Abstract
Microplastic (MP) pollution is a major concern in aquatic environments. Many studies have detected MPs in fishes; however, little is known about differences of microplastic uptake by fish in freshwater (FW) and those in seawater (SW), although physiological conditions of fish differ significantly in the two media. In this study, we exposed larvae (21 days post-hatching) of Oryzias javanicus (euryhaline SW) and Oryzias latipes (euryhaline FW), to 1-µm polystyrene microspheres in SW and FW for 1, 3, or 7 days, after which, microscopic observation was conducted. MPs were detected in the gastrointestinal tracts in both FW and SW groups, and MP numbers were higher in the SW group in both species. Vertical distribution of MPs in the water, and body sizes of both species exhibited no significant difference between SW and FW. Detection of water containing a fluorescent dye revealed that O. javanicus larvae swallowed more water in SW than in FW, as has also been reported for O. latipes. Therefore, MPs are thought to be ingested with water for osmoregulation. These results imply that SW fish ingest more MPs than FW fish when exposed to the same concentration of MPs.
Similar content being viewed by others
Introduction
Microplastics (MPs) are generally defined as plastic particles smaller than 5 mm1. In recent decades, MPs have become a major global issue in freshwater (FW) and marine environments2,3. MPs can have adverse effects on aquatic organisms, including fish. Recent studies have detected MPs in the bodies of both FW and marine fish4,5,6,7,8, mostly in the gastrointestinal tracts and gills of fish9,10,11.
Marine and FW fish experience very different physiological conditions, since osmotic pressure in the two media differs greatly. As marine fish are constantly dehydrated by hyperosmotic seawater (SW), they drink ambient SW almost continuously to compensate for the water loss. From ingested SW, marine fish adsorb sodium and chloride ions in the intestine, and then absorb water, the osmolality of which is reduced by this desalination. Absorbed monovalent ions are eliminates by chloride cells in the gill. In contrast, hypoosmotic FW tends to diffuse into FW fish, due to the osmotic pressure difference; thus, FW fish drink less water12,13. Such differences in water uptake and osmoregulation may influence MP ingestion. However, previous studies of MPs in fish have investigated either FW or marine species only14,15,16, thus the difference of MP ingestion in the two media was not clear because no reports have compared MP uptake in FW and SW using the same species.
Here, we employed medaka (Oryzias) fishes to demonstrate differences in MP uptake in the two different media. Medaka are an attractive model fish for ecotoxicology studies because of their low rearing cost, high fertility, ease in rearing of embryos, abundant developmental information, and availability of a complete genome sequence17,18,19,20,21. Moreover, a major advantage of medaka is the availability of related species that inhabit different osmotic environments with different euryhalinities22,23. In this study, we used two species, Japanese medaka (Oryzias latipes), which inhabits FW, but which adapts to SW, and Javanese medaka (Oryzias javanicus), which inhabits SW/brackish water, but which adapts to FW22,23. We used larvae (third larval stage; stage 42, 21 days post-hatching [dph])19 as a model animal. Larvae at this developmental stage have internal organs already developed as in adult fish, despite their relatively small body size, which is beneficial for easier in situ observation of ingested MPs. Here, we exposed medaka larvae reared in different media (SW and FW) to fluorescent-labeled polystyrene particles (1 µm in size) for 7 days and detected these particles in their bodies using a fluorescence microscope. We suggest that the difference in MP uptake in SW and FW environments is caused by the difference in water consumption.
Results
Microplastic distribution in the larval body
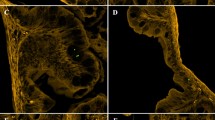
We exposed O. javanicus larvae (21 dph) that had been reared in 31-parts per thousand (ppt) SW (SW-Oj) and larvae acclimated to FW (FW-Oj) to 1-µm MPs for up to 7 days. After 1, 3, or 7 days of exposure, larvae were fixed and transparentized, and MP distribution was observed under a fluorescence microscope. We found that most MPs accumulated in the guts of both SW-Oj and FW-Oj, and that SW-Oj showed higher accumulations than FW-Oj (Fig. 1). We also observed some MPs near eye vesicles, but their positions were not determined precisely because tissues became less well-defined during transparentization (Supplementary Fig. S1). A similar experiment was conducted using O. latipes larvae reared in FW (FW-Ol) and those acclimated to 20-ppt SW (SW-Ol). We used SW-Ol acclimated to 20-ppt SW because exposure to 31-ppt SW caused high mortality in a preliminary experiment (Supplementary Fig. S2). Results were almost the same as with O. javanicus. MPs accumulated in the gut and were more abundant in SW-Ol than FW-Ol (Fig. 2). Fluorescent signals were not observed in transparentized negative control larvae of either species (Figs. 1, 2).
Microplastic distribution in the gastrointestinal tract of Oryzias javanicus (Oj) reared in seawater and freshwater. Images show Oj larvae exposed to fluorescently labeled microplastics at 0.25 mg/L for 1, 3, or 7 days. Images for negative controls were taken of Oj larvae cultured in water without microplastics for 1, 3, or 7 days. The scale bar indicates 0.5 mm. White and black arrowheads show the green fluorescent signal from microplastics in the gastrointestinal tracts of larvae. Caudal fins are not shown in this figure.
Microplastic distribution in the gastrointestinal tracts of Oryzias latipes (Ol) reared in seawater and freshwater. Images show Ol larvae exposed to fluorescently labeled microplastics at 0.25 mg/L for 1, 3, or 7 days. Images for negative controls were taken of Ol larvae cultured in water without microplastics for 1, 3, or 7 days. The scale bar indicates 0.5 mm. White and black arrowheads show the green fluorescent signal from microplastic in the gastrointestinal tracts of larvae. Caudal fins are not shown in this figure.
Comparison of microplastic numbers accumulated in larvae
MPs in larvae were extracted, filtered, and counted using a fluorescence microscope. Results were normalized by body weight (Fig. 3). No MPs were detected in negative controls in any exposure group, showing that no MP contamination occurred. In O. javanicus, MP numbers were higher in seawater exposure groups (SW-Oj) than freshwater groups (FW-Oj) throughout the experimental period (Fig. 3a). Similar results were also found in O. latipes. MP numbers in SW-Ol were higher than in FW-Ol fish (Fig. 3b). Differences in mean MP numbers between SW and FW fish in both species were statistically significant (P < 0.05) in all sampling periods. These results suggest that fish in SW ingest greater number of MPs when exposed to the same MP concentrations. MP uptake showed a gradual increase with exposure time (day 1, 3, and 7) in all exposure groups, and differences were statistically significant on days 1, 3, 7 for SW-Ol, and between days 1 and 7 for FW-Ol (Fig. 3b).
Microplastic uptake in medaka larvae. (a) Oryzias javanicus (Oj), (b) O. latipes (Ol). Larvae were exposed to microplastics (0.25 mg/L) for 1, 3, or 7 days. The black bar (SW-Oj, SW-Ol) indicates uptake concentrations in seawater groups, whereas the grey bar (FW-Oj, FW-Ol) shows uptake concentrations in freshwater groups. Each bar represents the mean of uptake concentration ± standard error of the mean (SEM). Different letters above the bars indicate significant differences, which were analyzed using one-way analysis of variance (ANOVA) (P < 0.05) followed by Tukey’s Honestly Significant Difference post-hoc test.
Vertical distribution of microplastics in seawater and freshwater
We considered the possibility that a difference in MP buoyancy in the two media might have contributed to the difference in MP uptake; therefore, MP vertical distribution was examined in FW and SW using the same beakers and aeration system as in the exposure experiment. This comparison revealed that MPs were distributed evenly from the surface to the bottom in SW (P = 0.4607) and FW (P = 0.2001) (Fig. 4). Accordingly, the difference in MP uptake in SW and FW cannot be attributed to MP buoyancy differences.
Microplastic vertical distribution. (a) Seawater, (b) Freshwater. Vertical distributions of polystyrene microplastics (0.25 mg/L) at the surface, middle, and bottom of the solution were measured using a microplate reader. The black bar indicates the MP particle number at the surface. The magenta bar indicates the MP particle number in the middle, whereas the blue bar shows the concentration at the bottom of the solution in seawater and freshwater. Differences between areas were analyzed for significance using one-way ANOVA (ns non-significant P > 0.05).
Comparison of morphological features between seawater and freshwater larvae
We also considered the possibility that larval body sizes may have differed between FW and SW, and that such a size difference might have caused the difference of MP uptake. Thus, total length, mouth width, head width, and body width were measured and compared among SW-Oj, FW-Oj, SW-Ol and FW-Ol larvae at 21 days post-hatching (Fig. 5). Nonetheless, no statistically significant differences in any measurements were found among groups. However, SW larval groups (SW-Oj and SW-Ol) showed slightly greater values in mouth and head width measurements compared to FW groups (FW-Oj and FW-Ol) (Fig. 5c,d).
Body Measurements of Oryzias javanicus and O. latipes larvae reared in freshwater and seawater. (a) Body length, (b) Body width, (c) Head width, (d) Mouth width. Morphological features of each medaka group were measured using a microscope. The black bar indicates seawater-reared O. javanicus (SW-Oj). The grey bar indicates freshwater-reared O. javanicus (FW-Oj). The brown bar shows seawater-reared O. latipes (SW-Ol), whereas the orange bar shows freshwater-reared O. latipes (FW-Ol). Differences between areas were analyzed for significance using one-way ANOVA (ns non-significant P > 0.05).
Water drinking observation
Water drinking in larvae of SW-Oj and FW-Oj was compared using SW and FW containing fluorescein isothiocyanate (FITC)-labeled dextran. After 3 h immersion in the respective media, FITC signals were observed under a fluorescence microscope. FITC signals were found in all SW-Oj larvae, except FW-Oj larvae (n = 3) (Supplementary Fig. S3).
Discussion
In this study, we compared MP uptake under different salinities using two species of medaka fishes, O. javanicus and O. latipes. O. javanicus larvae in SW (SW-Oj) ingested more MPs than those in FW (FW-Oj) (Figs. 1, 3a). Although O. javanicus can adapt to FW22,23, its native habitat is SW or brackish water (BW)24. Considering that O. javanicus is under stress in FW, the same experiment was conducted using O. latipes, which is a FW fish that can adapt to SW. When exposed to MPs, larvae adapted to 20-ppt SW accumulated more MPs than those reared in FW (Figs. 2, 3b). We used O. latipes larvae acclimatized to 20-ppt SW because exposure to concentrations higher than 20-ppt caused high mortality (Supplementary Fig. S2). Although it has been reported that adult O. latipes can adapt to 35-ppt SW22, larvae 7 to 21 dph may be less adaptable to SW25. Salinity of 20-ppt is unquestionably hyperosmotic for O. latipes larvae26. Thus, we concluded that larvae of both medaka fishes ingest more MPs in SW than FW, irrespective of whether the native habitat is SW, BW, or FW.
As SW and FW have different densities (Supplementary Table S1), we assumed that the vertical distribution of MPs might differ in the two media. Since medaka prefer the water surface24,27, the vertical distribution of MPs might have caused a difference in uptake. Therefore, we compared the vertical distribution of MPs in FW and SW under our experimental conditions. However, we found that MPs were uniformly distributed from the surface to the bottom in both SW and FW, suggesting that vertical MP distribution was not a factor.
We also compared total length, body width, head width, and mouth width of SW- and FW-acclimated larvae of both species, considering the possibility that osmotic stress affected body size, because growth hormone is involved in seawater adaptation in some fishes28. However, none of these parameters differed significantly, indicating that differences of MP uptake were not caused by differences of body size. Interestingly, mouth and head widths of seawater larvae (SW-Oj and SW-Ol) tended to be greater compared than those of the freshwater medaka (FW-Oj and FW-Ol) (Fig. 5), although these differences were not statistically significant. Implications of this result will be discussed later.
Previous studies have proposed several pathways of MP uptake in fish, such as by ingestion due to food/prey misidentification, transfer via the food chain, and passive ingestion via water drinking29. In all four experimental groups, MP particles were detected predominantly in the gut. This suggests that particles were ingested. The 1-µm MP particles used in this study were too small to be mistaken for food; therefore, we think that the main factor affecting MP uptake in O. javanicus and O. latipes larvae is water drinking for osmoregulation. Basically, osmolality of fish body fluids is maintained at approximately one third that of SW, in both SW fish and FW fish; thus, SW is hyperosmotic and FW is hypoosmotic for teleosts. Accordingly, teleosts must regulate body fluid osmolality in opposite directions in SW and FW, and euryhaline fishes can conduct both FW- and SW-type osmoregulation30. Teleosts in SW drink water to compensate for water loss in high salinity environments. They desalinize ingested SW in the esophagus and intestine, and then absorb water12. Thus, drinking is critical for adaptation to hyperosmotic environments. In contrast, FW fishes drink less water because a large amount of water enters their tissues by osmosis31. In O. latipes larvae, more water drinking in SW than FW has been reported32. In the present study, we compared water drinking in SW- and FW-acclimated O. javanicus and demonstrated higher water uptake in SW than in FW (Supplementary Fig. S3). Moreover, in this study, the mass of MPs in the guts of SW-Oj was approximately 4.5 times higher than of FW-Oj, and that of SW-Ol was 6 times higher than FW-Ol. Higher water drinking rates in SW than in FW have been reported in many other euryhaline teleosts13,33,34,35,36. Therefore, increased MP uptake in SW is likely to occur generally in euryhaline teleosts, and marine teleosts may ingest more MPs than FW teleosts, given the same concentration of MPs in the environment.
The tendency for wider bodies and mouths in SW-reared larvae, mentioned above, may be related to active drinking rates. Larvae in SW are presumed to open their mouths more frequently, to drink water, than those in FW. Bone staining of teleost larvae in previous reports show that the heads and mouths of teleost larvae initially consist of cartilaginous tissues, and ossification of the head and mouth commences later37,38. Medaka larvae used in this study correspond to stages with cartilaginous heads and mouths. Since cartilage is soft, elastic, flexible skeletal tissue, we assumed that enhanced drinking in early developmental stages may influence head and mouth structure. This idea is consistent with previous articles, reporting that physical activity can affect cartilage development in healthy human children39.
The concentration of MPs ingested in this study showed a gradual increase with exposure time in all exposure groups, especially in O. latipes (Fig. 3). This result was consistent with a previous report, using seabass, showing increased organ MP accumulation with longer exposure40. These results imply that the rate of elimination of MP particles is slower than the rate of ingestion, and that fish in both FW and SW accumulate MP particles, although rates of accumulation may differ, depending on particle size, shape, and concentration41.
Recent studies using different species of freshwater and seawater fish also reported that MP gut retention time varies by species42,43. Therefore, differences in uptake of MP particles cannot be inferred by simple comparisons of different species of marine and FW fishes. In this study, using two euryhaline Oryzias species, we clearly demonstrated that fish in SW ingest more MP particles than those in FW.
Most MP pollution originates on land as a result of anthropogenic activity, and MPs are carried by rivers to the sea44. The mean MP concentration in 14 Asian countries ranged from 100 items m−3 to more than 5000 items m−3 in FW environments45. The mean MP concentration was estimated as 0.44 items m−3 globally in marine environment46, much lower than the concentration in FW. Additionally, MP masses range from 0.4–250 μg/L in SW47 and 30–1790 μg/L in FW48. Accordingly, the concentration of 1-µm PS-MPs used in this study was within environmentally relevant levels. We suggest that MP uptake is higher in marine ecosystems than in freshwater environments. Therefore, even though concentrations of MPs in freshwater are higher than in seawater, the risk to marine ecosystems should not be dismissed, and marine regions with high MP concentrations should be monitored carefully.
Materials and methods
Experimental organisms
Javanese medaka (Oryzias javanicus; Oj) strain (RS831) from Penang, Malaysia, were obtained from the National Bioresource Project (NBRP) MEDAKA at the National Institute for Basic Biology, Okazaki, Japan. These fish were maintained in an aquarium system (Iwaki Co., Tokyo, Japan) with recirculating seawater. Seawater salinity was maintained at ~ 31-ppt. Additionally, an orange-red variant of O. latipes (Oryzias latipes; Ol) was purchased from a commercial source (Charm Co., Ltd., Gunma, Japan). O. latipes were cultured in freshwater using a 30-L tank equipped with standard aquarium filters and an air pump. Both species were fed twice a day with brine shrimp (Artemia nauplii) (INVE Aquaculture, Belgium) larvae. The photoperiod was set to 14/10 h day/night at 26 °C. Under such conditions, adult fish of both species spawn every morning.
All animal experiments complied with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). Care and use of animals were conducted according to Guidelines for the Care and Use of Animals from The University of Tokyo. All experimental procedures were approved by the Animal Ethics Committee of the Atmosphere and Ocean Research Institute of the University of Tokyo (permission no. P18-13).
Microplastic particles
Polystyrene MPs (PS-MPs; Diameter 1 µm with a coefficient of variation 2%) labeled with a fluorescent dye (yellow green; excitation 441.53 nm and emission 485.56 nm) were purchased from Polyscience, Inc., Warrington, PA, USA (Catalog No. 17154-10). PS microspheres (Density: 1.05 g/cm3) were provided as a suspension in water and were easily dispersed without aggregation. The PS-MP stock solution was stored at 4 °C.
Larval acclimation at different salinities
All acclimation processes were conducted at 26 °C. Fertilized eggs of O. javanicus, obtained by mass mating in 31-ppt SW, were incubated until hatching in petri dishes in 31-ppt SW. To acclimate larvae to FW, half the hatched larvae were maintained in 31-ppt for 7 days, and then acclimated to 15-ppt SW for 7 days, and to FW for 7 days (FW-Oj). Another half of O. javanicus larvae were maintained in 31-ppt SW for 21 days (SW-Oj). Fertilized eggs of O. latipes, obtained by mass mating in FW, were incubated until hatching in petri dishes in FW. To acclimate them to 20-ppt SW, half the hatched larvae were maintained in FW for 7 days, and subsequently acclimated to 10-ppt SW for 3 days, 12-ppt SW for 3 days, 15-ppt SW for 3 days, and were finally transferred to 20-ppt SW and maintained for 5 days (SW-Ol). The other half of O. latipes larvae were maintained in FW for 21 days (FW-Ol). As preliminary test, Ol larvae were also maintained in FW for 7 days, and then acclimated to 15-ppt SW for 7 days, and to 31-ppt SW for 7 days. Surviving and dead larvae were counted every day during the acclimation period.
Microplastic exposure
Freshwater and seawater used during tests were filtered before experiments using a 0.22 µm Millipore sterile vacuum filtration system (Sigma-Aldrich, Germany) to avoid particle contamination. Larval groups (21 days post-hatching; 7–8 mm in body length) were moved to 500-mL glass beakers equipped with aeration systems to keep MPs distributed evenly. During exposure tests, medaka larvae (n = 36) were exposed to 0.25 mg/L (108 particles/L) of 1-μm PS-MPs for 7-days. In addition, negative controls consisted of larvae (n = 12) in containers without MPs. Larvae were fed with fish larva powder food (Itosui, Tokyo, Japan) once a day. Also, water for negative control and exposure solutions were changed every day to maintain the salinity and MP concentrations during exposure tests. Moreover, water temperature and salinity were recorded before and during exposure tests (Supplementary Table S1). Densities of FW and SW used in this study were derived from temperature and salinity data49,50 (Supplementary Table S1). Larvae were randomly sampled (exposure group n = 12; negative control n = 4 in total) from each glass beaker at experimental days 1, 3, and 7. All exposure tests were conducted in triplicate.
Tissue transparentization and reagents
Fish larvae were bleached and transparentized according to previous reports51,52, with several modifications. Formalin (10% formalin solution), potassium hydroxide (KOH), hydrogen peroxide, Triton-X, phosphate-buffered saline (PBS), HEPES, and glycerin were purchased from Wako Pure Chemical Industries Ltd. (Tokyo, Japan). All other chemicals used in this study were of analytical grade. O. javanicus larvae (exposure group n = 6; negative control n = 2) were fixed in 10% formalin at 4 °C for 3 days. After removal of formalin, larvae were incubated in pre-bleaching solution (0.3% hydrogen peroxide in PBS) at room temperature overnight. Pre-bleaching solution was replaced with bleaching solution (3% hydrogen peroxide in PBS) and incubated at room temperature overnight. After bleaching, the bleaching solution was replaced with a tissue transparency solution (5% formalin, 5% Triton X-100, 1% KOH in PBS) and incubated at 42 °C for 24 h. In O. latipes, which has a less pigmented peritoneum than O. javanicus, bleaching was omitted, i.e., the tissue transparency solution was directly added after fixation, and incubated at 42 °C for 48 h. Transparency of each sample was confirmed under the microscope.
Microplastic extraction and counting
Fish (exposure group n = 6; negative control n = 2) were sacrificed on ice. Larvae were homogenized in 20 mM HEPES buffer solution (pH 7.0) using homogenizing pestles and then centrifuged at 10,000×g, 4 °C for 20 min to separate pellets and supernatants. Supernatants were discarded and 10% KOH was added to pellets, which were then reacted at 42 °C for 3 days to degrade tissues. To extract 1-μm PS-MPs, digested tissue solutions were then filtered using 0.22 µm Isopore™ polycarbonate membrane filters with vacuum filtration. Membrane filters were dried overnight and then set on glass slides with cover slips for particle counting.
Microplastic detection and counting
Detection and counting of MPs were conducted using an all-in-one fluorescence microscope (BZ-X800, KEYENCE, Osaka, Japan) equipped with a GFP filter (excitation 470/40 nm, emission 525/50 nm, dichroic 495 nm). MP distributions in transparentized larvae were observed at × 4 magnification using glass-based dishes. Glycerin was added on the sample to get clearer image and to prevent the sample from drying during observation. To count MPs extracted from homogenized bodies, images of MPs on membranes were automatically captured by the microscope (× 20 magnification), and the number of MPs was analyzed with the automatic counting system of the BZ-X800 Analyzer Software (KEYENCE), based on fluorescence intensity, shape, and size. MP counts were then normalized by the total mass of fish and compared between exposure groups.
Microplastic vertical distribution in seawater and freshwater
To estimate the cause of the difference in uptake, 0.25 mg/L (108 particles/L) of 1-μm PS-MPs were added to glass beakers of the same size as in the exposure experiment and filled with filtered 31-ppt SW or FW. These beakers were equipped with the same aeration system as the exposure experiment and covered with aluminum foil to avoid fluorescence signal loss due to light. Vertical MP distributions in SW and FW were examined by sampling water from the surface, middle, and bottom parts of the solution at 0, 6, 12, and 24 h. Fluorescence intensity of PS-MPs in the solutions were measured using a Microplate Reader Spectra iD3 (Molecular Devices, LLC, San Jose, CA, USA) at excitation and emission wavelengths of 441 nm and 486 nm. MP particle numbers were estimated by calibrating fluorescence intensities to a standard curve obtained with serial dilutions of fluorescent PS-MP suspensions.
Larval body measurements
To explore any differences in morphological features possibly related to MP uptake, head and body sizes of larvae in SW-Oj, FW-Ol, and FW-Oj groups were measured. Larvae of SW-Oj, FW-Ol, and FW-Oj (n = 10) were fixed in 10% formalin for 3 days. Morphological measurements of larval heads and bodies were conducted using the KEYENCE microscope and analyzer software. Body lengths (BL), body widths (BW), mouth widths (MW), and head widths (HW) were measured from the dorsal orientation. Body length was measured from the mouth tip to the caudal fin. Body width was measured as the width between the pectoral fins. Mouth width was the width between the two maxillary bones in the upper jaw, and head width was the width between eyes. BL, BW, MW, and HW were compared between the four groups.
Water drinking observation
Larvae of SW-Oj and FW-Oj at 21 dph were immersed in respective media containing 1-µM fluorescein isothiocyanate (FITC)-labeled dextran (Average Molecular Weight 70,000, Sigma, 46945), which served as an inert marker for drinking. Morphological observations on drinking were made after 3 h exposure. After exposure, larvae were removed and rinsed with FW or SW for 5 min to remove excess FITC-dextran, and then anesthetized on ice. Larvae were fixed in 10% formalin overnight before observation. Individual larvae were placed in a glass-based dish, and the presence of FITC-dextran in their intestines was examined from a ventral orientation using an all-in-one fluorescence microscope (KEYENCE) equipped with a GFP filter.
Statistical analysis
Microsoft Office 2010 and GraphPad Prism 9 were used for statistical analysis. Data are expressed as the mean ± standard error of the mean (SEM). Significant differences between seawater and freshwater groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's Honestly Significant Difference post-hoc test. Statistical analyses of MP vertical distribution and larval body measurements were also performed using one-way ANOVA. The level of statistical significance was set at P < 0.05.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Law, K. L. & Thompson, R. C. Microplastics in the seas. Science 345, 144–145 (2014).
Andrady, A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011).
Tang, Y. et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 766, 142572 (2021).
Tanaka, K. & Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 6, 1–8 (2016).
McNeish, R. E. et al. Microplastic in riverine fish is connected to species traits. Sci. Rep. 8, 1–12 (2018).
Cordova, M. R., Riani, E. & Shiomoto, A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 161, 111763 (2020).
Andreas, et al. Microplastic contamination in the Skipjack Tuna (Euthynnus affinis) collected from Southern Coast of Java, Indonesia. Chemosphere 276, 130185 (2021).
Buwono, N. R., Risjani, Y. & Soegianto, A. Spatio-temporal patterns of occurrence of microplastics in the freshwater fish Gambusia affinis from the Brantas River, Indonesia. Environ. Pollut. 311, 119958 (2022).
Jaafar, N. et al. Occurrence, distribution and characteristics of microplastics in gastrointestinal tract and gills of commercial marine fish from Malaysia. Sci. Total Environ. 799, 149457 (2021).
Kılıç, E. & Yücel, N. Microplastic occurrence in the gastrointestinal tract and gill of bioindicator fish species in the northeastern Mediterranean. Mar. Pollut. Bull. 177, 113556 (2022).
Su, L. et al. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J. Hazard. Mater. 365, 716–724 (2019).
Grosell, M. Intestinal transport. In The Physiology of Fishes (ed. Claiborne, J. B. et al.) 175–204 (Taylor & Francis Group, 2013).
Evans, D. H. Measurement of drinking rates in fish. Comp. Biochem. Physiol. 25, 751–753 (1968).
Mak, C. W., Ching-Fong Yeung, K. & Chan, K. M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 182, 1–10 (2019).
Wang, J. et al. Adaptation of life-history traits and trade-offs in marine medaka (Oryzias melastigma) after whole life-cycle exposure to polystyrene microplastics. J. Hazard. Mater. 414, 125537 (2021).
Zhang, C. et al. Size effects of microplastics on embryos and observation of toxicity kinetics in Larvae of Grass Carp (Ctenopharyngodon idella). Toxics 10, 76 (2022).
Rochman, C. M., Hoh, E., Kurobe, T. & Teh, S. J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 1–7 (2013).
Rochman, C. M., Kurobe, T., Flores, I. & Teh, S. J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 493, 656–661 (2014).
Murata, K., Kinoshita, M. Kamei, Y., Tanaka, M., Naruse, K. Medaka: Biology, Management, and Experimental Protocols (Wiley, 2020).
Takehana, Y. et al. Genome sequence of the euryhaline Javafish Medaka, Oryzias javanicus: A small aquarium fish model for studies on adaptation to salinity. G3 Genes Genomes Genet. 10, 907–915 (2020).
Lee, B. Y. et al. The genome of the Java medaka (Oryzias javanicus): Potential for its use in marine molecular ecotoxicology. Mar. Pollut. Bull. 154, 111118 (2020).
Inoue, K. & Takei, Y. Diverse adaptability in Oryzias species to high environmental salinity. Zool. Sci. 19, 727–734 (2002).
Inoue, K. & Takei, Y. Asian medaka fishes offer new models for studying mechanisms of seawater adaptation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 136, 635–645 (2003).
Yusof, S., Ismail, A., Koito, T., Kinoshita, M. & Inoue, K. Occurrence of two closely related ricefishes, Javanese medaka (Oryzias javanicus) and Indian medaka (O. dancena) at sites with different salinity in Peninsular Malaysia. Environ. Biol. Fishes 93, 43–49 (2012).
Watanabe, S., Matsui, K. & Kihara, R. Salinity measurement on euryhaline fish ranging in brackish water using micro conductivity loggers. Comp. Physiol. Biochem. 36, 64–71 (2019) (in Japanese with English abstract).
Miyanishi, H., Inokuchi, M., Nobata, S. & Kaneko, T. Past seawater experience enhances seawater adaptability in medaka, Oryzias latipes. Zool. Lett. 2, 1–10 (2016).
Heilig, A. K. et al. Wnt11 acts on dermomyotome cells to guide epaxial myotome morphogenesis. Elife 11, 1–34 (2022).
Sakamoto, T. & McCormick, S. D. Prolactin and growth hormone in fish osmoregulation. Gen. Comp. Endocrinol. 147, 24–30 (2006).
Roch, S., Friedrich, C. & Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 10, 1–12 (2020).
Takei, Y., Hiroi, J., Takahashi, H. & Sakamoto, T. Diverse mechanisms for body fluid regulation in teleost fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R778–R792 (2014).
Takei, Y. From aquatic to terrestrial life: Evolution of the mechanisms for water acquisition. Zool. Sci. 32, 1–7 (2015).
Kaneko, T. & Hasegawa, S. Application of laser scanning microscopy to morphological observations on drinking in freshwater medaka larvae and those exposed to 80% seawater. Fish. Sci. 65, 492–493 (1999).
Carrick, S. & Balment, R. J. The renin-angiotensin system and drinking in the euryhaline flounder, Platichthys flesus. Gen. Comp. Endocrinol. 51, 423–433 (1983).
Perrott, M. N., Grierson, C. E., Hazon, N. & Balment, R. J. Drinking behaviour in sea water and fresh water teleosts, the role of the renin–angiotensin system. Fish Physiol. Biochem. 10, 161–168 (1992).
Malvin, R. L., Schiff, D. & Eiger, S. Angiotensin and drinking rates in the euryhaline killifish. Am. J. Physiol. 239, R31–R34 (1980).
Potts, W. T. W. & Evans, D. H. Sodium and chloride balance in the killifish (Fundulus heteroclitus). Biol. Bull. 133, 411–425 (1967).
Gunter, H. M., Koppermann, C. & Meyer, A. Revisiting de Beer’s textbook example of heterochrony and jaw elongation in fish: Calmodulin expression reflects heterochronic growth, and underlies morphological innovation in the jaws of belonoid fishes. EvoDevo 5, 1–13 (2014).
Maradonna, F. et al. Probiotic supplementation promotes calcification in Danio rerio larvae: A molecular study. PLoS One 8, e83155 (2013).
Jones, G. et al. Knee articular cartilage development in children: A longitudinal study of the effect of sex, growth, body composition, and physical activity. Pediatr. Res. 54, 230–236 (2003).
Zitouni, N. et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 403, 124055 (2021).
Liu, Y. et al. Uptake and depuration kinetics of microplastics with different polymer types and particle sizes in Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 212, 112007 (2021).
Ohkubo, N., Ito, M., Hano, T., Kono, K. & Mochida, K. Estimation of the uptake and gut retention of microplastics in juvenile marine fish: Mummichogs (Fundulus heteroclitus) and red seabreams (Pagrus major). Mar. Pollut. Bull. 160, 111630 (2020).
Okamoto, K., Nomura, M., Horie, Y. & Okamura, H. Color preferences and gastrointestinal-tract retention times of microplastics by freshwater and marine fishes. Environ. Pollut. 304, 119253 (2022).
Jambeck, J. et al. Plastic waste inputs from land to the ocean. Science 347, 768–771 (2015).
Phuong, N. N. et al. Microplastics in Asian freshwater ecosystems: Current knowledge and perspectives. Sci. Total Environ. 808, 151989 (2022).
Bohdan, K. Estimating global marine surface microplastic abundance: Systematic literature review. Sci. Total Environ. 832, 155064 (2022).
Goldstein, M. C., Rosenberg, M. & Cheng, L. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol. Lett. 8, 817–820 (2012).
Lasee, S. et al. Microplastics in a freshwater environment receiving treated wastewater effluent. Integr. Environ. Assess. Manag. 13, 528–532 (2017).
Jones, F. E. & Harris, G. L. ITS-90 density of water formulation for volumetric standards calibration. J. Res. Natl. Inst. Stand. Technol. 97, 335 (1992).
UNESCO, Background papers and supporting data on the International Equation of State of Seawater 1980, UNESCO Technical Papers in Marine Science 38, 191–192 (1981).
Sakata-Haga, H. et al. A rapid and nondestructive protocol for whole-mount bone staining of small fish and Xenopus. Sci. Rep. 8, 1–10 (2018).
Futami, K., Furukawa, O., Maita, M. & Katagiri, T. Application of hydrogen peroxide-melanin bleaching and fluorescent nuclear staining for whole-body clearing and imaging in fish. Fish Pathol. 54, 101–103 (2019).
Acknowledgements
This work was supported by The University of Tokyo FSI-Nippon Foundation Research Project on Marine Plastics, and JSPS Core-to-core CREPSUM JPJSCCB20200009 to K.I. and the Honjo International Scholarship Foundation to H.M.P. The authors thank Mr. Abdul Rauf for his help in fish maintenance.
Author information
Authors and Affiliations
Contributions
H.M.P., T.T., and K.I. conceived and designed the study. H.M.P. performed all experiments and statistical analyses. S.R. prepared the fish samples. H.M.P. and K.I. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pratiwi, H.M., Takagi, T., Rusni, S. et al. Euryhaline fish larvae ingest more microplastic particles in seawater than in freshwater. Sci Rep 13, 3560 (2023). https://doi.org/10.1038/s41598-023-30339-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30339-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.