Abstract
The single and comparative intradermal tuberculin tests (SITT and CITT) are official in vivo tests for bovine tuberculosis (TB) diagnosis using bovine and avian purified protein derivatives (PPD-B and PPD-A). Infection with bacteria other than Mycobacterium tuberculosis complex (MTC) can result in nonspecific reactions to these tests. We evaluated the performance of the skin test with PPDs and new defined antigens in the guinea pig model. A standard dose (SD) of Rhodococcus equi, Nocardia sp., M. nonchromogenicum, M. monacense, M. intracellulare, M. avium subsp. paratuberculosis, M. avium subsp. avium, M. avium subsp. hominissuis, M. scrofulaceum, M. persicum, M. microti, M. caprae and M. bovis, and a higher dose (HD) of M. nonchromogenicum, M. monacense, M. intracellulare, M. avium subsp. paratuberculosis were tested using PPD-B, PPD-A, P22, ESAT-6-CFP-10-Rv3615c peptide cocktail long (PCL) and fusion protein (FP). The SD of R. equi, Nocardia sp., M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis did not cause any reactions. The HD of M. nonchromogenicum, M. monacense, M. intracellulare, and M. avium subsp. paratuberculosis and the SD of M. avium subsp. hominissuis, M. scrofulaceum and M. persicum, caused nonspecific reactions (SIT). A CITT interpretation would have considered M. avium complex and M. scrofulaceum groups negative, but not all individuals from M. nonchromogenicum HD, M. monacense HD and M. persicum SD groups. Only animals exposed to M. bovis and M. caprae reacted to PCL and FP. These results support the advantage of complementing or replacing PPD-B to improve specificity without losing sensitivity.
Similar content being viewed by others
Introduction
Animal tuberculosis (TB) is a globally spread zoonotic disease caused by species encompassed by the M. tuberculosis complex (MTC), most importantly by M. bovis and M. caprae. MTC includes other animal-adapted variants, namely M. microti, M. pinnipedii, M. orygis, M. mungi, M. suricattae, the Dassie bacillus and the chimpanzee bacillus1. It is a notifiable disease listed in the Terrestrial Animal Health Code (TAHC) of the World Organisation for Animal Health (WOAH). The disease has a great economic impact in the livestock industry and is a recognized public health problem due to its zoonotic nature1. One of the reasons for its economic impact lies in the huge costs derived from the implementation and application of strategies to control the disease. Apart from post-mortem inspection of animals at slaughterhouses in search of lesions compatible with TB, the tuberculin skin test (TST) is the standard diagnostic method for in vivo detection of infected individuals in TB eradication programs2. The European Communities Commission recognizes the single intradermal tuberculin test (SITT) and the comparative intradermal tuberculin test (CITT) as the official assays in the Member States, and the interferon-gamma (IFN-γ) assay as an alternative test that needs to be carried out in the laboratory (Commission delegated Regulation (EU) 2020/689 of 17 December 2019). In the SITT, the tuberculin known as purified protein derivative (PPD) from M. bovis (PPD-B) is injected intradermally and the skin-fold thickness is measured. When an animal is infected with or has been exposed to MTC bacteria, a delayed hypersensitivity reaction occurs causing an increase in the skin-fold thickness and/or clinical signs (diffuse or extensive edema, exudation, necrosis, pain or inflammation of the lymphatic ducts in that region or of the lymph nodes) at the PPD-B injection site. In the CITT both PPD-B and a PPD obtained from M. avium (PPD-A) are injected for comparison of the reactions. Sensitization with some non-tuberculous mycobacteria (NTM) or vaccination against TB or paratuberculosis, among other factors, have been pointed out as factors that can affect the specificity of the SITT3,4, leading to the slaughter of non MTC-infected animals and causing economic losses and great concern among farmers and authorities. The CITT can solve some of these issues by turning into negative some unspecific reactions to PPD-B. Its specificity can reach values of up to 99.98%5 but can miss some MTC-infected individuals reducing its sensitivity2. Antigenic reagents other than standard PPDs have been developed. Some have the capability to differentiate infected from Bacille Calmette-Guérin (BCG)–vaccinated animals (DIVA) because they are based on regions that are absent or not immunogenic in BCG vaccine strains (ESAT-6, CFP-10 and Rv3615c)6, like the defined antigens peptide cocktail long (PCL) and triple fusion protein (FP) used in previous studies7,8. A protein complex affinity-purified from the PPD-B called P22 has been developed as an alternative antigen in TB antibody based diagnosis9. This antigen has been recently used also in cellular response based tests10. Some NTM have been isolated from animals that reacted to PPDs11. However, few experimental studies on cross reactive immune responses induced by NTM exposure have truly demonstrated their ability to interfere with tuberculins in the skin test or the IFN-γ release assay12,13. Thus, the objective of this study was to evaluate the cross-reactivity of different NTM and other bacteria that were previously isolated from non-MTC-infected cattle reacting to skin testing or displaying TB-compatible lesions with official and new defined skin test antigens in guinea pigs exposed to these microorganisms.
Results
Skin test
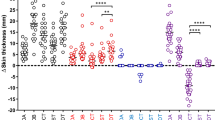
No animals, experimental units or data points were excluded from the study. Guinea pigs from control group (no sensitization) did not display any reaction to any of the skin test antigens used. The sizes of skin reactions (erythemas) seen after 24 h in response to intradermal inoculation of skin test antigens are shown in Fig. 1. No reaction was detected at the site where saline solution was inoculated in any of the groups. No large deviations from the mean were observed in each group. Most inoculated animals developed skin reactions to PPD-B and P22, except for non-mycobacterial agents and standard dose (SD) mycobacteria groups that were repeated (M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis) using a higher sensitization dose (HD). These HD inoculations were performed because, in contrast with previous reports and findings (i.e. isolation of these NTM from reactor cattle not infected with MTC), no reactions were observed with the SD and testing a HD was deemed necessary. Testing a HD of non-mycobacterial agents or of the remaining NTM was ruled out because the hypothesis was not planned beforehand, there was limited availability of animal resources and biosafety level 3 animal facilities at the time the second experiment could be conducted and the potential information that could be gained was not considered sufficient to ask for a modification of the authorization for animal experimentation and outweigh the “reduce” principle of the three Rs. All MTC-sensitized groups other than M. microti group developed strong reactions to antigens PCL and FP, especially to FP, while no reaction was detectable in the remaining guinea pigs groups. The specific response of animals sensitized with M. microti to PPD-B and P22 was much lower than that displayed by the other two MTC organisms. Erythematous area size analysis of variance showed a significant effect of antigen (p < 0.0001), sensitizing organism (p < 0.0001) and time (p < 0.0001). Antigen by organism (p < 0.0001) and antigen by time (p = 0.0481) were significant interactions, but not organism by time (p = 0.8525) nor antigen by organism by time (p = 0.9935) (Table 1). The mean PPD-B erythema area in M. bovis-sensitized group significantly differed from the remaining groups, except for the M. kansasii complex (MKC) member M. persicum (p = 0.5581) (see Table 2). Using P22, M. persicum and M. avium subsp. paratuberculosis HD-sensitized groups were not significantly different from M. bovis in their erythema area size. Comparison of PPD-B response of M. caprae-sensitized guinea pigs with other groups yielded smaller but still significant differences, except with M. persicum (p = 0.8688), M. avium subsp. hominissuis (p = 0.0837) and M. intracellulare HD (p = 0.1415) groups. This was not the case for P22, for which no differences were found with any group displaying reactions. The highest mean reactivity of non-MTC sensitizations (M. persicum group) was lower than specific M. bovis or M. caprae mean reactivity towards PPD-B. However, the erythema area of one guinea pig from the group sensitized with M. persicum fell within the range of M. caprae-sensitized group. Trying to set a cutoff with P22 for differentiating between positive and negative individuals was more difficult because there were two sensitizations (M. persicum and M. avium subsp. paratuberculosis HD) that caused reactions even higher than the expected specific one with M. caprae. MTC-sensitized groups (except the M. microti group) developed strong reactions to antigens PCL and FP, especially to FP, while no reactions were detected in groups exposed to bacteria other than MTC. The specific response (PPD-B and P22) of animals sensitized with M. microti was much lower than that displayed by the other two MTC members.
Reactivity according to inoculated organism and test antigen. Individual (I, II and III) and mean (M) erythema areas read 24 h after skin test antigens inoculation are plotted. The panel at the left on the top serves to represent the control group that was not inoculated any organisms (Ø), and the standard dose (SD) of Rhodococcus equi (Rho), Nocardia sp. (Noc), M. nonchromogenicum (Mno), M. monacense (Mmo), M. intracellulare (Min) and M. avium subsp. paratuberculosis (Map). The rest of results correspond to the SD of the organism indicated except for M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis (M. avium subsp. paratb) that correspond to inoculations with the higher dose (HD). B‒A indicates the result of subtracting PPD-A result to PPD-B result (PPD-B‒PPD-A).
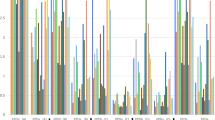
Antigen mean reading at 24 h (31.57 mm2) significantly decreased by 28% at 48 h (22.77 mm2) (p < 0.0001) (Table 1). Both values were well correlated when the first reading was above 50 mm2, but most reactions below this value vanished by 48 h (Fig. 2).
Effect of the time of reading (24 or 48 h) on the erythematous area according to inoculated organism and intradermal antigen. Results refer to inoculations with the standard dose (SD) except for M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis that correspond to inoculations with the higher dose (HD).
Post mortem analysis
Post-mortem results are summarized in Table 3. No macroscopic or microscopic lesions were detected among guinea pigs from Nocardia sp., R. equi, M. nonchromogenicum SD and HD, M. monacense SD and HD, M. intracellulare SD, M. avium subsp. paratuberculosis SD and M. scrofulaceum groups and, none of them yielded any isolate. Two M. intracellulare HD-sensitized individuals showed microscopic lesions compatible with mycobacterial infection that were not confirmed as being of mycobacterial origin by Ziehl–Neelsen staining (ZN) or culture. All three were culture negative. One guinea pig from the M. avium subsp. paratuberculosis HD group showed granulomatous lesions in liver and mesenteric LN that were not confirmed by ZN though the pool containing liver (pool 3) was culture positive with a single colony growing on a Herrold’s Egg Yolk Agar slant. Pool 1 containing the mesenteric LN of this animal was culture negative. In the M. avium subsp. avium group, two individuals were pool 3 culture positive and one of them had also lesions in muscle tissue at the inoculation site that was confirmed by culture. Similarly, the culture of pool 3 of two guinea pigs included in the M. avium subsp. hominissuis group produced isolates. One of them had granulomatous lesions in liver and spleen and, though ZN was negative, the isolation of M. avium subsp. hominissuis from pool 3 suggested that lesions could be due to this mycobacterium. As for the M. persicum group, gross and microscopic lesions were observed in one guinea pig (liver and mesenteric lymph nodes) and the bacterium was isolated from pools 1 and 3 in 2 individuals, from pool 2 in one animal and from the inoculation site also in one case. With regard to MTC, in the M. microti group lesions were only detected in the liver of one guinea pig but the bacterium was not isolated from any of these animals. In contrast, all guinea pigs belonging to M. caprae and M. bovis groups displayed lesions and were culture positive (Table 3).
Discussion
Exposure to or infection with some NTM or other bacteria have been considered as potential causes of nonspecific sensitization to PPD-B in the skin and other diagnostic tests of TB in cattle3,13,14. In spite of this, few controlled experiments have been carried out to demonstrate the implication of non-tuberculous organisms in the occurrence of cross-reactions with PPD-B and to evaluate the degree of interference of these nonspecific reactions in the diagnosis of TB. In addition, apart from some studies focused mainly on M. kansasii complex15,16,17, reports on the performance of antigens other than PPD (i.e. defined antigens) in tests based on cellular immune response and focused on non-MTC sensitizations are lacking. In this study we wanted to assess the degree of cross-reactions caused by sensitization (or infection) with some NTM strains and other non-mycobacterial microorganisms in response to skin test inoculation of standard PPDs and newer defined antigens using guinea pigs and isolates recovered from TB-negative cattle reacting to the skin test or with TB-compatible lesions.
Inoculation with the SD of Rhodococcus equi, Nocardia sp., M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis did not cause any visible skin reactions. According to these results these microorganisms would not cause interferences in the skin test. However, in the light of previous research11,13,14 that associated the appearance of cross-reactive responses in TB diagnostic tests with the detection of these NTM in the animals displaying those responses, we decided to increase the amount of mycobacteria administered to test whether this was due to a dose effect. Due to the limitations mentioned before (in skin test results), we had to prioritize and performed HD inoculations with the specified NTM and discarded Nocardia sp. and C. pseudotuberculosis. The latter two microbes have been associated with interferences in the diagnosis of bovine TB3,18, but their interferences might be more important as confounders in abattoir inspection (causing TB-like lesions) than in triggering cross-reactive responses18,19. Inoculations with M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis were repeated on new guinea pig groups with a HD and animals did develop visible reactions to PPDs and P22. This indicates that the response to diagnostic antigens is dependent on the dose of sensitizing bacteria (at least with respect to these species). Accordingly, nonspecific responses are expected to be greater the higher the sensitizing dose used is. Little is known on the dose of NTM needed to establish an infection or to induce a detectable reactive response in an animal under natural conditions. However, high amounts of NTM are found in soil or dust (up to 106 CFU/g), different types of water (up to 104 CFU/ml), biofilms (up to 106 CFU/cm2), plants (> 106 CFU/g), contaminated feed (e.g. feed stored in contact with domestic fowl), invertebrates, etc.3,20,21,22,23. Animals can get exposed to these sources by occasional or repeated contact through the respiratory or digestive tract mucosae or skin injuries. Unfortunately, there is a lack of information on the concentration of NTM harbored by infected animals; most studies dealing with NTM detection both in livestock and wildlife only report the prevalence or the number of culture positive individuals/tissues. Altogether, our results show that sensitization with M. nonchromogenicum HD, M. monacense HD, M. intracellulare HD, M. avium subsp. paratuberculosis HD, M. avium subsp. hominissuis SD, M. scrofulaceum SD and M. persicum SD could make animals react to the SITT. Most of these species have been previously pointed out as potential causes of nonspecific skin test reactions3,13,14. In contrast to previous research3,14, none of the animals from M. avium subsp. avium group displayed reactions to PPD-B or P22, despite their strong response to PPD-A. This isolate was obtained from a reactor cow confirmed as not infected with MTC. It could be that the standard dose was sufficient to produce top responses towards PPD-A but insufficient to cause visible responses to PPD-B. An alternative explanation for this would be that infection of guinea pigs with M. avium subsp. avium induced an immune response specific to targets in PPD-A that are not present (or under represented) in PPD-B and P22, at least in this experiment.
Interestingly, a comparative interpretation based on PPDs (CITT) would have deemed skin test negatives all individuals from M. avium complex and M. scrofulaceum groups as well as some from M. nonchromogenicum HD, M. monacense HD and M. persicum SD groups. Regardless of the specificity gain of this comparative interpretation, other research showed that sensitivity can be affected because animals co-infected with NTM (especially M. avium subspecies) and MTC can go undetected for having greater responses to PPD-A than to PPD-B24,25,26. In any case, since PPD-B erythema areas were greater than those seen for PPD-A, M. nonchromogenicum HD, M. monacense HD and, in particular, M. persicum could still have the potential to interfere also in the CITT. Another relevant point to be highlighted is that many of the animals belonging to NTM groups and reacting to the skin test did not show lesions nor yielded any isolates. The impossibility of confirming or ruling out that a reactor animal’s response is due to sensitizations with non-tuberculous organisms represents a setback for eradication programs, additional to the occurrence of some truly MTC-infected reactors for which tuberculous infection cannot be confirmed due to the low sensitivity of confirmatory tests14.
The results presented here show a high level of consistency and a relatively low variability that allowed obtaining statistical support to the differentiation of reactions. But the limited number of animals in each group precluded us from determining diagnostic cutoffs and thus, estimating the diagnostic performance parameters of antigens reliably. A general drop of erythema areas read at 24 h was observed at 48-h reading, with most reactions below the size of 50 mm2 disappearing by 48 h (Fig. 2). But some unspecific reactions to PPD-B still remained above 50 mm2 after 48 h (M. monacense HD, M. intracellulare HD, M. avium subsp. paratuberculosis HD, M. avium subsp. hominissuis SD and M. persicum SD). This and other considerations aside, we used 24-h readings comparisons following the recommendations of the WOAH for tuberculin potency testing in guinea pigs27. Mean PPD-B reactions were significantly greater in the group of M. bovis compared to the rest of groups except for that of M. persicum (p = 0.5581). Previous research showed that the skin-fold thickness increase in response to PPD-B was significantly smaller (p > 0.05) in MKC-challenged cattle than in M. bovis-challenged cattle15. This disagreement may be explained by the differences in challenge doses and bacterial strains used in each study. This was not the case for M. caprae group, with mean responses not significantly different not only in comparison with M. persicum but also with M. avium subsp. hominissuis or M. intracellulare HD groups. When it came to determine a cutoff to consider individuals as reactors or non-reactors, some of the animals sensitized with M. persicum, M. avium subsp. hominissuis and M. monacense HD showed reactions almost equal or even greater than those observed in one M. caprae guinea pig, which would have reduced the specificity or the sensitivity of PPD-B depending on the cutoff selected.
P22 has been proven to be useful and more specific than PPD-B in detecting the humoral response of infected animals9,28. However, its performance as a skin test reagent has not improved that of PPD-B in these experiments with guinea pigs. It did not improve specificity, with reactions not significantly different between M. persicum, M. avium subsp. paratuberculosis HD and M. bovis groups and between M. caprae and the rest of groups. It is striking that the difference between reaction sizes to PPD-B (larger) and P22 (smaller) is notably greater in the M. caprae group than in the M. bovis group. This may be because P22 is immunopurified from M. bovis-derived PPD-B and some of the antigens selected to compose this protein sub-complex may be less abundant, less immunogenic or down expressed in our M. caprae isolate. In spite of this, the mean erythema area of M. caprae group in response to PPD-B and P22 was greatly influenced by the guinea pig that had the largest reaction to PPD-B and the smallest one to P22 in its group. A field study on M. caprae-infected goats reported a sensitivity of 94% and 87% for SITT and P22 skin test, respectively10. But the mean and median SITT (PPD-B) reactions of goats infected with M. bovis can be bigger than in goats infected with M. caprae as well29.
Both FP and PCL showed the highest performance parameter estimates with all animals in M. bovis and M. caprae groups being clear reactors (100% apparent sensitivity), while no reaction was observed in any of the remaining guinea pigs (100% specificity). In contrast to P22, the mean erythema areas caused by PCL and FP were similar in M. bovis and M. caprae-sensitized guinea pigs. In addition, FP reactions in these two groups were similar to or even greater than those caused by PPD-B, which suggests that this antigen would have a comparable sensitivity with a greater specificity.
MKC is of great significance in terms of induction of nonspecific reactions in TB diagnosis because it seems to be cross-reactive not only with PPD, but also with defined antigens based on ESAT-6 or CFP-1015,17,30. The isolate from the complex used in this study, M. persicum, was obtained from a cow with no recent skin test available but showing gross lesions indistinguishable from typical bovine TB lesions at slaughter. Inoculation of guinea pigs with this isolate did not induce any responses to PCL or FP. Previous research has shown that both M. kansasii (former Type I M. kansasii) and M. persicum (former Type II) harbor ESAT-6 and CFP-1030,31. Their ESAT-6 and CFP-10 nucleotide sequences are almost equal (different only in 3 and 2 base positions, respectively) and result in identical amino acid sequences, although expression level is lower in M. persicum. Furthermore, a recent report concluded that the type-VII secretion system ESX-1-related EspACD locus, which is required for the secretion and function of EsxA (ESAT-6) and EsxB (CFP-10), is absent from M. persicum isolates32. Despite this, the effect of increasing the challenge dose of this isolate should be investigated also.
As expected, M. microti sensitization did not produce visible reactions towards these defined antigens as a result of its genomic deletions33. But it was also a low sensitizing power organism (including PPDs) at least using the SD sensitization, with guinea pigs mostly undetectable with confirmatory tests. The low responses to PPD-B in this group might not be in line with expectations. It is not clear if this could be due to differences between the abundance of the antigens present in PPD-B9 and those secreted by M. microti33 (M. microti in general or this specific strain) or to a less successful sensitization. These conclusions should be reassessed using higher sensitizing doses and other strains. Notwithstanding, the epidemiologic situation of M. microti in certain geographic regions does not seem to be irrelevant34,35,36. Thus, a combined use of defined antigens together with PPD-B might help distinguish between M. bovis or M. caprae infections and M. microti infections if they can be suspected.
In conclusion, in light of these and previous reports there is a need to at least complement the standard tuberculins with new defined antigens PCL and FP7 or additional antigens or diagnostic interpretation criteria. Further research is necessary to assess the effects of sensitization with more NTM and bacteria other than mycobacteria (e.g. Corynebacterium sp. and Nocardia sp.), at different sensitizing doses inoculated through different routes, including co-sensitization with non-MTC and MTC bacteria and with a special focus on microorganisms already suspected of causing interferences in the diagnosis of TB, as well as to estimate analytical and epidemiologic performance parameters of defined PCL and FP antigens, all of that considering animal species subjected to routine TB testing (i.e. cattle and goats).
Methods
Guinea pigs
Two consecutive experiments were carried out. Thirty-nine and fifteen specific pathogen-free Dunkin Hartley (HsdDhl:DH) female guinea pigs weighing 300–349 g were purchased from Envigo (Envigo, Horst, Netherlands) and used in the first and the second experiments, respectively. Since all were experimental animals, no inclusion/exclusion criteria were set a priori except sex and weight. Animals were randomly distributed (no specific randomization method) in groups of three individuals each (I, II and III) and housed separately in GP-SUITE cage racks (Tecniplast S.p.A., Buguggiate, Varese, Italy) with ad libitum water and food supply at the biosafety level 3 animal facilities in NEIKER. All animals had a two-week adaptation period. Thirteen groups were used in the first experiment, including standard dose (SD) Nocardia sp., Rhodococcus equi, M. monacense, M. nonchromogenicum, M. intracellulare, M. avium subsp. avium, M. avium subsp. hominissuis, M. avium subsp. paratuberculosis, M. scrofulaceum, M. persicum, M. microti and M. caprae groups as well as a negative control group. The second experiment comprised five groups that included higher dose (HD) groups of M. monacense, M. nonchromogenicum, M. intracellulare and M. avium subsp. paratuberculosis and an SD group of M. bovis (see “Intramuscular inoculation of bacteria” section). The number of animals per group was the minimum. It was determined by the minimum number of individuals needed for a Latin Square design allowing for changing the antigen injection position (6 antigens) between the animals of each group, but considering each flank as an independent experimental unit in order to reduce the number of animals needed (see “Skin test” section for more detail). Each experiment started after a 2-week adaptation period. The order of treatments and measurements and animal/cage location were not considered as potential confounders. Only two investigators performed the intramuscular inoculation of bacteria and were aware of the treatment and control groups allocation (M.V.G. and I.A.S.).
Animal housing and care, as well as all the experimental procedures were carried out in compliance with the European, National and Regional Law and Ethics Committee regulations. The experimental design underwent ethical review and approval by NEIKER’s Animal Care and Use Committee (NEIKER-OEBA-2020-010) and by the competent local authority, the Department of Agriculture of Diputación Foral de Bizkaia (2020/52-BFA).
Bacteria
The bacterial strains used to inoculate the guinea pigs are listed in Table 4. All NTM were isolated from SITT reactor cattle or cattle with TB-compatible lesions that were negative to MTC in culture. All mycobacteria were grown in Middlebrook 7H9 (M7H9) broth (Becton, Dickinson and Company, Sparks, MD, USA) supplemented with 10% Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (Becton, Dickinson and Company), 0.2% glycerol and 0.05% Tween 80 (Sigma-Aldrich, Co. Ltd., Haverhill, United Kingdom). M. avium subsp. paratuberculosis culture broth was further supplemented with 2 mg/l mycobactin J (IDvet, Grabels, France). Nocardia sp. and Rhodococcus equi isolates were propagated in 5% sheep blood Columbia agar plates (bioMérieux, Marcy-l’Étoile, France) and re-suspended in phosphate-buffered saline containing 0.2% glycerol and 0.05% Tween 80 (PBS-GT). The concentration of bacteria was normalized using the pelleted wet weight method proposed earlier for M. avium subsp. paratuberculosis as follows37. The bacterial cells were harvested by centrifugation at 3000×g for 10 min in a pre-weighed cone-bottomed centrifugation tube. The supernatant was decanted and the tubes drained for 10–15 min on sterile absorbent material. The tube was reweighed and the wet weight of the bacterial pellet determined. Bacteria were re-suspended at a final concentration of 2 mg/ml in PBS-GT and stored at − 80 °C until use. The concentration of colony-forming units (CFU) of these stock suspensions was estimated by plating serial dilutions onto agar-solidified M7H9 with 10% OADC and 0.2% glycerol (mycobacteria) or onto 5% sheep blood Columbia agar (non-mycobacteria) the day of inoculation.
Intramuscular inoculation of bacteria
Bacteria inocula were prepared the same day of inoculation by diluting the frozen 2 mg/ml stocks (− 80 °C) in sterile saline (NaCl 0.9%) solution (B. Braun Medical S.A., Rubí, Barcelona, Spain). The procedure for guinea pig sensitization and skin testing was based on the recommendations of the WOAH for tuberculin potency testing27. In the groups of the first experiment and in the second experiment’s M. bovis group, animals were inoculated 0.5 ml of saline solution containing a SD (0.0001 mg wet weight corresponding to approximately 103 CFU) of the corresponding microorganism by a deep intramuscular injection made on the medial side of the thigh of the right hind limb. Inoculations with M. nonchromogenicum, M. monacense, M. intracellulare and M. avium subsp. paratuberculosis were repeated in the second experiment using new guinea pig groups and a HD of these bacterial species (0.1 mg wet weight corresponding to approximately 106 CFU). Animals in the negative control group were inoculated with 0.5 ml of the same saline solution used to prepare bacteria inocula.
Skin test
Five weeks after inoculation both flanks of guinea pigs were depilated. According to our previous experience and to the literature, the skin test reaction magnitude is not significantly influenced by the location in the flank of the guinea pig or the flank itself (right or left) where an antigen is injected38. However, skin test antigens were inoculated in a Latin square design in agreement with WOAH recommendations but considering each flank of each guinea pig as independent experimental units (see Fig. 3). Antigens were diluted in saline solution to deliver the desired dose of each antigen in 0.1 ml intradermal injections using MYJECTOR (29G × 1/2′′) 1 ml insulin syringes (Terumo Europe N. V., Leuven, Belgium). In the left flank, 2 µg of PPD-B (50 IU), 1 µg of PPD-A (50 IU) (CZ Vaccines, Pontevedra, Spain) and 2 µg of P229 were injected in alternate positions. In the right flank, the defined antigens peptide cocktail-long (PCL) (1 µg each peptide) (GenScript Biotech, Piscataway, NJ, USA) covering the sequences of ESAT-6, CFP-10, and Rv3615c7 and ESAT-6-CFP-10-Rv3615c fusion protein (FP) (3 µg) (Lionex Ltd., Braunschweig, Germany) as well as a no-antigen negative control (saline solution) were injected. Skin test responses were measured after 24 h and 48 h by the same investigator (J.M.G.; unaware of grouping) and using calipers. The area (mm2) of erythema was calculated according to its shape (circumference A = π × r2; ellipse A = π × major r × minor r).
Post-mortem analysis
After skin test response readings, animals underwent deep general anaesthesia with intramuscular xylazine (5 mg/kg; XILAGESIC 2%, Laboratorios Calier SA, Barcelona, Spain) and ketamine (50 mg/kg; Anesketin 100 mg/ml, Dechra Veterinary Products, Barcelona, Spain). Guinea pigs were then euthanized by intracardiac administration of sodium pentobarbital (200 mg/kg; Dolethal 200 mg/ml, Vetoquinol Especialidades Veterinarias, Madrid, Spain). All animals were systematically and thoroughly necropsied.
Gross pathology and histopathology
Guinea pigs’ tissues were visually inspected in search of macroscopic pathological changes. Whenever possible, samples from retropharyngeal, parotid, cervical, prescapular, tracheobronchial, mediastinal, axillary, iliac, mesenteric, hepatic, prefemoral, inguinal and popliteal lymph nodes (LNs), as well as from lungs, liver, spleen, kidneys, intestine and muscle tissue from the inoculation injection site were collected for pathological and microbiological analysis. However, sample representation might have not been equal for histopathological and microbiological analysis always. Thus, a sample showing microscopic lesions could have been underrepresented in culture or inversely, a culture positive sample could have been underrepresented in histopathological analysis.
Tissue samples were fixed in 10% buffered formalin and subsequently dehydrated through a graded alcohol series before being embedded in paraffin wax. Sections, 3–5 μm thick, were stained with Carazzi's haematoxylin and eosin (HE) for histopathological studies and with ZN method for acid‐fast bacilli (AFB) detection.
Culture
A pool including tissues from the digestive system (pool 1; intestine portion including the ileocecal junction and mesenteric LNs), a pool with tissues from the respiratory tract (pool 2; lungs and associated lymph nodes) and a pool including tissues most related with potential lymphatic or hematogenous circulation of bacteria (pool 3: liver, spleen and retropharyngeal, parotid, cervical, prescapular, axillary, iliac, hepatic, prefemoral, inguinal and popliteal LNs) were cultured. If macroscopic pathological changes were observed in muscle tissue from the site where bacteria were injected, this tissue was also cultured.
Tissues (0.2–2 g) were thoroughly homogenized in 10 ml of sterile distilled water using a GentleMACS™ Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Tissue homogenates from mycobacteria-exposed guinea pigs were decontaminated with the BD BBL™ MycoPrep™ kit (Becton, Dickinson and Company, Sparks, MD, USA) following the instructions of the manufacturer. The resulting pellet was re-suspended in 1 ml sterile distilled water. The suspension was used to inoculate one BBL™ mycobacteria growth indicator tube (MGIT™) supplemented with BACTEC™ MGIT™ growth supplement and PANTA™ antibiotic mixture (Becton, Dickinson and Company), one Coletsos tube (Difco, RPD SL, Sentmenat, Barcelona, Spain) and one tube of Lowenstein-Jensen medium with pyruvate (Difco). Samples from M. avium subsp. paratuberculosis-exposed animals were also cultured in BD Herrold’s Egg Yolk Agar with Mycobactin, amphotericin, nalidixic acid and vancomycin (Becton, Dickinson and Company). Tissue homogenates from guinea pigs exposed to Nocardia sp. and R. equi were seeded onto 5% sheep blood Columbia agar and BD MacConkey agar (Becton, Dickinson and Company) plates. MGIT cultures were introduced in a BACTEC™ MGIT™ 960 System (Becton, Dickinson and Company) and the remaining solid cultures in a standard incubator at 37 °C.
PCR identification of isolated mycobacteria
One milliliter of BACTEC positive MGITs was centrifuged at 16,000×g for 3 min and the pellets re-suspended in 250 µl of distilled water. Colonies grown on solid media were re-suspended directly in distilled water. Suspensions were transferred to microcentrifuge tubes containing 0.3–0.4 ml zirconia/silica beads (0.1 mm diameter) (BioSpec Products Inc., Bartlesville, OK, USA), inactivated at 90 °C for 20 min and shaken in a TissueLyser II (Qiagen, GmbH, Hilden, Germany) for 10 min at 30 Hz. After centrifugation at 16,000×g for 5 min, 5 µl of supernatants was analyzed using a real-time PCR that simultaneously detects the genus Mycobacterium, the M. avium subspecies, the MTC and an internal amplification control39,40. DNA from MTC-confirmed isolates was subsequently submitted to standard spoligotyping41.
Statistics
Skin swelling diameter readings were submitted to a first general analysis of variance with the SAS GLM procedure (SAS, Inc. Cary, N. Caroline, USA) for each sensitizing agent (18 levels: M. bovis, M. caprae, M. avium subsp. avium, M. avium subsp. hominissuis, M. persicum, M. microti, M. avium subsp. paratuberculosis standard and high dose (SD and HD), M. intracellulare SD and HD, M. monacense SD and HD, M. nonchromogenicum SD and HD, M. scrofulaceum, Nocardia sp., Rhodococcus equi and blank control), reading time (two levels: 24 and 48 h) and diagnostic reagent (six levels: PPD-B, PPD-A, P22, PCL, FP and PBS) (Table 1). Then a simplified significant model was used for final analysis where factors without significant effect and sensitizing levels without any response were excluded (Tables 1, 2). Time was also excluded once verified that readings decreased by 48 h. Finally, post-hoc PPD-B and P22 responses least square means pairwise comparisons with the Student’s t test Tukey correction for multiple comparisons were applied for the sensitizations with M. bovis and M. caprae in comparison to the other sensitization groups (Table 2).
Ethics declaration
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. The study is reported in accordance with ARRIVE guidelines.
Data availability
All the data are available in the publication, including the tables and figures.
References
Malone, K. M. & Gordon, S. V. Mycobacterium tuberculosis complex members adapted to wild and domestic animals. Adv. Exp. Med. Biol. 1019, 135–154 (2017).
Schiller, I. et al. Bovine tuberculosis: A review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound. Emerg. Dis. 57, 205–220 (2010).
de la Rua-Domenech, R. et al. Ante mortem diagnosis of tuberculosis in cattle: A review of the tuberculin tests, gamma-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci. 81, 190–210 (2006).
Garrido, J. M. et al. Paratuberculosis vaccination causes only limited cross-reactivity in the skin test for diagnosis of bovine tuberculosis. PLoS ONE 8, 80985 (2013).
Goodchild, A. V., Downs, S. H., Upton, P., Wood, J. L. & Rua-Domenech, R. Specificity of the comparative skin test for bovine tuberculosis in Great Britain. Vet. Rec. 177, 258 (2015).
Vordermeier, H. M., Jones, G. J., Buddle, B. M., Hewinson, R. G. & Villarreal-Ramos, B. Bovine tuberculosis in cattle: Vaccines, DIVA tests, and host biomarker discovery. Annu. Rev. Anim. Biosci. 4, 87–109 (2016).
Srinivasan, S. et al. A defined antigen skin test for the diagnosis of bovine tuberculosis. Sci. Adv. 5, 4899 (2019).
Jones, G. J. et al. Test performance data demonstrates utility of a cattle DIVA skin test reagent (DST-F) compatible with BCG vaccination. Sci. Rep. 12, 1–8 (2022).
Infantes-Lorenzo, J. A. et al. Proteomic characterisation of bovine and avian purified protein derivatives and identification of specific antigens for serodiagnosis of bovine tuberculosis. Clin. Proteom. 14, 36 (2017).
Arrieta-Villegas, C. et al. Evaluation of P22 antigenic complex for the immuno-diagnosis of tuberculosis in BCG vaccinated and unvaccinated goats. Front. Vet. Sci. 7, 374 (2020).
Bercovier, H. & Vincent, V. Mycobacterial infections in domestic and wild animals due to Mycobacterium marinum, M. fortuitum, M. chelonae, M. porcinum, M. farcinogenes, M. smegmatis, M. scrofulaceum, M. xenopi. M. kansasii. Rev. Sci. Technol. 20, 265–290 (2001).
Corner, L. A. & Pearson, C. W. Response of cattle to inoculation with atypical mycobacteria of bovine origin. Aust. Vet. J. 54, 379–382 (1978).
Biet, F. & Boschiroli, M. L. Non-tuberculous mycobacterial infections of veterinary relevance. Res. Vet. Sci. 97, S69–S77 (2014).
Varela-Castro, L. et al. Beyond tuberculosis: Diversity and implications of non-tuberculous mycobacteria at the wildlife-livestock interface. Transbound. Emerg. Dis. 69, 2978–2993 (2022).
Waters, W. R. et al. Immune responses to defined antigens of Mycobacterium bovis in cattle experimentally infected with Mycobacterium kansasii. Clin. Vaccine Immunol. 13, 611–619 (2006).
Waters, W. R. et al. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin. Vaccine Immunol. 17, 247–252 (2010).
Vordermeier, H. M. et al. Assessment of cross-reactivity between Mycobacterium bovis and M. kansasii ESAT-6 and CFP-10 at the T-cell epitope level. Clin. Vaccine Immunol. 14, 1203–1209 (2007).
Bezos, J. et al. Lack of interference with diagnostic testing for tuberculosis in goats experimentally exposed to Corynebacterium pseudotuberculosis. Vet. J. 205, 113–115 (2015).
Michelet, L. et al. Second line molecular diagnosis for bovine tuberculosis to improve diagnostic schemes. PLoS ONE 13, e0207614 (2018).
Falkinham, J. O. 3rd. Ecology of nontuberculous mycobacteria. Microorganisms 9, 2262 (2021).
Loret, J. F. & Dumoutier, N. Non-tuberculous mycobacteria in drinking water systems: A review of prevalence data and control means. Int. J. Hyg. Environ. Health 222, 628–634 (2019).
Kazda, J. The Ecology of Mycobacteria (Springer, 2000).
Pavlik, I., Ulmann, V., Hubelova, D. & Weston, R. T. Nontuberculous mycobacteria as sapronoses: A review. Microorganisms 10, 1345 (2022).
Aranaz, A. et al. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M. avium subsp. paratuberculosis. Vet. Res. 37, 593–606 (2006).
Alvarez, J. et al. Effect of paratuberculosis on the diagnosis of bovine tuberculosis in a cattle herd with a mixed infection using interferon-gamma detection assay. Vet. Microbiol. 135, 389–393 (2009).
Hope, J. C. et al. Exposure to Mycobacterium avium induces low-level protection from Mycobacterium bovis infection but compromises diagnosis of disease in cattle. Clin. Exp. Immunol. 141, 432–439 (2005).
World Organization for Animal Health. Bovine Tuberculosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (World Organization for Animal Health, 2018).
Barral, T. D. et al. P22 protein complex in the serodiagnosis of animal tuberculosis: Antigenic stability and cross-reactivity with Corynebacterium pseudotuberculosis infection. Comput. Immunol. Microbiol. Infect. Dis. 90–91, 101891 (2022).
Bezos, J. et al. Goats challenged with different members of the Mycobacterium tuberculosis complex display different clinical pictures. Vet. Immunol. Immunopathol. 167, 185–189 (2015).
Scherrer, S., Landolt, P., Friedel, U. & Stephan, R. Distribution and expression of esat-6 and cfp-10 in non-tuberculous mycobacteria isolated from lymph nodes of slaughtered cattle in Switzerland. J. Vet. Diagn. Investig. 31, 217–221 (2019).
Jagielski, T. et al. Genomic insights Into the Mycobacterium kansasii complex: An update. Front. Microbiol. 10, 2918 (2020).
Tagini, F., Pillonel, T., Bertelli, C., Jaton, K. & Greub, G. Pathogenic determinants of the Mycobacterium kansasii complex: An unsuspected role for distributive conjugal transfer. Microorganisms 9, 1–22 (2021).
Garcia-Pelayo, M. C. et al. Microarray analysis of Mycobacterium microti reveals deletion of genes encoding PE-PPE proteins and ESAT-6 family antigens. Tuberculosis 84, 159–166 (2004).
Smith, N. H., Crawshaw, T., Parry, J. & Birtles, R. J. Mycobacterium microti: More diverse than previously thought. J. Clin. Microbiol. 47, 2551–2559 (2009).
Tagliapietra, V. et al. Mycobacterium microti at the environment and wildlife interface. Microorganisms 9, 2084 (2021).
Michelet, L., de Cruz, K., Tambosco, J., Hénault, S. & Boschiroli, M. L. Mycobacterium microti interferes with bovine tuberculosis surveillance. Microorganisms 8, 1–7 (2020).
Hines, M. E. et al. Experimental challenge models for Johne’s disease: A review and proposed international guidelines. Vet. Microbiol. 122, 197–222 (2007).
Chandran, A. et al. Development of a diagnostic compatible BCG vaccine against Bovine tuberculosis. Sci. Rep. 9, 17791 (2019).
Sevilla, I. A. et al. Detection of mycobacteria, Mycobacterium avium subspecies, and Mycobacterium tuberculosis complex by a novel tetraplex real-time PCR assay. J. Clin. Microbiol. 53, 930–940 (2015).
Sevilla, I. A. et al. Detection of mycobacteria by culture and DNA-based methods in animal-derived food products purchased at Spanish supermarkets. Front. Microbiol. 8, 1030 (2017).
Kamerbeek, J. et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35, 907–914 (1997).
Acknowledgements
Authors thank Mercedes Domínguez and Jose Antonio Infantes-Lorenzo from Instituto de Salud Carlos III, Madrid, Spain, and Vivek Kapur from Pennsylvania State University, PA, USA, for kindly providing P22 and PCL antigens, respectively. We also want to thank Amaia Etxezarreta, Mertxe Bascones and Jose Carlos Ibabe for laboratory technical support and Sergio Ayuso, Félix Blanco and Fidel Goiri for animal care and handling.
Funding
This study was supported with funds from the Spanish Ministry of Science and Innovation (MCIN/AEI/10.13039/501100011033) (Research Project PID2019-105155RB-C33) and the INTERREG POCTEFA 2014–2020 Program (Research Project EFA357/19 INNOTUB; co-funded by the European Regional Development Fund). L.F.V. holds a pre-doctoral fellowship from the Department of Economic Development, Sustainability and Environment of the Basque Government.
Author information
Authors and Affiliations
Contributions
J.M.G. and I.A.S. conceived and planned the study and obtained the funding. B.P.V., L.M., M.L.B., J.B., G.J.J., H.M.V., J.M.G. and I.A.S. contributed to choosing isolates and/or deciding skin test protocols and antigens. B.P.V., L.M. and M.L.B. provided isolates. G.J.J. and H.M.V. provided antigens. L.F.V., M.F., M.V.G., J.M.G. and I.A.S. performed the experiments and laboratory analysis and compiled the data. R.A.J., J.M.G. and I.A.S. analyzed and interpreted the results. R.A.J., J.M.G. and I.A.S. wrote the original draft of the manuscript. All authors reviewed the original draft and contributed to writing and editing the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any competing interests. G.J.J. and H.M.V. are employed by the Animal and Plant Health Agency (APHA) who holds 3 patents for the use of Rv3615c in diagnostic tests for bovine TB (WO/2009/060184, WO/2011/135369 and WO/2012/010875).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-Veiga, L., Fuertes, M., Geijo, M.V. et al. Differences in skin test reactions to official and defined antigens in guinea pigs exposed to non-tuberculous and tuberculous bacteria. Sci Rep 13, 2936 (2023). https://doi.org/10.1038/s41598-023-30147-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30147-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.