recommended to perform echocardiography during index admission and during the follow-up period annually after AMI using commercially available ultrasound systems. Assessment of systolic function was performed according to ASE/EACVI recommendations17. Follow-up data was recorded during the 36-month of follow-up after discharge. The data was completed by telephone interview if patients did not visit on their scheduled day of follow-up. Using a web-based case report form in the internet-based Clinical Research and Trial management system (iCReaT), independent clinical research coordinators collected all baseline data and clinical events up to 36-month follow-up (iCReaT Study No. C110016).
Study outcomes
The primary outcome was a composite of all-cause death, spontaneous MI, or rehospitalization for HF. Secondary endpoints included individual components of primary outcome. Spontaneous MI was defined as the recurrence of symptoms or the presence of electrocardiographic changes in association with a rise in cardiac biomarker levels above the upper limit of normal, and periprocedural MI was not included as a clinical outcome. All endpoints were defined according to the Academic Research Consortium definitions18. All clinical events were evaluated by an independent event adjudicating committee. The definition of study endpoints and the process of event adjudication is described in the previous publication of KAMIR-NIH investigators16,19.
Statistical analysis
Categorical variables were presented as numbers and relative frequencies (percentages) and were compared using the Chi-squared test. Continuous variables were expressed as mean ± standard deviation and were compared using the analysis of variance (ANOVA). Repeated measure ANOVA test was used to compare the overall difference of repeated measurements of systolic and diastolic blood pressures, heart rate, cardiac workload (calculated by multiplying systolic blood pressure and heart rate), LVEF, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) among the groups. Cumulative incidence of events at 36 months was calculated based on Kaplan–Meier censoring estimates, and comparison of clinical outcomes among groups was performed with the log-rank test. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using univariable and multivariable Cox proportional hazard models. Covariates included in multivariable model were selected if they were significantly different among the 3 groups or considered to have significant predictive values, which were as follows: age, sex, hypertension, diabetes mellitus, and LVEF at baseline. The Cox proportional hazard regression in a propensity-score matched cohort for main comparators (Maintain-RAASi group vs. Stop-RAASi group) was performed. A multivariable logistic regression model was used to generate propensity-scores which indicate the probability that one would be in the Stop-RAASi group. The logistic regression model included age, sex, body mass index, hypertension, diabetes mellitus, dyslipidemia, previous MI, previous angina, previous atrial fibrillation, previous cerebrovascular accident, current smoking, familial history of coronary artery disease, Killip class, presented as STEMI, cardiogenic shock, 3-vessel disease, left main coronary artery disease, use of ECMO, LVEF at baseline, and LVEF at 1-year as covariates. A 1:1 matching process without replacements was performed by a greedy algorithm with a caliper width of 0.25 standard deviations, yielding 105 patients in the Stop-RAASi group matched with 105 controls in the Maintain-RAASi group. All probability values were two-sided and P values < 0.05 were considered statistically significant. All analyses were conducted using Stata software, version 14.0 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Baseline characteristics
Baseline characteristics are described in Table 1. We analyzed 726 AMI patients whose LVEF was below 50% at baseline but restored at 12-month follow-up according to treatment strategy of RAASi (544 maintained RAASi at 12-month [Maintain-RAASi group], 108 stopped RAASi at 12-month, based on restored EF [Stop-RAASi group], and 74 did not use RAASi at baseline or during 12-month follow-up [RAASi-Not-Used group]). Starting from the date of 12-month follow-up echocardiography, median follow-up duration of the study population was 740.0 days. Except for a higher proportion of hypertension in the Maintain-RAASi group, proportions of cardiovascular risk factors were similar among the 3 groups.
Patients who presented with cardiogenic shock or received cardiopulmonary resuscitation were more prevalent in RAASi-Not-Used group than others. However, procedural characteristics including use of mechanical hemodynamic support were not different among the 3 groups. Except RAASi, profiles of discharge medications were similar among groups. At discharge, 73.9% used angiotensin-converting enzyme inhibitors and 26.1% angiotensin receptor blockers, while the proportion of angiotensin receptor blockers increased to 53.9% at 12-month and angiotensin-converting enzyme inhibitors decreased to 46.1%.
Change of systemic hemodynamics and biomarkers during follow-up
Systolic blood pressure, diastolic blood pressure, and cardiac workload consistently increased after the index procedure during the follow-up (Fig. 2). Among the 3 groups, Maintain-RAASi and Stop-RAASi groups showed higher systolic and diastolic blood pressure than RAASi-Not-Used group at baseline, 12-month, and 36-month follow-up. Conversely, cardiac workload was similar among the 3 groups at baseline, 12-month, and 36-month follow-up.
Changes of systemic hemodynamics according to treatment strategy of RAAS inhibitor after post-AMI 12-month. Serial change of systemic hemodynamic parameters, (A) SBP, (B) DBP, (C) heart rate, and (D) cardiac workload is presented in 3 groups; Maintain-RAASi, Stop-RAASi, and RAASi-Not-Used groups. Abbreviations: b.p.m., beats per minute; DBP, diastolic blood pressure; SBP, systolic blood pressure; otherwise as in Fig. 1.
LVEF at 12-months restored similarly among all groups (Maintain-RAASi group, 57.6 ± 6.2%; Stop-RAASi group, 57.2 ± 5.7%; RAASi-Not-Used group, 57.2 ± 5.9%), and tended to decrease more in RAASi-Not-Used group (52.8 ± 5.8%) than Stop-RAASi group (55.3 ± 9.0%) and Maintain-RAASi group (56.0 ± 8.7%) at 36-month (Fig. 3). The NT-proBNP level was similar among the 3 groups at baseline, however, Stop-RAASi group showed increased NT-proBNP level than Maintain-RAASi group at both 12-month and 36-month. RAASi-Not-Used group showed the highest level of NT-proBNP during the follow-up period (Fig. 3).
Changes of LV systolic function and levels of NT-proBNP according to Treatment Strategy of RAAS Inhibitor after Post-AMI 12-Month. Serial change of (A) LV ejection fraction, and (B) concentration of NT-proBNP is presented in 3 groups; Maintain-RAASi, Stop-RAASi, and RAASi-Not-Used groups. Abbreviations: LV, left ventricular; NT-proBNP, N-terminal pro-B-type natriuretic peptide; otherwise as in Fig. 1.
Outcomes at 36-month according to treatment strategy of RAAS inhibitor
At 36-month after index procedure, the risk of all-cause death, spontaneous MI, or rehospitalization due to HF in Stop-RAASi group was significantly higher than Maintain-RAASi group (11.4% vs. 5.4%; adjusted HR 2.09, 95% CI 1.02–4.28, P = 0.043), mainly driven by increased risk of all-cause death in the Stop-RAASi group. Regarding the main comparator groups (Stop-RAASi group vs. Maintain-RAASi group), the proportionality assumptions were not violated for all outcomes (Supplementary Fig. 1 and 2). The cumulative incidence of primary outcome was similar between Stop-RAASi group and RAASi-Not-Used group (11.4% vs. 12.1%; adjusted HR 1.18, 95% CI 0.47–2.99, P = 0.725) (Table 2 and Fig. 4). Comparison of secondary outcomes showed similar trends (Supplementary Fig. 3). However, outcomes related with coronary revascularization, especially target vessel or target lesion revascularization, were similar among three groups (Supplementary Table 4). The results were consistently observed in a well-balanced 1:1 propensity-score matched cohort (Supplementary Table 1 and 2). The results were also consistent in another multivariable model including aspirin, beta-blocker, and statin use at 1-year (Supplementary Table 3).
Comparison of composite of all-cause death or spontaneous MI after post-AMI 12-month according to treatment strategy of RAAS inhibitor. Comparison of cumulative incidence and Kaplan–Meier curves of (A) a composite of all-cause death, spontaneous MI, or rehospitalization for HF, and (B) all-cause death or spontaneous MI among the 3 groups; Maintain-RAASi, Stop-RAASi, and RAASi-Not-Used groups, are presented. Abbreviations: HF, heart failure, otherwise as in Fig. 1.
Discussion
In this nationwide, multicenter, and prospective AMI registry, we evaluated the prognostic impact of discontinuation of RAASi in HFresEF patients. Major findings were as follows: (1) Although LVEF at 36 months was similar between the Maintain-RAASi group and the Stop-RAASi group, NT-proBNP level at 36 months was the lowest in the Maintain-RAASi group among the 3 groups; (2) the Stop-RAASi group had a significantly higher cumulative incidence of all-cause death, MI, or HF readmission compared with the Maintain-RAASi group; and (3) cumulative incidence of all-cause death, MI, or HF readmission in the Stop-RAASi group was similar with that of RAASi-Not-Used group.
Discontinuation of GDMT after recovery of LV systolic function in HF patients
GDMT for HF can improve LVEF by > 10% in more than one-third of patients with HF20,21. Reverse remodeling of the LV, which is defined by decrease in LV size, regression of hypertrophy and fibrosis, and improvement in systolic function, is well known to be associated with improved symptoms and clinical outcomes20,22. In this regard, recent guidelines developed a new subset of HFrEF patients whose LVEF is restored (HFresEF)23,24. The need for continuation of HF medications after recovery of LVEF can sometimes be questioned due to adverse drug reactions, financial burden, polypharmacy, and uncertain benefit of indefinite HF medications in patients with HFresEF25.
To date, a few anecdotal studies and one randomized trial investigated the impact of discontinuation of medications in various subgroups of HF patients25,26,27,28,29. Discontinuation of HF medications after recovery of LVEF in DCM patients was associated with relapse of HF and LV dysfunction25,29. In a small study with 15 patients with HFresEF, discontinuing beta-blockers resulted in a substantial decrease in LVEF, which was then restored after resumption of the beta-blockers27. Recently, the TRED-HF trial exclusively evaluated DCM patients and reported that approximately half of patients in the medication discontinuation group eventually had a substantial decline in LV systolic function, a rise in LV end-diastolic volume or NT-proBNP level, or clinical deterioration25. Furthermore, in the substudy of TRED-HF trial, medication discontinuation resulted in rapid remodeling of LV, with early tissue and functional changes, even among patients who did not relapse30. However, these studies were primarily focused on patients with DCM, and more importantly, could not investigate the impact of medication discontinuation on mortality.
Continuation of GDMT in Post-AMI Patients with HFresEF
In contrast to DCM patients for whom GDMT is almost their only therapeutic option, successful revascularization in post-AMI patients with HFrEF can help recovery of viable myocardium and LV systolic function. Therefore, the prognostic impact of discontinuation of HF medications after recovery of LVEF can be different between the 2 patient populations. However, there has been no previous study focusing on post-AMI patients with HFresEF. In the current study, discontinuation of RAASi was associated with significantly higher NT-proBNP levels and cumulative incidence of all-cause death, MI, or HF readmission compared with continuation of RAASi in post-AMI patients with HFresEF. Importantly, discontinuation of RAASi was associated with almost 3 times higher risk of death than continuation of RAASi in this patient population. Interestingly, patient prognosis in the Stop-RAASi group was comparable with that in the RAASi-Not-Used group.
Our findings corroborate prior studies from DCM patients demonstrating that the recovery of systolic function per se should not be taken as a cure of HF but rather as a remission or temporary discontinuation of a negative stimulus31, providing a rationale for indefinite GDMT without interruption in patients with HFrEF due to their ischemic etiology even after successful revascularization32. Notably, the present study was particularly important since it first demonstrated (1) impact of treatment discontinuation in the homogenous population of ischemic HF occurred after AMI and (2) increased mortality after discontinuation of HF medications in patients with HFresEF. Another issue regarding the prognostic impact of RAASi in patients with ischemic HF is reduction in additional coronary events, such as spontaneous MI25. In the current results, the 2-year risk of spontaneous MI was numerically higher in the Stop-RAASi group than the Maintain-RAASi group without statistical significance (3.5% vs. 2.1%, respectively). Larger prospective studies with longer follow-up duration are required to clarify whether discontinuation HF medications, especially RAASi, would increase future coronary events.
Possible mechanisms and future perspectives
In general, relapsing HF and related events in patients with HFresEF might be caused by the partial reversal of the HF phenotypes superimposed on the permanent myocardial damage33. It is known that even with continued GDMT, many HFresEF patients have remaining impaired cardiac mechanics and are still at significant risk for clinical events34. Recent TRED-HF substudy presented that short-term unfavorable remodeling after discontinuation of RAASi might be facilitated by cellular alterations such as calcium dysregulation, energetic malfunction, or sarcomeric dysfunction30. In the present study, although LVEF was similar between the Maintain-RAASi group and the Stop-RAASi group (56.0 ± 8.7% vs. 55.3 ± 9.0%, respectively), there was substantial difference in NT-proBNP levels between the 2 groups at 24-month follow-up (36 months after AMI), which suggested unfavorable cardiac mechanics and remodeling with discontinuation of RAASi. Although our data did not include any parameters of cardiac structure or function other than LVEF, these results strongly suggest that recovered LV function alone is not a clinically useful indicator that may advise discontinuation of HF medications, even in post-AMI HFresEF patients32. Also, these observations implied the importance of regular surveillance and continuation of GDMT including RAASi in HFresEF patients35. Future studies should determine which component of GDMT is more critical to prevent relapse, and also should focus on elucidating the characteristics that distinguish remission from permanent recovery in post-AMI HFresEF patients. In addition, further analysis to evaluate the change of cardiac structural and functional parameters and the subclinical markers of LV systolic function such as LV strain after discontinuing RAASi in HFresEF.
Study limitations
Several limitations should be discussed. First, since we analyzed an observational prospective cohort, unmeasured confounding factors may have altered the results. Furthermore, the limited sample size of the Stop-RAASi groups and borderline statistical significance in the propensity score matched group analysis should be interpreted with caution. Until future randomized controlled trial is available, the current study results should be interpreted as hypothesis-generating study. Second, selection bias cannot be excluded since a substantial number of participants were omitted due to a lack of echocardiography data at post-AMI 12-month follow-up. Third, the number of analyzed patients in this study might not be sufficient to identify significant differences in LVEF or NT-proBNP level among the 3 groups. Fourth, the reason for discontinuing RAASi of each patient was not clearly reported. Finally, the effect of different proportions of patients who were taking beta-blockers or statins at 12-month after AMI between the Maintain-RAASi and the Stop-RAASi groups cannot be ignored.
Conclusion
In post-AMI HF patients with restored LV systolic function, discontinuation of RAASi was associated with a significantly increased risk of all-cause death, MI, or rehospitalization for HF. This result strongly suggests the importance of continuation of RAASi in post-AMI patients with HFrEF, even after recovery of LVEF. Further randomized controlled trial is needed to confirm the results from the current hypothesis-generating study.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the data management policy of the KAMIR investigators, but are available from the corresponding author on reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- CI:
-
Confidence interval
- DCM:
-
Dilated cardiomyopathy
- EF:
-
Ejection fraction
- HFresEF:
-
Heart failure with restored ejection fraction
- HR:
-
Hazard ratio
- MI:
-
Myocardial infarction
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- PCI:
-
Percutaneous coronary intervention
- RAASi:
-
Renin–angiotensin–aldosterone-system-inhibitors
References
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 42, 3599–3726 (2021).
Garg, R. et al. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 273, 1450–1456 (1995).
Packer, M. et al. Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation 100, 2312–2318 (1999).
Investigators*, S. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325, 293–302 (1991).
Ghio, S. et al. Long-term left ventricular reverse remodelling with cardiac resynchronization therapy: Results from the CARE-HF trial. Eur. J. Heart Fail. 11, 480–488 (2009).
Kramer, D. G. et al. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: A meta-analytic approach. J. Am. Coll. Cardiol. 56, 392–406 (2010).
Nijst, P. et al. Withdrawal of neurohumoral blockade after cardiac resynchronization therapy. J. Am. Coll. Cardiol. 75, 1426–1438 (2020).
Basuray, A. et al. Heart failure with recovered ejection fraction: Clinical description, biomarkers, and outcomes. Circulation 129, 2380–2387 (2014).
Florea, V. G., Rector, T. S., Anand, I. S. & Cohn, J. N. Heart failure with improved ejection fraction: Clinical characteristics, correlates of recovery, and survival: Results from the valsartan heart failure trial. Circ. Heart Fail. 9, e003123 (2016).
Kalogeropoulos, A. P. et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 1, 510–518 (2016).
Punnoose, L. R. et al. Heart failure with recovered ejection fraction: A distinct clinical entity. J. Cardiac Fail. 17, 527–532 (2011).
Halliday, B. P. et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. The Lancet 393, 61–73 (2019).
Wilcox, J. & Yancy, C. W. Stopping medication for heart failure with improved ejection fraction. The Lancet 393, 8–10 (2019).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiologia Polska (Polish Heart J.) 74, 1037–1147 (2016).
Adamo, L. et al. Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ. Heart Fail. 10, e003788 (2017).
Kim, J. H. et al. Multicenter cohort study of acute myocardial infarction in Korea-interim analysis of the Korea acute myocardial infarction registry-national institutes of health registry. Circ. J. 80, 1427–1436. https://doi.org/10.1253/circj.CJ-16-0061 (2016).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Garcia-Garcia, H. M. et al. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation 137, 2635–2650. https://doi.org/10.1161/CIRCULATIONAHA.117.029289 (2018).
Lee, J. M. et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J. Am. Coll. Cardiol. 71, 844–856. https://doi.org/10.1016/j.jacc.2017.12.028 (2018).
Merlo, M. et al. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J. Am. Coll. Cardiol. 57, 1468–1476. https://doi.org/10.1016/j.jacc.2010.11.030 (2011).
Wilcox, J. E. et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: Findings from IMPROVE HF. Am. Heart J. 163, 49-56 e42. https://doi.org/10.1016/j.ahj.2011.10.001 (2012).
Tayal, U. et al. Predictors of left ventricular remodelling in patients with dilated cardiomyopathy—A cardiovascular magnetic resonance study. Eur. J. Heart Fail. 22, 1160–1170. https://doi.org/10.1002/ejhf.1734 (2020).
Yancy, C. W. et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 68, 1476–1488. https://doi.org/10.1016/j.jacc.2016.05.011 (2016).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016).
Halliday, B. P. et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomised trial. Lancet 393, 61–73. https://doi.org/10.1016/S0140-6736(18)32484-X (2019).
Waagstein, F., Caidahl, K., Wallentin, I., Bergh, C. H. & Hjalmarson, A. Long-term beta-blockade in dilated cardiomyopathy. Effects of short- and long-term metoprolol treatment followed by withdrawal and readministration of metoprolol. Circulation 80, 551–563. https://doi.org/10.1161/01.cir.80.3.551 (1989).
Swedberg, K., Hjalmarson, A., Waagstein, F. & Wallentin, I. Adverse effects of beta-blockade withdrawal in patients with congestive cardiomyopathy. Br. Heart J. 44, 134–142. https://doi.org/10.1136/hrt.44.2.134 (1980).
Pflugfelder, P. W., Baird, M. G., Tonkon, M. J., DiBianco, R. & Pitt, B. Clinical consequences of angiotensin-converting enzyme inhibitor withdrawal in chronic heart failure: A double-blind, placebo-controlled study of quinapril The Quinapril Heart Failure Trial Investigators. J. Am. Coll. Cardiol. 22, 1557–1563. https://doi.org/10.1016/0735-1097(93)90578-O (1993).
Moon, J. et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can. J. Cardiol. 25, e147-150. https://doi.org/10.1016/s0828-282x(09)70497-0 (2009).
Halliday, B. P. et al. Myocardial remodelling after withdrawing therapy for heart failure in patients with recovered dilated cardiomyopathy: Insights from TRED-HF. Eur. J. Heart Fail. 23, 293–301. https://doi.org/10.1002/ejhf.2063 (2021).
Wilcox, J. E. & Yancy, C. W. Heart failure—A new phenotype emerges. JAMA Cardiol. 1, 507–509. https://doi.org/10.1001/jamacardio.2016.1356 (2016).
Wilcox, J. & Yancy, C. W. Stopping medication for heart failure with improved ejection fraction. Lancet 393, 8–10. https://doi.org/10.1016/S0140-6736(18)32825-3 (2019).
Mann, D. L., Barger, P. M. & Burkhoff, D. Myocardial recovery and the failing heart: Myth, magic, or molecular target?. J. Am. Coll. Cardiol. 60, 2465–2472. https://doi.org/10.1016/j.jacc.2012.06.062 (2012).
Adamo, L. et al. Abnormal global longitudinal strain predicts future deterioration of left ventricular function in heart failure patients with a recovered left ventricular ejection fraction. Circ. Heart Fail. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003788 (2017).
Enzan, N. et al. Beta-blocker use is associated with prevention of left ventricular remodeling in recovered dilated cardiomyopathy. J. Am. Heart Assoc. 10, e019240. https://doi.org/10.1161/JAHA.120.019240 (2021).
Acknowledgements
This research was supported by a fund by Research of Korea Centers for Disease Control and Prevention (2016-ER6304-01).
Author information
Authors and Affiliations
Consortia
Contributions
S.H.L., T-M.R., and J.M.L. wrote the main manuscript text and prepared tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S.H., Rhee, TM., Shin, D. et al. Prognosis after discontinuing renin angiotensin aldosterone system inhibitor for heart failure with restored ejection fraction after acute myocardial infarction. Sci Rep 13, 3539 (2023). https://doi.org/10.1038/s41598-023-30700-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30700-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.