Abstract
The optimal time to initiate adjuvant therapy (AT) in elderly patients with glioblastoma (GBM) remains unclear. We investigated the impact of timing to start AT on overall survival (OS) using two national-scale datasets covering elderly GBM populations in the United States. A total of 3159 and 8161 eligible elderly GBM patients were derived from the Surveillance, Epidemiology and End Results (SEER)—Medicare linked dataset (2004–2013) and the National Cancer Database (NCDB) (2004–2014), respectively. The intervals in days from the diagnosis to the initiation of AT were categorized based on two scenarios: Scenario I (quartiles), ≤ 15, 16–26, 27–37, and ≥ 38 days; Scenario II (median), < 27, and ≥ 27 days. The primary outcome was OS. We performed the Kaplan–Meier and Cox proportional hazards regression methods for survival analysis. A sensitivity analysis was performed using Propensity Score Matching (PSM) method to achieve well-balanced characteristics between early-timing and delayed-timing in Scenario II. Improved OS was observed among patients who underwent resection and initiated AT with either a modest delay (27–37 days) or a longer delay (≥ 38 days) compared to those who received AT immediately (≤ 15 days) from both the SEER-Medicare dataset [adjusted hazard ratio (aHR) 0.74, 95% CI 0.64–0.84, P < 0.001; and aHR 0.81, 95% CI 0.71–0.92, P = 0.002] and the NCDB (aHR 0.83, 95% CI 0.74–0.93, P = 0.001; and aHR 0.87, 95% CI 0.77–0.98, P = 0.017). The survival advantage is observed in delayed-timing group as well in Scenario II. For elderly patients who had biopsy only, improved OS was only detected in a longer delay (Scenario I: ≥ 38 days vs. ≤ 15 days) or the delayed-timing group (Scenario II: ≥ 27 days vs. < 27 days) in the NCDB while no survival difference was seen in SEER-Medicare population. For the best timing to start AT in elderly GBM patients, superior survivals were observed among those who had craniotomy and initiated AT with a modest (27–37 days) or longer delays (≥ 38 days) following diagnosis using both the SEER-Medicare and NCDB datasets (Scenario I). Such survival advantage was confirmed when categorizing delayed-timing vs. early-timing with the cut-off at 27 day in both datasets (Scenario II). The increased likelihood of receiving delayed AT (≥ 27 days) was significantly associated with tumor resection (STR/GTR), years of diagnosis after 2006, African American and Hispanics races, treatments at academic facilities, and being referred. There is no difference in timing of AT on survival among elderly GBM patients who had biopsy in the SEER-Medicare dataset. In conclusion, initiating AT with a modest delay (27–37 days) or a longer delay (≥ 38 days) after craniotomy may be the preferred timing in the elderly GBM population.
Similar content being viewed by others
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor, accounting for approximately 48.6% of primary malignant brain tumors1. The median overall survival (OS) of GBM patients range from 14.6 to 20.9 months for randomized clinical trial (RCT) participants, 11 and 9.3 months in all GBM patients and elderly GBM patients in real world setting, respectively2,3,4,5.
The standard of care of newly diagnosed GBM is maximum safe resection followed by concurrent radiation and oral daily temozolomide (TMZ) chemotherapy and then adjuvant TMZ, 5 days on and 23 days off for 6–12 cycles1,2. Biopsy is offered to patients with tumors’ eloquent locations, significant comorbidity or frail health. Identification of the best timing to initiate adjuvant therapy (AT) has been considered as an important factor in aiding GBM control. Traditionally, treating physicians start AT soon after pathological diagnosis, surgical wound recovery and discharge from rehabilitation facility. Other factors delaying AT due to logistic reasons include visiting radiation oncologist and neuro-oncologist or medical oncologist as well insurance approval of radiation and chemotherapy plus dispensary of TMZ from pharmacy6. Further delay may occurs when patients need second opinions, transfer care to a tertiary medical center or participate clinical trials. Most patients started AT approximately 3–6 weeks after craniotomy and sooner for patient who had biopsy only (35 days2, within 5 weeks + 28 days7, 28 days after last RT8, 29–48 days9, 3.8 months5). Prior studies have investigated the association between timing of AT and GBM patients’ outcomes by retrospective data analyses only as there is no clinical trial performed addressing this question10,11,12,13. However, these retrospective studies generated controversial findings. Five studies demonstrated the association of delayed-timing of radiation (RT)/chemoradiation (CRT) with an improved GBM survival10,11,12,13,14, while one study reported an inferior outcome of long-delayed initiation of concurrent chemoradiation (CCRT) on GBM survival15. Three studies found no difference in GBM survival across different timings (Table S1)16,17,18. There is few study addressing this issue in elderly GBM patients only.
Several limitations existed in the aforementioned nine studies: (1) Only one study focused on elderly GBM patients16 and included GBM patients diagnosed from 1991 to 2002 only, which was prior to the era of Stupp protocol of concurrent RT with TMZ based on EORTC-NCIC trial2; (2) All of these studies applied different thresholds of timing groups and none of them validated their results in another comparable dataset; (3) Only three studies explored the predictors of delayed RT/CRT timing and could not reach a consistent conclusion12,13,16; (4) Four studies had limited sample sizes (N < 700) including RCT data, local hospital records, or TCGA11,15,17,18.
Therefore, the optimal AT timing and the related predictors of delayed AT remain uncertain for GBM patients, particularly the elderly GBM population. The objectives of the present study are to investigate the survival impact of AT timing as well as the related potential predictors using two large national cohorts of elderly GBM patients from the SEER-Medicare and NCDB datasets.
Materials and methods
Datasets and populations
This is a retrospective cohort study of elderly GBM patients derived from the SEER-Medicare (2004–2013) and NCDB datasets (2004–2014). The SEER-Medicare linked dataset collected cancer data across 18 population-based cancer registries, covering approximately 34% of the U.S. population. The Medicare program is a federally funded primary health insurance for approximately 97% elderly patients aged 65 years and older in the U.S. The NCDB is a nation-wide, hospital-based oncology database that collects surveillance cancer data as previously described19. The variable settings of core parameters in the NCDB are similar to those collected in the SEER. We chose the NCDB dataset from 2004 to 2014 to best match the data period collected in SEER-Medicare (2004–2013).
Study subjects and study periods
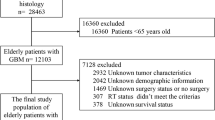
De-identified newly diagnosed elderly GBM patients (age at diagnosis ≥ 65) from the SEER-Medicare between January 2004 and December 2013 and from the NCDB Participant User File (PUF) between January 2004 and December 2014 were queried. GBM was defined by the International Classification of Disease for Oncology, third edition (ICD-O-3) coded as 9440, 9441, or 944220, with topography codes C710–C71921,22. Exclusion criteria are described in Supplementary Materials and Fig. S1.
Predictors, covariates, and outcomes
Primary exposure variables
For the SEER-Medicare database, time to AT was calculated as days from the date of diagnosis to the date of AT initiation, and it was categorized based on two scenarios: Scenario I (quartiles, days): 0–15, 16–26, 27–37, and ≥ 38; Scenario II (median, days): < 27 (early-timing), and ≥ 27 (delayed-timing).For the NCDB database, we classified the timing of AT using the same thresholds of the SEER-Medicare, which is used as a validation dataset.
Covariates
For the SEER-Medicare dataset, covariates include socio-demographics, facility/SEER registry features, and clinical treatments. Surgery and AT were identified by MEDPAR (Medicare Provider Analysis and Review), NCH (National Claims History), outpatient, DME (Durable Medical Equipment), and Part D Event files in Medicare claims. The details of coding were presented in Supplemental Table S2. We included the Medicare claims from 1999 and successfully evaluated the comorbidity during one year of claims prior to diagnosis for all cases. This data was used to calculate pre-diagnostic Charlson Comorbidity Scores.
For the NCDB database, socio-demographics settings are similar to the SEER-Medicare data. Facility features included facility location, facility type, residence-hospital distance, and care transition. Charlson/Deyo comorbidity score was a weighted score derived from the sum of the scores for each of comorbid conditions and classified into 0, 1, and ≥ 2. Care transition/treatment referral occurred if a patient underwent treatment at multiple facilities. The utilization of adjuvant RT or chemotherapy was defined as yes or no. More details about the definition of covariates were presented in supplementary materials.
Outcomes
OS was measured as the time interval in months from diagnosis to death or last visit. Those who were still alive by the last date of follow-up (December 31, 2014) were censored. Additionally, we examined the predictors associated with improved survival related to delayed-timing (≥ 27 days) versus early-timing (< 27 days).
Statistical analysis
The Mann–Whitney U tests and Pearson's χ2 tests were performed to compare patient characteristics by groups of AT timing. Univariable and multivariable binary logistic regression models were utilized to identify the predictors of delayed AT. The Hosmer–Lemeshow test was applied to check the goodness-of-fit of the regression models. OS was assessed by using Kaplan–Meier methods, and the difference across survival functions was tested by a two-sided log-rank test.
Multivariable Cox proportional hazards regression analyses were conducted to assess the impact of time to AT on survival by controlling covariates. Stratification analysis by surgery type (biopsy or resection) were also examined. The regression models rendered adjusted hazard ratio (aHR) estimates and 95% confidence interval (CI). A sensitivity analysis was performed using PSM method to achieve well-balanced characteristics between early-timing and delayed-timing (supplementary materials). Statistical analyses were performed with Stata IC 15.1 (StataCorp, College Station, TX). P values were two-sided and considered statistically significant at P < 0.05.
Ethics statement
This study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. All methods were performed in accordance with the relevant guidelines and regulations. The need for informed consent was waived by Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston due to retrospective nature of the study.
Results
SEER-Medicare dataset
A total of 3159 elderly GBM patients were included, with a median time to AT of 26 days (range 0–89 days). The median age at diagnosis was 73 years (IQR 69–78, range 65–95, years). The majority of patients were Caucasians (91.7%), married (69.1%), resided in metropolitan areas (82.3%), and treated at teaching hospitals (64.9%). In Table 1, the distributions of surgery (resection vs. biopsy), AT (non-CRT vs. CRT), and chemotherapy agents (None vs. TMZ vs. Other chemotherapy agents) varied significantly across times to start AT (All P < 0.001). Patients who underwent gross total resection (GTR) or subtotal resection (STR) were more likely to experience delayed-timing (≥ 27 days) of AT compared to those who underwent biopsy (Table S7).
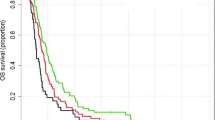
The median OSs were 5, 6, 6, and 7 months across the 4 quartiles of AT timing for all patients in SEER-Medicare, respectively (P < 0.001) in Scenario I (Table 3). For the resection cohort, patients who started AT between 27–37 days experienced the longest median OS (9 months) compared to the remaining 3 groups (≤ 15 days: 6, 16–26 days: 7, ≥ 38 days: 8 months; P < 0.001). Similar results were observed in Scenario II (median OS, < 27 days vs. ≥ 27 days: 7 vs. 8 months; P < 0.001). The Kaplan–Meier plots of survival curves are presented in Fig. 1A–C (Scenario I) and Fig. 2A–C (Scenario II).
OS of GBM patients across time to AT (four-category) by applying the Kaplan–Meier method. *“−” was used when the number of patients was 11 or fewer based on privacy policy of both SEER-Medicare and NCDB. (A) OS of GBM across time to AT in patients undergoing biopsy from SEER-Medicare. (B) OS of GBM across time to AT in patients undergoing resection from SEER-Medicare. (C) OS of GBM across time to AT among total patients from SEER-Medicare. (D) OS of GBM across time to AT in patients undergoing biopsy from NCDB. (E) OS of GBM across time to AT in patients undergoing resection from NCDB. (F) OS of GBM across time to AT among total patients from NCDB.
OS of GBM patients across time to AT (two-category) by applying the Kaplan–Meier method. *“−” was used once the number of patients was 11 or fewer based on privacy policy of both SEER-Medicare and NCDB. (A) OS of GBM across time to AT in patients undergoing biopsy from SEER-Medicare. (B) OS of GBM across time to AT in patients undergoing resection from SEER-Medicare. (C) OS of GBM across time to AT among total patients from SEER-Medicare. (D) OS of GBM across time to AT in patients undergoing biopsy from NCDB. (E) OS of GBM across time to AT in patients undergoing resection from NCDB. (F) OS of GBM across time to AT among total patients from NCDB.
As shown in Table 4, in the resection cohort, patients who began AT between 27 and 37 days (aHR 0.74, 95% CI 0.64–0.84, P < 0.001) and ≥ 38 days (aHR 0.81, 95% CI 0.71–0.92, P = 0.002) experienced improved OS. This survival benefit is observed in delayed-timing group in Scenario II ( ≥ 27 days vs. < 27 days, aHR 0.82, 95% CI 0.74–0.90, P < 0.001). Similar findings were observed in the total cohort: Scenario I, aHR 0.86, 95% CI 0.78–0.96, P = 0.005 (27–37 days vs. ≤ 15 days), aHR 0.86, 95% CI 0.78–0.95, P = 0.004 (≥ 38 days vs. ≤ 15 days); Scenario II, aHR 0.88, 95% CI 0.82–0.94, P < 0.001 (≥ 27 days vs. < 27 days). No survival difference across timing groups was detected in the biopsy cohort (neither Scenario I, nor Scenario II).
The model statistics for all covariates (aHR, 95% CI, and p-value) in multivariable Cox models were shown in Tables S3 (cutoff using quartiles) and S4 (cutoff using median). After performing the PSM method, the sample size of matched sub-cohort by early-timing versus delayed-timing was 3048 from the SEER-Medicare. The impact of delayed-timing as opposed to early-timing on GBM survival remained significant after repeating the Cox modeling in the PSM matched subsamples (Table S9, total patients after PSM).
NCDB dataset
A total of 8161 GBM patients were included after exclusions, with a median time to AT at 33 days (range: 0–91 days). The median age at diagnosis was 71 years (IQR: 67–75, range: 65–90) and most of patients were Caucasians (90.0%), residents in metropolitan areas (81.7%), treated at academic centers (45.6%), and Medicare beneficiaries (82.1%). They were treated at academic facilities (45.6%) and were not referred (69.5%). In Table 2, the distributions of all the covariates except gender, insurance, and Charlson/Deyo Score varied significantly across times to start AT. Patients who were diagnosed from 2007 to 2014, blacks and Hispanics, treated at academic facilities, referred, and underwent resection were more likely to receive AT 27 days or later. In addition, patients treated at facilities in the South, Midwest, and Western areas of the USA, had a reduced likelihood of delayed AT compared to those treated at Northeast facilities (Table S8).
As presented in Table 3, the median OS were 6.4, 9.8, 11.2, and 10.8 months corresponding to their respective timing groups for total patients (P < 0.001). For patients who underwent biopsy, the median OSs were prolonged from ≤ 15 days to ≥ 38 days (6.3, 7.2, 9.3, and 11.1 months, respectively, P = 0.003). Patients (resection group) who received AT between 27 and 37 days (11.4 months) following diagnosis achieved the longest OS compared to the remaining three intervals (≤ 15 days: 6.4, 16–26 days: 10.2, and ≥ 38 days: 10.8, months; P < 0.001). Figures 1D,E,F and 2D,E,F display Kaplan–Meier plots of survival curves for the NCDB cohort.
For the total cohort, the adjusted HRs indicated that the risk of death decreased by 17% in patients who received AT after a modest delay (27–37 days) from diagnosis (aHR 0.83, 95% CI 0.75–0.92, P < 0.001) and decreased by 16% in patients with the longest delay (≥ 38 days) (aHR 0.84, 95% CI 0.77–0.94, P = 0.002) compared to the shortest delay group (≤ 15 days). For the biopsy cohort, the risk of death for patients received AT ≥ 38 days was reduced by 23% in contrast to those in the group of ≤ 15 days (aHR 0.77, 95% CI 0.61–0.97, P = 0.024). For the resection group, patients with modest delay and the longest delay had 17% (aHR 0.83, 95% CI 0.74–0.93, P = 0.001) and 13% (aHR 0.87, 95% CI 0.77–0.98, P = 0.017) reduction in the risk of mortality, respectively (Table 4). The detailed model statistics for all covariates (aHR 95% CI and P value) are shown in Tables S5 and S6. Similar findings were observed in Scenario II (≥ 27 days vs. < 27 days): Biopsy, aHR 0.84, 95% CI 0.74–0.95, P = 0.006; Resection, aHR 0.89, 95% CI 0.84–0.94, P < 0.001; Total patients, aHR 0.88, 95% CI 0.83–0.93, P < 0.001 (Table 4) and in the PSM-matched sub-cohort (Table S9). After performing the PSM method, the sample size of matched sub-cohort by early-timing versus delayed-timing was 4776 from the NCDB.
Discussion
We explored the optimal timing of AT in elderly GBM patients using two large-scale national datasets. Our findings demonstrated that elderly GBM patients who underwent craniotomies and then received AT with a modest delay and a longer delay experienced a superior OS compared to those who were treated with AT immediately after surgery. After determining delayed-timing and early-timing using the cut-off of 27 days (median) (Scenario II), the survival benefit remained significant for patients received AT 27 days or later. More than 80% of patients received AT less than 2-month in the SEER-Medicare (80.6%) and the NCDB (83.3%). Significant predictors related to delayed timing (≥ 27 days) included the extent of resection (EoR), year of diagnosis, race, facility location, facility type, and care transition.
As shown in Table S1, prior studies revealed that various initiations within 4–6 weeks of AT might not be necessarily related to prolonged GBM survival based on RCT data10,11, public-accessible datasets12,13,15,16, data from health insurers14, and single-hospital/multi-center electronic health records (EHR)17,18. Those studies applied different thresholds to classify AT timing groups, including continuous form18, median15,16,18, tertiles17, quartiles10,12,15,16,18, incremental waiting days by 6 days from 15 to 42 days13, incremental waiting days by 2 weeks from 4 to 13 weeks14, and significant cut-off defined based on previous findings (42 days)15. However, the conclusions derived from these studies remain controversial.
Among all prior studies, only Lai et al. explored the association between RT timing and survival for elderly GBM patients in the SEER-Medicare dataset (1991–2002)16, which was before the era of the Stupp protocol2. They found no significant survival difference across the timing groups based on quartiles and median. Compared to the RTOG-based secondary analysis10 and the study using the Clinformatics Data Mart Database14, our study had a similar cut-off of timing groups and subsequent findings. Blumenthal et al. classified RT timing using quartiles and identified a 3.3-month survival benefit for patients with longer delay of RT initiation (> 4 weeks) over those received RT early (0–2 weeks) (median OS across Q1–Q4: 9.2, 10.8, 11.7, and 12.5 months, P < 0.001)10. The explanation of better median OSs results across four intervals in the RTOG-based clinical trial study than the OSs reported in this study is likely related to discrepancies between clinical trial participants and the general GBM population, as reported by many others23. Furthermore, a health insurer study in the U.S. demonstrated that CRT beginning within 4 weeks of craniotomy was associated with significantly inferior survival as compared to the middle delayed group (4–6 weeks) among HGG patients diagnosed between 2005 and 201414. However, the study included not only GBM, but also grade III anaplastic astrocytoma and oligodendroglioma.
Based on the NCDB dataset, two studies investigated the effect of AT timing on GBM survival12,13. Osborn et al. identified that initiation of CCRT during 31–37 days was associated with improved GBM survival by 7% versus the reference group (0–24 days)12. However, they included GBM patients only diagnosed between 2010 and 2012, and excluded those underwent biopsies or received non-CRT and sequential CRT (SCRT). Pollom et al. categorized AT timing into six groups using an incremental of 6 days from 15 to 42 days and stratified the survival analysis by EoR13. Their results indicated that survival patterns varied across CRT timing groups by biopsy [< 15 days vs. > 42 days (ref.): HR, 1.67, P < 0.001] and resection [15–21 days vs. > 42 days: HR 0.82, P = 0.030]13. This study excluded GBM patients diagnosed from 2005 to 2009, which is similar to the exclusion criteria of Osborn et al. study. Considering the Stupp protocol was initiated in 2005, the removal of GBM patients diagnosed from 2005 to 2009 might lead to inaccurate estimates of time to AT and restrict the findings limited to a sub-population of treated with Stupp protocol.
Four pilot studies with limited sample sizes had generated contrary conclusions, such as moderate delay of CCRT (30–34 days over 0–30 days) improve GBM survival11, no survival difference across timing groups17,18, and longer delay (> 42 days vs. 0–42 days) decreased GBM survival15. In short, based on prior studies, the optimal timing of GBM AT still remain unclear due to lack of RCT data, limited sample sizes11,15,17,18, restricted database10, truncated period of GBM diagnosis12,13, lack of stratification by EoR10,11,15,17,18. Those publications have reported contrary conclusions (e.g., Sun et al. > 42 days related to worse survival than 0–42 days15; Pollom et al. > 42 days related to better survival than 15–21 days13).
Additionally, only three studies explored the predictors of delayed AT timing. Significant factors associated with delayed initiation of AT included blacks12,13, tumor size > 3 cm12, EoR12,13,16, treatment at academic facilities12, residence in metropolitan areas13, distance between residence-hospital > 50 miles13, and Medicaid/non-insured/other government insurance13. Only the highest tier of income (≥ $48,000) was reported as a negative factor13. In this study, we detected the higher likelihood of delayed AT timing is associated with surgical resection, later years of diagnosis, blacks and Hispanics, treatment at academic facilities, and receipt of referral in the NCDB database. From the SEER-Medicare dataset, we found only STR/GTR as the positive factor associated with delayed-timing, which agrees with the conclusion by Lai et al. study16.
Although the mechanisms underlying the survival advantage of moderate delayed time to AT still remain unknown, the potential explanation to the survival advantage of moderate delayed time to AT might be related to the transient brain tumor/tissue removal/injury and subsequent recovery from surgical procedures. Hypoxia occurs due to vasculature disruption from surgical resection in the remaining brain tissue around the surgical area, which may reduce sensitivity of residual GBM cells to RT and TMZ24,25. Regarding the mechanisms of RT on brain tumors, ionizing radiation produces organic free radicals that form only in the presence of oxygen, which then results in DNA damage. Therefore, the efficacy of RT may be diminished since tumor DNA in hypoxic environments are less succeptible26. Peker et al. suggested that the rats that received RT within 1–2 weeks following brain surgery had significantly higher levels of tissue damage compared to those started RT ≥ 3 weeks after operation27. We speculate that early initiation of AT may diminish the killing capacity to GBM tumor cells by the RT and TMZ in a possible hypoxic environment, resulting in shorter patients’ survival. In addition, for patients underwent biopsy only, these patients usually were in poor health status or, tumor located in eloquent areas. The significant survival benefit of delayed AT on biopsy only group in the NCDB, but not in the SEER-Medicare dataset could be due to the differences in these two datasets on target population (SEER-Medicare: population-based data; NCDB: hospital-based data), data sources (SEER-Medicare: 18 cancer registries; NCDB: over 1500 CoC-accredited facilities), and proportion of general population (SEER-Medicare: covering elderly cancer patients from around 34% of the U.S. population; NCDB: around 70% of newly diagnosed cancer cases). Also, patients underwent biopsy only may be more likely to receive standard RT or shortened hypofractionated RT, which may make the mechanisms underlying the survival advantage of delayed AT more complex.
The present study is the first to comprehensively explore the optimal timing of AT for elderly GBM based on the SEER-Medicare dataset with a validation using the NCDB dataset. Also, we validated two classification methods of time to AT (quartiles or median) and obtained consistent results. Further, our analysis included the first full coverage of the Stupp protocol since 2005 and even extended one year prior to 2005 (SEER-Medicare: 2004–2013; NCDB: 2004–2014), which could cover the potential off-label usage of TMZ as well. Besides multivariable Cox proportional hazards model and stratification by biopsy and resection, we performed 1:1 PSM method as sensitivity analysis to minimize the impact of potential confounders or selection bias.
There are several limitations in the present study. First, our study is retrospective analyses of two large datasets including large proportion of elderly GBM patients, which might introduce selection bias or confounders. To minimize the impact of confounders/bias, we performed multivariable models, stratifications, and PSM method. However, PSM could not address the imbalance of unknown or unmeasured variables across timing groups. Second, the survival benefit detected in patients with delayed AT might be related to survival bias, a time-dependent variable for AT initiation which could be helpful to minimize the potential impact of survival bias in future studies. Lastly, there is no data provided regarding molecular profiles [e.g., isocitrate dehydrogenase1/2 (IDH1/2)28,29,30, or O6-methylguanine DNA methyltransferase (MGMT)31,32,33], the percent of resection or residual tumor volume34,35,36,37,38,39,40, Karnofsky Performance Scale (KPS)36,41,42, which may influence our findings on OS.
Conclusions
For the best timing in elderly GBM patients to start AT, superior survivals were observed among those who had craniotomy and initiated AT with a modest (27–37 days) or longer delay (≥ 38 days) following diagnosis based on analysis from both SEER-Medicare and NCDB datasets (Scenario I). Such survival advantage was confirmed in delayed-timing group in both datasets (Scenario II). The increased likelihood of receiving delayed AT (≥ 27 days) was significantly associated with tumor resection (STR/GTR), years of diagnosis after 2006, African American and Hispanics races, treatments at academic facilities, and being referred. There is no difference in timing of AT on survival among elderly GBM patients who had biopsy in the SEER-Medicare dataset. We conclude that initiating AT with a modest delay (27–37 days) or a longer delay (≥ 38 days) after craniotomy may be the preferred timing in the elderly GBM population.
Data availability
The datasets used and/or analyzed during the current study are available from the National Cancer Institute and American College of Surgeons (https://www.facs.org/quality-programs/cancer-programs/).
References
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro. Oncol. 22, 1–96 (2020).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Zhu, P. et al. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: A population-based study. Oncotarget 8, 44015–44031 (2017).
Perry, J. R. et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 376, 1027–1037 (2017).
Stupp, R. et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 318, 2306–2316 (2017).
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15(Suppl 2), 1–56 (2013).
Gilbert, M. R. et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 31, 4085–4091 (2013).
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 699–708 (2014).
Chinot, O. L. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722 (2014).
Blumenthal, D. T. et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: A secondary analysis from the radiation therapy oncology group database. J. Clin. Oncol. 27, 733–739 (2009).
Han, S. J. et al. The effect of timing of concurrent chemoradiation in patients with newly diagnosed glioblastoma. Neurosurgery 77, 248–253 (2015).
Osborn, V. W. et al. Impact of timing of adjuvant chemoradiation for glioblastoma in a large hospital database. Neurosurgery 83, 915–921 (2018).
Pollom, E. L. et al. Newly diagnosed glioblastoma: Adverse socioeconomic factors correlate with delay in radiotherapy initiation and worse overall survival. J. Radiat. Res. 59, i11–i18 (2018).
Nathan, J. K. et al. Early initiation of chemoradiation following index craniotomy is associated with decreased survival in high-grade glioma. J. Neurooncol. 135, 325–333 (2017).
Sun, M. Z. et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J. Neurosurg. 122, 1144–1150 (2015).
Lai, R. et al. The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro. Oncol. 12, 190–198 (2010).
Wang, T. J. C. et al. Timing of adjuvant radiotherapy in glioblastoma patients: A single-institution experience with more than 400 patients. Neurosurgery 78, 676–682 (2016).
Louvel, G. et al. Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother. Oncol. 118, 9–15 (2016).
Zhu, P. et al. Improved survival of glioblastoma patients treated at academic and high-volume facilities: A hospital-based study from the National Cancer Database. J. Neurosurg. 1, 1–12 (2019).
International Classification of Diseases for Oncology (ICD-O). http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=15&codcch=4350. Accessed 27 April 2019.
Iorgulescu, J. B. et al. The misclassification of diffuse gliomas: Rates and outcomes. Clin. Cancer Res. 25, 2656–2663 (2019).
Harary, M. et al. Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro Oncol. 22, 369–380 (2020).
Shahar, T. et al. The impact of enrollment in clinical trials on survival of patients with glioblastoma. J. Clin. Neurosci. 19, 1530–1534 (2012).
Hall, E. J. & Amato, J. Radiobiology for the radiologist, Seventh edition. (Wolters Kluwer Health/Lippincott Williams & Wilkins, 2012). https://trove.nla.gov.au/version/51355121. Accessed 28 April 2019.
Murray, D. et al. Influence of oxygen on the radiosensitivity of human glioma cell lines. Am. J. Clin. Oncol. 26, e169-177 (2003).
Gordillo, G. M. & Sen, C. K. Revisiting the essential role of oxygen in wound healing. Am. J. Surg. 186, 259–263 (2003).
Peker, S. et al. Irradiation after surgically induced brain injury in the rat: Timing in relation to severity of radiation damage. J. Neurooncol. 70, 17–21 (2004).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Turkalp, Z., Karamchandani, J. & Das, S. IDH mutation in glioma: New insights and promises for the future. JAMA Neurol. 71, 1319–1325 (2014).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Molenaar, R. J. et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 16, 1263–1273 (2014).
Chen, Y. et al. MGMT promoter methylation and glioblastoma prognosis: A systematic review and meta-analysis. Arch. Med. Res. 44, 281–290 (2013).
Sanai, N. & Berger, M. S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 62, 753–764 (2008).
Stummer, W. et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 7, 392–401 (2006).
Lacroix, M. et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 95, 190–198 (2001).
Li, Y. M. et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection?. J. Neurosurg. 124, 977–988 (2016).
Grabowski, M. M. et al. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 121, 1115–1123 (2014).
Marko, N. F. et al. Extent of resection of glioblastoma revisited: Personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J. Clin. Oncol. 32, 774–782 (2014).
Brown, T. J. et al. Association of the extent of resection with survival in glioblastoma a systematic review and meta-analysis. JAMA Oncol. 2, 1460–1469 (2016).
Lamborn, K. R., Chang, S. M. & Prados, M. D. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 6, 227–235 (2004).
Bauchet, L. et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 12, 725–735 (2010).
Acknowledgements
Abstract presentation, The 23rd Annual Meeting of the Society of Neuro-Oncology (SNO), New Orleans, Louisiana, U.S., November 2018; Abstract presentation, The 18th Biennial Canadian Neuro-Oncology Meeting, Banff, Canada, May 2018.
Funding
This project was supported in part by the Dr. Marine Rose Foundation (JJZ) and the Vivian L. Smith Neurosurgery Department Research Fund (YE).
Author information
Authors and Affiliations
Contributions
P.Z. and J.Z. contributed to the study conception and design. Data acquisition, preparation, and statistical analysis were performed by P.Z. The first draft of the manuscript was written by P.Z. and all authors commented on previous versions of the manuscript. All authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, P., Du, X.L., Hwang, Ly. et al. Impact of timing to initiate adjuvant therapy on survival of elderly glioblastoma patients using the SEER-Medicare and national cancer databases. Sci Rep 13, 3266 (2023). https://doi.org/10.1038/s41598-023-30017-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30017-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.