Abstract
ETV4, one of ETS proteins overexpressed in prostate cancer, promotes migration, invasion, and proliferation in prostate cells. This study identifies a series of previously unknown ETV4 alternatively spliced transcripts in human prostate cell lines. Their expression has been validated using several unbiased techniques, including Nanopore sequencing. Most of these transcripts originate from an in-frame exon skipping and, thus, are expected to be translated into ETV4 protein isoforms. Functional analysis of the most abundant among these isoforms shows that they still bear an activity, namely a reduced ability to promote proliferation and a residual ability to regulate the transcription of ETV4 target genes. Alternatively spliced genes are common in cancer cells: an analysis of the TCGA dataset confirms the abundance of these novel ETV4 transcripts in prostate tumors, in contrast to peritumoral tissues. Since none of their translated isoforms have acquired a higher oncogenic potential, such abundance is likely to reflect the tumor deranged splicing machinery. However, it is also possible that their interaction with the canonical variants may contribute to the biology and the clinics of prostate cancer. Further investigations are needed to elucidate the biological role of these ETV4 transcripts and of their putative isoforms.
Similar content being viewed by others
Introduction
Erythroblast Transformation Specific (ETS) proteins are a family of transcription factors, which can act as activators or repressors of their gene targets1. ETS proteins are involved in the regulation of various signaling cascades, which control diverse biological processes: proliferation, differentiation, development, transformation, apoptosis, migration, invasion, and angiogenesis2. These proteins are characterized by the presence of the ETS domain, a highly conserved DNA-binding domain that recognizes the purine-rich DNA motif 5’-GGA(A/T)-3′ 3,4,5. In addition to the ETS domain, these proteins contain a variety of diverse regulatory domains that characterize different subfamilies1. For example, the PEA3 subfamily, which includes the ETS translocation variant 4 (ETV4) gene, is characterized by two acidic domains, each one constituting the core of a transcriptional activation domain: the 32 aa acidic domain near the amino terminus is more potent than the 60 aa acidic domain near at the carboxyl terminus6,7,8. Moreover, the activity of the ETS domain and the activation domains are regulated by their flanking sequences6,7,8,9.
Aberrant expression of ETS genes is present in several types of cancer10. In prostate cancer, this is a frequent and -usually- early phenomenon11,12,13, which results from a chromosomal translocation involving any of several ETS genes14,15,16. The ETS genes most frequently involved in these translocations are ERG, ETV1, ETV4, and ETV5, with ERG being by large the most common13,14.
ETV4 (also called PEA3 or E1AF) is a member of the ETS transcription factor family, belonging to the PEA3 subfamily. In humans, the ETV4 gene maps to the long arm of chromosome 17 (band 17q21), and it consists of 13 exons encoding for 9 transcripts, which differ in the 5' UTR and the usage of different start codons17. ETV4 is expressed during embryogenesis and it contributes to motor axon guidance18. Furthermore, it is involved in developmental processes such as branching morphogenesis (mammary gland, lung, and kidney)19,20,21 and the formation of limb buds22. ETV4 overexpression has been correlated with the activation of cancer-related genes relevant to cell proliferation and to invasiveness23,24,25,26. Aberrant expression of ETV4 has been observed in several cancers (breast, prostate, ovary, lung, and gastrointestinal tract cancer)24,27,28, and it has been associated with metastatic disease and with poor prognosis29.
In prostate cancer, ETV4 is one of the ETS genes involved in chromosomal translocations30,31,32. Several studies in prostate cell lines have shown that ETV4 induces many neoplastic features: invasiveness, cell migration33,34, cell cycle progression, anchorage-independent growth, tumor growth in a xenograft model and also epithelial mesenchymal transition34. In addition, prostate expression of ETV4 results in the late development of mouse prostatic intraepithelial neoplasia in transgenic mice35 whereas occurrence of metastasis was associated with the late increase of ETV4 expression in a mouse model with several genetic alterations (pTen loss, NKX3.1 deletion, and a KRAS activating mutation) 36.
Alternative splicing is a process that generates multiple mRNA transcripts from a single gene, thus expanding the proteome37,38. Alternative splicing may rearrange the regulatory domains of several ETS genes to generate multiple isoforms with possible diverse functions39,40,41. Alternative splicing is a tightly regulated process that plays a relevant role in development and physiology42,43: in fact, its deregulation has been associated with a variety of diseases43,44,45,46. Recently, alternative splicing has been also included among the ‘hallmarks’ of cancer cells47. Alternative splicing has been described also in various genes that play a role in prostate cancer48,49,50, including members of the ETS gene family39. Multiple isoforms with distinctive oncogenic potential have been described for ERG51,52,53, ETV139 and ETS154,55, while data on ETV4 are not yet available.
We have investigated the expression of the alternative ETV4 transcripts in prostate cell lines and have found a number of previously unreported ETV4 transcripts. Furthermore, we analyzed the role of these ETV4 transcripts and studied the effects of their putative isoforms on some phenotypic neoplastic features.
Results
Identification of novel alternative ETV4 transcripts in cell lines
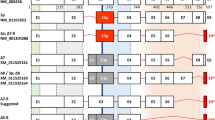
NCBI Reference Sequences (RefSeq) database56 for the human ETV4 (NC_000017.11 based on GRCh38.p13 Primary Assembly) reports 9 curated transcripts (one listed as “hypothetical”), which encode for 6 putative isoforms (Table 1, Fig. 1a,b).
Schematic representation of the 9 transcripts of ETV4 gene reported in the NCBI RefSeq database (https://www.ncbi.nlm.nih.gov/gene) according to the ETV4 genomic sequence NC_000017.11 based on the GRCh38.p13 Primary Assembly. The identification numbers for each transcript and each protein isoform are reported. The position of functional features is shown on the top: DNA-binding ETS motif (black bar); Acidic domain (hatched bars). The exonic structure is shown with coding exon sequences in grey and the 5′ and 3′ UTR in white. The size (in bp) of the first exon of each transcript is reported. (a) Transcripts of ETV4 curated and listed in NCBI RefSeq database. Three transcripts encode for isoform #1, two transcripts encode for isoform #2, each one of the other 3 transcripts encodes for isoform #3, isoform #4 and isoform #5. Transcripts that encode for the same isoform show different 5' UTR. Isoforms #4 and #5 differ to isoform #1 for the presence of a substitution (amino acid P instead of LA in isoform #4) or a deletion (DGAMG in isoform #5). Transcripts 7 and 8 differ from transcript 1 for some small nucleotide deletions. (b) Structure of the hypothetical transcript XM_047435592.1 that should encode for an isoform (X1) of 221 amino acids (24 kDa) due to a start codon in exon 7 restoring the reading frame in spite of a frameshift from skipping of exon 8. (c) ETV4 transcripts assessed in several human cancer (prostate: DU145, PC3, VCap, LNCap, 22RV1; breast: MDA-MB-231 indicated as MDA231, MCF7; leukemia: K562), and normal (prostate RWPE and breast MCF10) cell lines by RT-PCR. The primers, in exon 3 and 12, used to amplify the retro-transcribed ETV4 cDNA are reported in Supplementary Table. GAPDH was used as amplification control. H20: reagent control; M: 100 bp ladder. The sizes of the expected bands are reported on the right.
Seven of these NCBI RefSeq curated transcripts correspond to long canonical ETV4 transcripts (Fig. 1a): (i) transcripts 1, 2 and 6 differ in the 5’ UTR and encode the full-length ETV4 isoform #1 (484 aa); (ii) transcripts 3 and 4 share the 5’UTR with transcripts 2 and 1, respectively, but skipping of exon 2 should lead to a putative 445 aa isoform #2, with a start codon in exon 3; (iii) transcripts 7 and 8 are derivatives of transcript 1 from which they differ for a small in frame deletion of, respectively, 3 (isoform #4) and 15 nucleotides (isoform #5). The short transcript 5 should encode a putative 200 aa isoform #3 due to a start codon in exon 9 (Fig. 1a). Finally, NCBI RefSeq also reports a hypothetical transcript XM_047435592.1 that, in spite of a frameshift due to exon 8 skipping, should encode for an isoform (#X1) of 221 amino acids (24 kDa) due to a predicted start codon in exon 7 (Fig. 1b).
RT-PCR amplification of ETV4 cDNA from several prostate cell lines using primers in exon 3 and 12 (Supplementary Table 1) has shown the expected 1031 bp amplicon, corresponding to the above long ETV4 transcripts, and also several shorter amplicons that suggest the presence of additional transcripts (Fig. 1c). Expression of these shorter ETV4 amplicons has been found also in breast (MDA-MB231) and leukemic (K562) cell lines. In all investigated cell lines the shorter ETV4 amplicons were always fainter than the 1031 bp ETV4 amplicon. Cell lines in which ETV4 protein was not detected (VCap, LNCap and MCF7) were not investigated further (data not shown).
The presence of each ETV4 transcript reported in the NCBI RefSeq database was tested by using specifically designed primers, as reported in Supplementary Table 1. We found that almost all cell lines express the transcripts 1 (Fig. 2a), 2, 3 (Fig. 2b) and the short transcript 5 (Fig. 2c), with the notable absence of transcript 3 in 22RV1 (Fig. 2b) and of transcripts 1 and 3 in RWPE (Fig. 2a,b). We have not been able to detect transcript 4 and 6 in any of the cell lines (Fig. 2a). Finally, in all the investigated cell lines we have found an amplicon corresponding to the hypothetical XM_047435592.1 transcript (isoform #X1) that from now on will be named X1 (Fig. 2d).
Specific RT-PCR for detection of the ETV4 transcripts reported in the NCBI RefSeq database in selected cell lines (Du145, PC3, 22RV1, RWPE, MDA-231 and K562). (a) RT-PCR to detect Transcript 1 (T1, 314 bp), Transcript 4 (T4, 203 bp) and Transcript 6 (T6, 395 bp). Primers are located in the 5’UTR common to transcripts 1, 4 and 6, and in exon 5. The 314 bp amplicon (Transcript 1) was present in all the analyzed cell lines, except RWPE, while the 203 bp (Transcript 4) and the 395 bp (Transcript 6) amplicons were not detected in any of them. The band corresponding to the 314 bp amplicon may include also Transcripts 7 and 8, which differ from Transcript 1 for the deletion of few nucleotides. (b) RT-PCR to detect Transcript 2 (T2, 321 bp) and Transcript 3 (T3, 210 bp). Primers are located in the 5’UTR common to transcripts 2 and 3, and in exon 5. The 321 bp amplicon (Transcript 2) was present in all the analyzed cell lines. The 210 bp amplicon (Transcript 3) was present in all the analyzed cells (faint in RWPE) but in 22RV1. (c) Transcript 5 (T5, 332 bp) was detected in all analyzed cells. Primers are located in the 5’UTR of transcripts 5, and in exon 5. (d) RT-PCR to detect Transcripts 1 to 8, but 5, (T1-8, 637 bp) and Transcript X1 (371 bp), which were present in all the analyzed cells. Primers are located in exon 6 and in exon 10. The presence of additional amplicons suggests the existence of other alternative transcripts such as ETV4 ∆7 (475 bp), see Fig. 4. Primer sequences for the specific amplification of each transcript are reported in Supplementary Table 1. Lane M is the 100 bp ladder. Sizes of the expected bands are reported on the right. Ct: reagent control.
Thus, the cell lines we have analyzed express both the short alternative transcripts reported in NCBI RefSeq database (transcript 5 and X1). However, these two short transcripts do not account for the number of short ETV4 amplicons we have found (Figs. 1c, 2d), suggesting the expression of additional novel ETV4 transcripts. Identification and characterization of these potentially novel alternative ETV4 transcripts have been performed by cloning and sequencing the ETV4 RT-PCR products from PC3 and Du145 cells (primers in exon 3 and 13: Supplementary Table 1). The cloning and sequencing approach confirmed the results obtained with the specific RT-PCR (see above), including the presence of the hypothetical XM_047435592.1 transcript (isoform #X1), and revealed the presence of transcripts 7 and 8 in Du145 but not in PC3 cells (Table 1). Furthermore, this approach revealed also the presence of several additional alternative transcripts (Table 2), few of which having the potential to be transcribed into a protein due to in frame exon skipping: a transcript without exon 4 (ETV4 ∆4), one without exon 7 (ETV4 ∆7) and one without exons 6, 7 and 8 (ETV4 ∆6–8) (Fig. 3a). The presence of these three potentially productive transcripts (ETV4 ∆4, ETV4 ∆7 and ETV4 ∆6–8) has been tested by specific RT-PCR (Fig. 3b–d). The transcripts ETV4 ∆4, ETV4 ∆7 and ETV4 ∆6–8 should encode for putative ETV4 proteins of 468 aa (52 kDa), 430 aa (48 kDa) and 299 aa (33 kDa), respectively. In addition, ETV4 ∆6–8 should present the substitution of an aspartic acid with a histidine due to the junction between exons 5 and 9 (Fig. 3e). It is noteworthy that none of these potentially productive alternative ETV4 transcripts, except ETV4 ∆7 (ENST00000545089 in the ENSEMBL database), have been described so far.
Detection of the novel ETV4 alternative transcripts in selected cell lines (Du145, PC3, 22RV1, RWPE, MDA-231 and K562). (a) Schematic representation of the potentially productive splice transcripts of ETV4 found by cloning and sequencing in PC3 and Du145 cell lines. The exon structure is shown with coding exon sequences in grey and 5’ UTRs in white. The position of functional features is shown at the top: DNA-binding ETS motif (black bar); Acidic domain (hatched bars). The structure of the full-length ETV4 (ETV4 FL) and of the short X1 transcript (ETV4 X1) are reported for comparison. ETV4 ∆4, deletion of exon 4; ETV4 ∆7, deletion of exon 7; ETV4 ∆6–8, deletion of exons 6, 7 and 8. (b-d) Specific RT-PCR for detection of the novel alternative transcripts of ETV4 in cell lines. Primer sequences for the specific amplification of each alternative transcript are reported in Supplementary Table 1. Lane M is the 100 bp ladder. Sizes of the expected bands are reported on the right. In each panel the larger band is from the long ETV4 transcripts (ETV4-FL), the smaller from ETV4 ∆4 (b), from ETV4 ∆7 (c) and from ETV4 ∆6–8 (d). In panel (d) are present additional bands including the 621 bp (ETV4 ∆7) and the 517 bp (ETV4 X1) amplicons. (e) Nucleotides present at junction in the ETV4 ∆6–8 variant. The amino acids present at the junction are also reported. In the box is shown the amino acid substitution presents in the variant in respect to the full-length ETV4. Exon numbering is according to the ETV4 genomic sequence NC_000017.11 based on the GRCh38.p13 Primary Assembly.
Finally, in an exploratory experiment we have investigated the relative expression of these alternative ETV4 transcripts within the total ETV4 mRNA by quantitative RT-PCR; this very rough quantification showed that alternative transcripts accounted for 23% of total ETV4 mRNA in PC3 cells and up to 74% in 22RV1 cells (Supplementary Fig. 1). In all prostate cell lines ∆7 was the most expressed among the alternative transcripts, followed by ∆4 and X1, except for the 22RV1 cells, which express X1 at levels similar to ∆7. This expression pattern was also observed in the leukemic K562 cells, whereas the pattern in breast cancer MDA cells was more similar to that of 22RV1 (Supplementary Fig. 1). Due to the difficulty in obtaining reliable relative quantification of all ETV4 splice variants by quantitative RT-PCR, we resorted to long-read sequencing analysis using the Oxford Nanopore platform.
Explorative analysis of alternative ETV4 transcripts in cell lines by nanopore sequencing
In order to investigate the presence and the distribution of alternative ETV4 transcripts in an unbiased manner we have amplified the ETV4 cDNA using a primer in exon 3 and a common external one, followed by sequencing with the Oxford Nanopore platform. Nanopore sequencing has confirmed that the transcripts identified by cloning represent valid ETV4 transcripts (∆4, ∆7, ∆6–8 and X1) and has also revealed several other alternative transcripts, which possibly could encode for a protein product (Table 3). It is interesting that three of these additional transcripts combine exon 4 skipping with each one of the transcripts identified by cloning. Among alternative ETV4 transcripts identified by Nanopore sequencing, the one with exon 9 skipping was present at a frequency of around 1% in PC3 and RWPE prostate cell line as well as in breast cancer and leukemic cell lines; whereas, in most of the cell lines, all other transcripts have frequency below 0.5%. It is noteworthy that the non-productive ∆7–8 transcript is relatively abundant in all prostate cell lines besides PC3.
Nanopore sequencing allowed quantification of ETV4 transcripts (Table 3): among prostate cell lines canonical ETV4 were the most abundant transcripts in PC3 (76.1%) and RWPE (61.5%) and also in breast cancer (66.2%) and leukemic (85.2%) cell lines. Whereas in 22RV1, the most expressed transcript was the ∆6–8 (25.1%). Overall, these results were similar to the quantification obtained by quantitative RT-PCR (Supplementary Fig. 1).
Alternative ETV4 transcripts in patients with prostate cancer
In order to assess whether these novel alternative ETV4 transcripts were also present in prostate cancer tissues, we retrieved from the Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) the data on tumor prostate tissues from prostate cancer patients whose tumor tissue expressed ETV4. This analysis confirmed that most of the novel alternative ETV4 transcripts found in cell lines were also present in the tumor tissues of these patients (Table 4): ∆7 and ∆8(X1) transcripts were found in most of the patients (82 and 68%, respectively) and in 64% of patients were present both; also ∆4 and ∆6–8 transcripts were relatively frequent (25 and 32%, respectively). These four transcripts were present all together in 21% of patients. None of these transcripts was found in the tumor tissue of 18% of patients and in the normal peritumoral tissues of 60% of patients. In comparison, alternative transcripts were found in normal peritumoral tissues in a relatively small fraction (40%) of PC patients and only at low expression levels (Table 4): in any event, also in the “normal” peritumoral tissue the ∆7 and ∆8(X1) transcripts were the most frequent (23 and 17%, respectively).
Characterization of the isoforms translated from the alternative ETV4 transcripts
In order to analyze the biological characteristics of the putative isoforms translated from the novel ETV4 transcripts (∆4, ∆6–8, ∆7, and X1), we have cloned each of them in four expression vectors. These vectors, upon transient transfection, were able to express proteins of the expected size (Fig. 4a). Transcription ability, proliferation ability and subcellular localization of these isoforms have been compared with the full-length ETV4 (ETV4-FL)34 in transfected cells (Fig. 4b–e).
Functional characterization of proteins translated from the alternative ETV4 transcripts. (a) Western blot analysis of ETV4 isoforms expressed from 4 specific expression vectors: ETV4-∆4, ETV4-∆7, ETV4-∆6–8, and ETV4-X1. FL, cells transfected with a vector expressing the full-length ETV4 (ETV4-FL). CTL, cells transfected with the empty vector. (b-c) ETV4 splice variants transcriptional activity measured by luciferase reporter assays. The vectors encoding each one of ETV4 splice variants or ETV4-FL have been transfected in RWPE cells along with a vector containing part of CDKN1A (b) or TWIST1 (c) promoter upstream firefly luciferase. Relative luciferase units (R.L.U.) is the firefly/renilla ratio of each variant compared to that obtained by control (CTL, cells transfected with the empty vector). The data represent mean ± sem from at least three independent experiments performed in triplicate: *P < 0.05, **P < 0.01 (black asterisks: comparison with CTL; red asterisks: comparison with ETV4 FL). (d) Cell proliferation assessed by MTT assay in RWPE cells expressing the ETV4 isoforms. The values are expressed as increase of the initial cell number. Each value represents the average of 3 independent experiments. Cells transfected with any of ETV4-FL, ETV4 ∆7 and ETV4 ∆4 showed an increase of the proliferation rate, although this increase was statistically significant only for ETV4-FL and ETV4 ∆7 *P < 0.05, **P < 0.01. (e) Expression of the ETV4 isoforms in the nucleus (left) and in the cytoplasm (right) of RWPE cells transiently transfected with the indicated ETV4 isoforms (∆4, ∆7 and ∆6–8) compared with the ETV4-FL (FL). Fibrillarin and HSP90 have been used as loading control for nucleus and cytoplasm, respectively. M: molecular weight marker.
Transcription ability of ETV4 isoforms
We have investigated the transcriptional activity of these ETV4 isoforms using a dual luciferase reporter assay. We co-transfected RWPE normal prostate cells with the expression vector containing the coding sequence of each one of the above ETV4 transcripts alongside with one containing, upstream to the firefly gene, a fragment of the promoter of two known targets of ETV4. Specifically, we used the promoters of TWIST157 and CDKN1A (P21)35 genes, which are, respectively, positively and negatively regulated by ETV4. We compared the relative luciferase units (R.L.U.) of each ETV4 isoform with ETV4-FL (Fig. 4b, c). All these ETV4 isoforms, except X1, were able to reduce luciferase expression driven by a region of CDKN1A (P21) promoter with the same efficiency as the full-length ETV4 (ETV4-FL) (Fig. 4b). The weaker inhibition by X1 could be explained by the reduced amount of protein yielded by the expression vector: indeed, its inhibition ability was fully restored by increasing the amount of transfected vector (Fig. 4b). In contrast, ETV4-∆7 and ETV4-∆6–8 but not ETV4-∆4 and ETV4-X1 were able to increase luciferase expression driven by a region of TWIST1 promoter (Fig. 4c). Transfection with increased amounts of ETV4-X1 plasmid did not modify the effect of X1 on the TWIST1 promoter.
Proliferation induced by the novel ETV4 isoforms
We compared the ability of these ETV4 isoforms to increase the proliferation rate of RWPE cells. Transfection with either ETV4-∆7 or ETV4-∆4 was able to increase the proliferation rate of RWPE cells at levels similar to those induced by ETV4-FL (although only ETV4-FL and ETV4-∆7 reached a significant difference compared to control–RWPE cells transfected with empty vector). On the contrary, ETV4-∆6–8 and ETV4-X1 were unable to increase RWPE proliferation rate (Fig. 4d).
Subcellular localization of the novel ETV4 isoforms
Analysis of the subcellular localization of these isoforms in the RWPE cell line has shown that they were located mainly in the nucleus (Fig. 4e) compared to ETV4-FL, which was more homogenously distributed in both nuclear and cytoplasmatic compartments. It is interesting that a similar pattern has been observed also in the MCF7 breast cancer cell line (Supplementary Fig. 2).
Discussion
The impairment and the dysregulation of the splicing machinery have been recognized as one of the key features that characterize cancer cells. In fact, the presence of alternative mRNA splicing transcripts is quite common in cancer and can result either from mutations in splicing regulatory elements or from changes in the regulatory splicing machinery58,59. Several genes exhibit abnormal splicing pattern in cancer and, often, specific patterns may characterize particular types of cancer. Similar findings have been reported also for ETS transcription factors that play a pathogenic role in several kind of cancers10. In fact, alternative splicing, modifying the arrangement of ETS regulatory domains, may generate variants of an ETS transcription factor with different functions and different oncogenic potential39,51,54,60 that, eventually, can influence prognosis53.
ETV4 is an ETS transcription factor that plays an important oncogenic role in several tissues, especially in prostate and breast. Nine different ETV4 transcripts have been previously described, seven of which generate very similar canonical isoforms because of an alternative usage of 5′UTR (Fig. 1a,b; Table 1). However, compared with other ETS transcriptional factors, there is no data on the usage of alternative ETV4 transcripts in normal and cancer cells. Here, for the first time, the expression pattern of ETV4 alternatively spliced transcripts has been systematically analyzed.
Systematic analysis of the known ETV4 transcripts reported in RefSeq revealed that the pattern of expression of the canonical transcripts was variable in different prostate cell lines, whereas all cell lines expressed both the short transcripts (transcript 5 and X1). However, these two short transcripts do not explain the number of short fragments obtained from the amplification of the entire ETV4 mRNA (Fig. 1c). In fact, unbiased cloning and sequencing of the ETV4 RT-PCR products confirmed the presence of the known transcripts and further revealed that prostate cell lines express several novel alternative transcripts. Most of these transcripts could be transcribed into an ETV4 protein as a result of an in frame exon skipping (Table 2): exon 4 for ETV4 ∆4, exon 7 for ETV4 ∆7 and exons 6, 7 and 8 for ETV4 ∆6–8 (Fig. 3a).
In order to confirm these findings and to identify other possible alternative ETV4 transcripts we used high-throughput sequencing to perform an unbiased survey. Typically, with second-generation sequencing, untargeted transcript identification is performed through assembly of short reads spanning the gene of interest and extrapolation of the structure of the transcript based on splice junctions and read frequencies. These approaches may introduce bias and artifacts that could impede the determination of the real structure of the transcripts61. To avoid such possible bias and artifacts, we have used the Oxford Nanopore Technology platform, which is able to sequence long fragment of genetic material allowing precise characterization of the structure of the transcripts and quantification of their expression62,63.
Nanopore sequencing has confirmed that the transcripts identified by cloning represent valid ETV4 transcripts (∆4, ∆7, ∆6–8 and X1) and has revealed additional alternative transcripts that include both potentially productive (ETV4 ∆9 and those combining exon 4 skipping with each one of ∆7, ∆6–8 and X1) and non-productive (ETV4 ∆7–8 and ∆6;∆8) transcripts (Table 3). Analysis of ETV4 transcripts in tumor and peritumoral samples of prostate carcinoma patients from TCGA dataset revealed the presence of exon-to-exon junctions compatible with the ETV4 transcripts identified by cloning and nanopore sequencing (Table 4): this provides an additional, although indirect, evidence that they represent valid ETV4 transcripts. In addition, Nanopore sequencing has allowed also a more precise relative quantification of ETV4 transcripts (Table 3) with the respect to quantitative RT-PCR. Canonical ETV4 transcripts were more abundant than all other alternative transcripts in prostate cell lines PC3 (76.1%) and RWPE (61.5%) but not in DU145 (23.1%) and 22RV1 (14.7%). The patterns of expression of the potentially productive alternative transcripts varied in the investigated prostate cell lines: ∆4 was the most expressed in PC3 whereas X1 and ∆6–8 were the most frequent in the other cells (DU145, RWPE and in 22RV1). It is noteworthy that canonical, ∆7, X1, and ∆6–8 transcripts were expressed at similar levels in 22RV1 (∆6–8 was the most frequent) and that in this cell line also the non-productive ∆7–8 transcript, barely found in the other prostate cell lines, was relatively abundant (11.6%). For comparison, canonical ETV4 transcripts are the most abundant also in breast cancer (66.2%) and leukemic (85.2%) cell lines.
Various distinct molecular mechanisms may cause alternative splicing: activation of alternative 5′ and 3′ splice sites, intron retention, exon skipping and alternative exon usage. Most of previously known ETV4 transcripts encode for slightly different isoforms differing in the start codons because of the alternative usage of either exon 2 or exon 3 (Table 1). On the other hand, almost all the novel alternative ETV4 transcripts found in prostate cell lines and in human prostate tumors derive from exon skipping mechanism (Tables 2, 3 and 4). All the potentially productive alternative ETV4 transcripts retain the ETS domain, encoded by exons 11, 12 and 13: thus, their encoded isoforms should retain the ability to drive transcription. Nevertheless, these splice variants could potentially have different biological roles compared to the canonical isoforms thanks to loss of inhibitory or activating sequences in the skipped exons. It is also possible that exon skipping could affect ETV4 mRNA/protein stability or its ability to localize in the nucleus where ETV4 exerts the transcriptional activity.
The biological properties of the putative proteins encoded by the alternative transcripts, which are more frequent in prostate cell lines and in tumor tissues (ETV4 ∆4, ∆6–8, ∆7, and X1) have been investigated in vitro. Transfection experiments in prostate cell lines showed that the ability to increase proliferation rate was retained only by ETV4 ∆7 and, to a lesser extent, by ETV4 ∆4 (Fig. 4d), in spite of the fact that all splice variants were preferentially located into the nucleus (Fig. 4e). We have previously shown that ETV4 is able to promote prostate cell proliferation also through p21 downregulation mediated by direct binding of ETV4 to the CDKN1A (p21) promoter35. Therefore, it is somehow surprising that, despite their very different ability to promote cell proliferation, all these splice variants were able to exert a negative regulation of CDKN1A (P21) promoter with efficiency apparently comparable to the canonical ETV4 (Fig. 4b). This confirms that the ability of ETV4 to promote proliferation is only partially due to direct downregulation of the CDKN1A (p21) promoter35.
ETV4, analogously to the other member of the ETS family, interacts with various complexes of transcriptional co-factors and contains multiple regulatory domains that modify its translational capability and allows a tight regulation of the target genes. The N-terminal inhibitory domain (NID), spanning ETV4 exons 7 and 8, is one of the intramolecular autoinhibitory modules that regulates ETV4 activity: in fact, NID represses ETV4 DNA binding via transient interactions with the DNA-recognition helix of the ETS domain, interaction that is released by the acetylation of Lysine (Lys226 or Lys260)64. However, lack of a large portion of NID did not result in a significant increase of the effects on ETV4 target promoters. In fact, the ETV4 variants missing exons 7 and 8 (∆6–8 and ∆7) did not show, in comparison with canonical variants, any increased ability in repressing the CDKN1A (P21) promoter or in promoting the TWIST1 promoter (Fig. 4b,c). In addition, despite a discrete effect on the CDKN1A promoter repression, ETV4 Δ6-8 was unable to promote cell proliferation. This suggests that exons 7 and 8 include, together with the autoinhibitory NID, also additional structures with diverse and likely opposite functions.
Exon 4 encodes for a large part of the amino-terminal transactivation domain (TAD) responsible for the interaction of ETV4 with MED25, a subunit of the evolutionary conserved multi-subunit RNA polymerase II transcriptional regulator named Mediator65. It is intriguing that the two splice variants lacking exon 4, ETV4 ∆4 and ETV4 X1, were unable to exert the positive regulation of the TWIST1 promoter (Fig. 4c). Thus, it is possible that absence of exon 4 (ETV4 ∆4, ETV4 X1) prevents ETV4 binding to MED25 and –consequently– to Mediator, thus hindering transcriptional activation of the TWIST1 promoter. Moreover, all the investigated splice variants, irrespective of exon 4 status, were able to downregulate the CDKN1A (P21) promoter suggesting that TAD-Mediator interaction is not necessary for the negative regulation of this and, possibly, other promoters, e.g. ERBB2 and collagenase-1, usually negatively regulated by ETV466,67. This phenotypic characterization of the alternative ETV4 variants has revealed subtle differences in their biological features that could provide insight in the functional organization of ETV4. However, a deeper functional characterization is still needed, especially to evaluate whether the interaction between canonical and alternative ETV4 variants could affect their function similarly to what as has been shown for other ETS proteins68.
None of these splice variants have completely lost their transcriptional ability, yet our partial functional characterization does not seem to support the notion that some ETV4 alternatively spliced variants have higher neoplastic potential with respect to the canonical variants. Nevertheless, at least one alternative ETV4 transcript is expressed in the tumor tissues of most of prostate cancer patients (82%) and one in four of these patients expressed at least 4 different alternative transcripts; at variance, alternative ETV4 transcripts were found expressed, usually at very low levels, in the peritumoral tissues of only 40% of patients. Thus, the abundance of alternative ETV4 transcripts seems specific of prostate tumors.
In conclusion, we have identified and characterized a previously unknown set of ETV4 alternatively spliced transcripts, most of which are expected to encode ETV4 protein isoforms. These transcripts are much more abundant in human prostate tumors than in normal tissues. This pattern of expression with such abundance of alternative ETV4 transcripts in prostate tumors is likely to be secondary to the deranged splicing machinery often present in tumors. However, although none of the alternative splice variants appear to have acquired key neoplastic abilities, it is possible that molecular interactions between canonical and alternative variants contribute to influence the neoplastic features of prostate cancer. Further investigations are needed to completely define the role, if any, of the alternative ETV4 variants in defining the features and the development trajectories of prostate cancer.
Materials and methods
Cell culture
Du145, PC3, LnCap, MCF7, MDA-MB231, K562 (IST “Cell Bank and Cell Factory”, Genoa, Italy), V-Cap, 22RV1, RWPE, MCF10 (American Type Culture Collection) human cell lines were cultured according to cell-bank instructions. Du145, LnCap, 22RV1 and K562 cell lines were maintained in RPMI-1640 medium; MDA-MB231, MCF7 and VCap cell lines were maintained in DMEM high glucose medium; PC3 cell line were maintained in HamF12 medium; all these media were supplemented with 10% fetal bovine serum, 4 mM of L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin. RWPE cell line was maintained in serum-free KER medium supplemented with Epidermal Growth Factor and Bovine Pituitary Extract (Gibco, Carlsband, CA, USA). MCF10 cell line was maintained in DMEM medium, supplemented with 20% fetal bovine serum, 25 U insulin, 0.5 μg/mL hydrocortisone and 5 nM EGF (Gibco). RWPE and MCF10A media were supplemented with 4 mM of L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted with RNeasy Mini Kit (Qiagen, Germantown, MD, USA) and reverse transcribed using the Gene Amp RNA PCR Kit (Life Technologies-Thermo Fisher Scientific, Monza, Italy). Briefly, 1 µg of total RNA, in a reaction mixture (total volume 20 µl) containing 2.5U of reverse transcriptase with its buffer, 2.5 uM of random-examer primers and 100 µM of each deoxynucleoside triphosphates (dNTP) (Life Technologies), was reverse transcribed at 42 °C for 40 min; the enzyme was inactivated at 99 °C for 5 min, and the samples were immediately chilled at 4 °C.
The cDNA was used as template to amplify the ETV4 transcripts with the primers listed in Supplementary Table 1. Briefly, 1 µl of the above template was amplified in 50 µl reaction mixture containing 1.25 U of Taq Polimerase with its buffer and 2 mM MgCl2 (Life Technologies-Thermo Scientific), 100 µM of each dNTP, 0.4 µM of each primers. PCR amplification was performed for 35 cycles (94 °C for 20 s, 57 °C for 20 s, and 72 °C 45 s) on MyCycler (Bio-Rad Hercules, CA, USA).
Identification of ETV4 splice variants and construction of variant expression vectors
In order to identify possible ETV4 splice variants, ETV4 cDNAs from the RNA from PC3 and DU145 cell lines were amplified (see above) by primers in exon 3 and 13 (Fig. 1a) and the products were cloned using the TOPO TI cloning Kit into the pCRII cloning vector (Life Technologies) according to the manufacturer's protocol. The cloned inserts were characterized by Sanger sequencing. The presence of the splice variants identified by this approach was tested in the various cell lines by RT-PCR using the primers specific for each variant, which are listed in Supplementary Table 1.
Some of the identified novel variants (ETV4 ∆4, ∆7, ∆6-7-8, and X1: see “Results” for details) were cloned in a previously described expression vector encoding the full-length ETV4 (ETV4-FL) corresponding to the NCBI Transcript 134. Specifically, the BtrI-FspAI fragment contained in the ETV4-FL vector was replaced with the BtrI-FspAI fragment from the pCRII plasmids containing the splice variants. Each vector has been sequenced. Sequence and exon numbering is according to the NC_000017.11 sequence based on the GRCh38.p13 Primary Assembly.
Reverse transcription quantitative PCR (RT-qPCR)
ETV4 transcripts were quantified by RT-qPCR performed on CFX96 thermocycler (Bio-Rad Hercules, CA, USA). Briefly, 3 µl of a 1:30 dilution of the cDNA template, produced as described above, was used in a qPCR reaction mixture (total volume, 20 µl) containing the SsoFast EvaGreen Supermix (Bio-Rad) and 0.3 uM of each primer (see below and supplementary Table 1). Thermal conditions: 98 °C for 30 s followed by 45 cycles (98 °C for 6 s, 60 °C for 10 s). Relative expression levels of the target genes were calculated by the 2-ΔΔCt method using the glyceraldehyde phosphate dehydrogenase (GAPDH) as reference gene. Each RT-qPCR has been performed 3 times in triplicate.
To quantify ETV4 splice variants we designed, for each variant, a primer recognizing only the specific variant paired with another appropriate primer. Primer specificity is due to its complementarity to the sequence of the junction derived from the alternative splicing. Primer pairs for ETV4 ∆4, ∆7, ∆6-7-8, and X1 splice variants are listed in Supplementary Table 1.
In order to roughly quantify total ETV4 transcripts we designed a pair of primers that amplifies an ETV4 amplicon from exon 11 to exon 12, which encompasses the ETS domain and is present in all variants: thus, it may serves as a proxy for total ETV4 transcripts.
To estimate relative proportions of each splice variant in the respect of the total ETV4 transcripts in a cell line, the relative expression level of each variant was normalized against that of the total ETV4 transcripts (see above). However, since the amplification efficiency of each amplicon has not been demonstrated this technique provides only a very rough estimate of the proportion of the splice variant in each cell line. After this exploratory experiment a proper quantification of the expression of ETV4 splice variants was performed by Nanopore sequencing (see below).
Cell transfection
Transient transfection was performed on 70% confluent cells using 5 μg of expression vector (ETV4-FL, ETV4 ∆4, ∆7, ∆6-7-8 and X1) and 12 μl of X-tremeGENE HP DNA transfection reagent (Merck, Darmstadt, Germany) according to manufacturer instructions. Cell lysates were prepared and analyzed 48 h after transfection.
Identification and quantification of ETV4 transcripts by Nanopore Sequencing
Total RNA (1–3 µg) was extracted and retro-transcribed using a reverse primer containing a sequence complementary to an ETV4 sequence (exon 13: 5′-AGTGGGACAAAGGGACTGTG-3’), a specific barcode for each cell line followed by a common shared sequence (5’-GACCACGCGTATCGATGTCGAC-3’). The reaction mixture (total volume 20 µl) contained 100 U of Maxima H minus Reverse transcriptase with its buffer (Life Technologies), 20 U of RNAse inhibitor, 500 µM of each dNTP (Life Technologies) and 20 pmol of the above primer. ETV4 cDNA was amplified using a forward primer in exon 3 (5′-GCCGCCCCTCGACTCTGAA-3′) and a reverse primer containing only the common shared sequence (see above). The reaction mixture (total volume 50 µl) contained 1 U of the high fidelity thermo-stable KOD Hot-start DNA polymerase with its buffer and 1.5 mM of MgSO4 solution (Merck), 200 µM of each dNTP, 0.3 µM of each primer. To maintain a linear proportion of each transcript, ETV4 cDNA was subjected to 20 amplification cycles. ETV4 cDNA from Du145 cell line was subjected to 35 amplification cycles, allowing only a qualitative analysis of ETV4 transcripts.
Barcoded PCR products were purified using 1.8 X Agencourt AMPure XP beads (Agentcourt). Purified PCR products were quantified using Qubit High Sensitivity Kit (Thermo Fisher, Waltham, MA, USA). Based on their concentrations, equimolar concentration of the PCR products were pooled together. Libraries were prepared from these pooled PCR products using the SQK-LSK109 kit (Oxford Nanopore Technologies), according to the manufacturer protocol. The libraries were run on Flongle flow cells (v.9.4.1 pore), using the MK1B sequencer on the MinKNOW software (v.19.2.2) (Oxford Nanopore Technologies). Base calling was performed using GUPPY (v.3.0.3) to generate FASTQ files from the FAST5 files. Reads were then aligned to the reference genome and analyzed using minimap2 (v.2.17)69. For IGV visualization, BAM files were sorted according to mapped chromosomal location using sort from SAMtools70 to visualize the reads mapped on to the reference genome71. Moreover, sequence reads were aligned to each individual barcode and exon/intron on the ETV4 gene using LAST (version 1045)72,73:
lastdb -uNEAR -cR01 target target.fasta.
last-train -Q1 target reads.fastq > target.par.
lastal -P4 -Q1 -p target.par target reads.fastq | last-split -m1 > myaln.maf.
The alignment files were then used to demultiplex the reads and to build an exon/intron matrix for each read with a custom script (Supplementary methods, Script 1). Reads that matched ETV4 exons/introns in the same orientation and in the expected proper order were used to reconstruct the structure of the originating transcripts. Finally, aggregated tables containing each reconstructed transcripts with the corresponding number of reads were generated.
The final analysis was limited to the transcripts found with a frequency ≥ 1% in at least one cell line.
Western blot analysis and subcellular localization
Cells were lysed 24 h after transfection (lysis solution: NaCl 150 mM; Nonidet 40 1%; Sodium deoxycholate 0.5%; SDS 0.1%; Tris–HCl pH8,0 5 mM; EDTA pH8.0 5 mM, PMSF 2 uM; Na3Vo4 1 mM; protease inhibitors cocktail 0,1%); total protein concentration was measured by bicinchoninic acid assay (Thermo Scientific). The proteins were then separated by 10% SDS–PAGE, transferred to PVDF membranes (Bio-Rad), hybridized with primary antibodies and then with horseradish-peroxidase-conjugated secondary antibodies. The signals were detected using Immobilon Crescendo Western HRP Substrate (Merck) and Chemidoc XRS plus (Bio-Rad Hercules, CA, USA). Proteins were detected by the following primary antibodies: ETV4 Monoclonal Antibody raised against the entire human ETV4 (clone H00002118-M01. Abnova Atlanta, GA, USA) and actin Polyclonal Antibody (clone A2066. Merck). Densitometric quantifications have been performed by using the software ImageJ (https://imagej.nih.gov/ij/).
For the subcellular localization analysis, we obtained nuclear and cytoplasmatic lysates using the NE-PER nuclear and cytoplasmic extraction reagent (Life Technologies) according to the manufacturer's protocol. The lysates were analyzed, as described above, with the monoclonal antibody for ETV4 and, as loading control, monoclonal antibodies for the nuclear Fibrillarin (clone sc-374022. Santa Cruz Biotechnology, Heidelberg, Germany) and for the cytoplasmic HSP90 (clone sc-13119. Santa Cruz Biotechnology).
Cell Proliferation by MTT assay
Cells were seeded on 96 well plates; cell viability was assessed by the colorimetric assay method based on Thiazolyl Blue Tetrazolium Blue (MTT) (Merck). MTT reagent was added to the cells at a concentration of 0.2 mg/mL. The cells were incubated at 37 °C for 4 h. Crystallized MTT was then dissolved in acidified isopropanol and following the absorbance at 570 nm was measured using Victor X5 Plate Reader (Perkin Elemer, Milan, Italy). After background reading subtraction, the data were expressed as percentage of initial cell number. The experiments were performed 3 times in triplicate.
Transfection and Dual luciferase reporter assays
The RWPE cells were cultured in 12-well plates to about 60% confluence before transfection. The cells were transfected with plasmids containing cDNA from the full-length or the splice variants of ETV4 in combination with a plasmid containing part of the promoter of two known targets of ETV4 upstream the firefly gene: TWIST157 or CDKN1A (p21)35. A 845 bp fragment that includes the proximal ETV4 binding site at -676/-671 from transcriptional start site has been used for CDKN1A35 and a 761 bp fragment that includes the putative ETV4 binding sites at −125/−120; −239/−232 and −261/−256 from transcriptional start site has been used for TWIST157. We compared the relative luciferase units (R.L.U.) of each variant to that obtained by ETV4-FL. Cells were also co-transfected with Renilla luciferase pRL-TK reporter vector (Promega, Madison, WI, USA) (ratio 10:1) to normalize luciferase activity. The cells were transfected with the X-tremeGENE HP DNA transfection reagent (Merck) and, after 24 h, were harvested and lysed. Luciferase activity was quantified on the cell lysate using the Dual-Glo Luciferase Assay System (Promega) and the GloMax 20/20 Luminometer (Promega). We calculated the relative luciferase units or R.L.U (firefly/renilla) of each variant and we compared the R.L.U. of each variant to that obtained with ETV4-FL.
Analysis of ETV4 transcripts in patients with prostate carcinoma (TCGA dataset)
We have selected from the TCGA dataset (https://www.cancer.gov/tcga) samples from patients with prostate cancer for which RNA-seq data were available and that expressed ETV4 (n = 551). Among these samples we have further selected prostate tumor samples with ETV4 expression above the 95th percentile (n = 28) and all the peritumoral prostate tissue samples expressing ETV4 (n = 52). The aligned sequences were downloaded for the analysis. Reads encompassing non-canonical splicing from ETV4 RNA-seq data have been selected and counted by visual inspection using the “Integrative Genomics Viewer (IGV)”71,74.
Statistical analysis
All data are expressed as mean ± sem. Student t-test was performed using GraphPad Prism v.5.0 for Windows (GraphPad Software, La Jolla, California, USA). Statistical significance was accepted for P ≤ 0.05.
Data availability
Nanopore sequencing data have been uploaded to the NCBI SRA database (BioProject PRJNA890000) and are available at the following link: https://www.ncbi.nlm.nih.gov/sra/PRJNA890000.
References
Hollenhorst, P. C., McIntosh, L. P. & Graves, B. J. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu. Rev. Biochem. 80, 437–471. https://doi.org/10.1146/annurev.biochem.79.081507.103945 (2011).
Findlay, V. J., LaRue, A. C., Turner, D. P., Watson, P. M. & Watson, D. K. Understanding the role of ETS-mediated gene regulation in complex biological processes. Adv. Cancer Res. 119, 1–61 (2013).
Karim, F. D. et al. The ETS-domain: A new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 4, 1451–1453. https://doi.org/10.1101/gad.4.9.1451 (1990).
Sharrocks, A. D. The ETS-domain transcription factor family. Nat. Rev. Mol. Cell. Biol. 2, 827–837 (2001).
Wei, G.-H. et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 29, 2147–2160 (2010).
Laget, M. P. et al. Two functionally distinct domains responsible for transactivation by the Ets family member ERM. Oncogene 12, 1325–1336 (1996).
Janknecht, R., Monté, D., Baert, J. L. & de Launoit, Y. The ETS-related transcription factor ERM is a nuclear target of signaling cascades involving MAPK and PKA. Oncogene 13, 1745–1754 (1996).
Bojović, B. B. & Hassell, J. A. The PEA3 Ets transcription factor comprises multiple domains that regulate transactivation and DNA binding. J. Biol. Chem. 276, 4509–4521 (2001).
Greenall, A., Willingham, N., Cheung, E., Boam, D. S. & Sharrocks, A. D. DNA binding by the ETS-domain transcription factor PEA3 is regulated by intramolecular and intermolecular protein protein interactions. J. Biol. Chem. 276, 16207–16215 (2001).
Sizemore, G. M., Pitarresi, J. R., Balakrishnan, S. & Ostrowski, M. C. The ETS family of oncogenic transcription factors in solid tumours. Nat. Rev. Cancer 17, 337–351 (2017).
Clark, J. et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene 27, 1993–2003. https://doi.org/10.1038/sj.onc.1210843 (2008).
Perner, S. et al. TMPRSS2-ERG fusion prostate cancer: An early molecular event associated with invasion. Am. J. Surg. Pathol. 31, 882–888. https://doi.org/10.1097/01.pas.0000213424.38503.aa (2007).
Nicholas, T. R., Strittmatter, B. G. & Hollenhorst, P. C. Oncogenic ETS factors in prostate cancer. Adv. Exp. Med. Biol. 1210, 409–436. https://doi.org/10.1007/978-3-030-32656-2_18 (2019).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Rubin, M. A., Maher, C. A. & Chinnaiyan, A. M. Common gene rearrangements in prostate cancer. J. Clin. Oncol. 29, 3659–3668 (2011).
Arora, K. & Barbieri, C. E. Molecular subtypes of prostate cancer. Curr. Oncol. Rep. 20, 58. https://doi.org/10.1007/s11912-018-0707-9 (2018).
Ishida, S. et al. Genomic structure and promoter activity of the E1AF gene, a member of the ETS oncogene family. Biochem. Biophys. Res. Commun. 339, 325–330. https://doi.org/10.1016/j.bbrc.2005.11.024 (2006).
Livet, J. et al. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35, 877–892 (2002).
Chotteau-Lelievre, A. et al. PEA3 transcription factors are expressed in tissues undergoing branching morphogenesis and promote formation of duct-like structures by mammary epithelial cells in vitro. Dev. Biol. 259, 241–257 (2003).
Lu, B. C. et al. Etv4 and Etv5 are required downstream of GDNF and Ret for kidney branching morphogenesis. Nat. Genet. 41, 1295–1302. https://doi.org/10.1038/ng.476 (2009).
Liu, Y., Jiang, H., Crawford, H. C. & Hogan, B. L. Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev. Biol. 261, 10–24. https://doi.org/10.1016/s0012-1606(03)00359-2 (2003).
Mao, J., McGlinn, E., Huang, P., Tabin, C. J. & McMahon, A. P. Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic Hedgehog and promoting outgrowth of the vertebrate limb. Dev. Cell 16, 600–606. https://doi.org/10.1016/j.devcel.2009.02.005 (2009).
Kaya, M. et al. A single ets-related transcription factor, E1AF, confers invasive phenotype on human cancer cells. Oncogene 12, 221–227 (1996).
Hiroumi, H. et al. Expression of E1AF/PEA3, an Ets-related transcription factor in human non-small-cell lung cancers: Its relevance in cell motility and invasion. Int. J. Cancer 93, 786–791 (2001).
Discenza, M. T., Vaz, D., Hassell, J. A. & Pelletier, J. Activation of the WT1 tumor suppressor gene promoter by Pea3. FEBS Lett. 560, 183–191 (2004).
Jiang, J. et al. E1AF promotes breast cancer cell cycle progression via upregulation of Cyclin D3 transcription. Biochem. Biophys. Res. Commun. 358, 53–58 (2007).
Davidson, B. et al. PEA3 is the second Ets family transcription factor involved in tumor progression in ovarian carcinoma. Clin. Cancer Res.: An Off. J. Am. Assoc. Cancer Res. 9, 1412–1419 (2003).
Baker, R. et al. Pea3 transcription factors and wnt1-induced mouse mammary neoplasia. PLoS ONE 5, e8854 (2010).
de Launoit, Y. et al. The PEA3 group of ETS-related transcription factors Role in breast cancer metastasis. Adv. Exp. Med. Biol. 480, 107–116 (2000).
Tomlins, S. A. et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 66, 3396–3400 (2006).
Han, B. et al. A fluorescence in situ hybridization screen for E26 transformation-specific aberrations: Identification of DDX5-ETV4 fusion protein in prostate cancer. Cancer Res. 68, 7629–7637 (2008).
Hermans, K. G. et al. Two unique novel prostate-specific and androgen-regulated fusion partners of ETV4 in prostate cancer. Cancer Res. 68, 3094–3098 (2008).
Hollenhorst, P. C., Paul, L., Ferris, M. W. & Graves, B. J. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer 1, 1044–1052 (2011).
Pellecchia, A. et al. Overexpression of ETV4 is oncogenic in prostate cells through promotion of both cell proliferation and epithelial to mesenchymal transition. Oncogenesis 1, e20 (2012).
Cosi, I. et al. ETV4 promotes late development of prostatic intraepithelial neoplasia and cell proliferation through direct and p53-mediated downregulation of p21. J. Hematol. Oncol. 13, 112 (2020).
Aytes, A. et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc. Natl. Acad. Sci. USA 110, E3506-3515 (2013).
Maniatis, T. & Tasic, B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature 418, 236–243. https://doi.org/10.1038/418236a (2002).
Nilsen, T. W. & Graveley, B. R. Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463. https://doi.org/10.1038/nature08909 (2010).
Coutte, L. et al. Characterization of the human and mouse ETV1/ER81 transcription factor genes: Role of the two alternatively spliced isoforms in the human. Oncogene 18, 6278–6286 (1999).
Boti, M. A., Adamopoulos, P. G., Tsiakanikas, P. & Scorilas, A. Nanopore sequencing unveils diverse transcript variants of the epithelial cell-specific transcription factor Elf-3 in human malignancies. Genes (Basel) https://doi.org/10.3390/genes12060839 (2021).
Kishimoto, Y. et al. A novel tissue specific alternative splicing variant mitigates phenotypes in Ets2 frame-shift mutant models. Sci. Rep. 11, 8297. https://doi.org/10.1038/s41598-021-87751-5 (2021).
Kalsotra, A. & Cooper, T. A. Functional consequences of developmentally regulated alternative splicing. Nat. Rev. Genet. 12, 715–729. https://doi.org/10.1038/nrg3052 (2011).
Paronetto, M. P., Passacantilli, I. & Sette, C. Alternative splicing and cell survival: From tissue homeostasis to disease. Cell Death Differ. 23, 1919–1929. https://doi.org/10.1038/cdd.2016.91 (2016).
Scotti, M. M. & Swanson, M. S. RNA mis-splicing in disease. Nat. Rev. Genet. 17, 19–32. https://doi.org/10.1038/nrg.2015.3 (2016).
Dvinge, H., Kim, E., Abdel-Wahab, O. & Bradley, R. K. RNA splicing factors as oncoproteins and tumour suppressors. Nat Rev Cancer 16, 413–430. https://doi.org/10.1038/nrc.2016.51 (2016).
Bonnal, S. C., López-Oreja, I. & Valcárcel, J. Roles and mechanisms of alternative splicing in cancer–implications for care. Nat. Rev. Clin. Oncol. 17, 457–474. https://doi.org/10.1038/s41571-020-0350-x (2020).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011).
Dehm, S. M., Schmidt, L. J., Heemers, H. V., Vessella, R. L. & Tindall, D. J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 68, 5469–5477. https://doi.org/10.1158/0008-5472.can-08-0594 (2008).
Rajan, P., Elliott, D. J., Robson, C. N. & Leung, H. Y. Alternative splicing and biological heterogeneity in prostate cancer. Nat. Rev. Urol. 6, 454–460. https://doi.org/10.1038/nrurol.2009.125 (2009).
Paschalis, A. et al. Alternative splicing in prostate cancer. Nat. Rev. Clin. Oncol. 15, 663–675 (2018).
Owczarek, C. M. et al. Detailed mapping of the ERG-ETS2 interval of human chromosome 21 and comparison with the region of conserved synteny on mouse chromosome 16. Gene 324, 65–77 (2004).
Zammarchi, F., Boutsalis, G. & Cartegni, L. 5’ UTR control of native ERG and of Tmprss2:ERG variants activity in prostate cancer. PLoS ONE 8, e49721 (2013).
Hagen, R. M. et al. Quantitative analysis of ERG expression and its splice isoforms in formalin-fixed, paraffin-embedded prostate cancer samples: association with seminal vesicle invasion and biochemical recurrence. Am. J. Clin. Pathol. 142, 533–540 (2014).
Laitem, C. et al. Ets-1 p27: a novel Ets-1 isoform with dominant-negative effects on the transcriptional properties and the subcellular localization of Ets-1 p51. Oncogene 28, 2087–2099. https://doi.org/10.1038/onc.2009.72 (2009).
Adler, D., Ochsenfahrt, J., Fuchs, K. & Wernert, N. Novel identification of the ETS-1 splice variants p42 and p27 in prostate cancer cell lines. Oncol. Rep. 27, 1321–1324. https://doi.org/10.3892/or.2012.1667 (2012).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733-745. https://doi.org/10.1093/nar/gkv1189 (2016).
Howe, L. R., Watanabe, O., Leonard, J. & Brown, A. M. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 63, 1906–1913 (2003).
Urbanski, L. M., Leclair, N. & Anczuków, O. Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdiscip Rev. RNA 9, e1476. https://doi.org/10.1002/wrna.1476 (2018).
Hershberger, C. E. et al. Complex landscape of alternative splicing in myeloid neoplasms. Leukemia 35, 1108–1120 (2021).
Koizumi, S. et al. Isoforms of the human ets-1 protein: Generation by alternative splicing and differential phosphorylation. Oncogene 5, 675–681 (1990).
Oikonomopoulos, S. et al. Methodologies for transcript profiling using long-read technologies. Front. Genet. 11, 606 (2020).
Athanasopoulou, K., Adamopoulos, P. G. & Scorilas, A. Structural characterization and expression analysis of novel MAPK1 transcript variants with the development of a multiplexed targeted nanopore sequencing approach. Int. J. Biochem. Cell. Biol. 150, 106272. https://doi.org/10.1016/j.biocel.2022.106272 (2022).
Ito, Y. et al. Nanopore sequencing reveals TACC2 locus complexity and diversity of isoforms transcribed from an intronic promoter. Sci. Rep. 11, 9355. https://doi.org/10.1038/s41598-021-88018-9 (2021).
Currie, S. L. et al. Structured and disordered regions cooperatively mediate DNA-binding autoinhibition of ETS factors ETV1, ETV4 and ETV5. Nucleic Acids Res. 45, 2223–2241. https://doi.org/10.1093/nar/gkx068 (2017).
Currie, S. L. et al. ETV4 and AP1 transcription factors form multivalent interactions with three sites on the MED25 activator-interacting domain. J. Mol. Biol. 429, 2975–2995. https://doi.org/10.1016/j.jmb.2017.06.024 (2017).
Benbow, U., Schoenermark, M. P., Orndorff, K. A., Givan, A. L. & Brinckerhoff, C. E. Human breast cancer cells activate procollagenase-1 and invade type I collagen: Invasion is inhibited by all-trans retinoic acid. Clin. Exp. Metastasis 17, 231–238 (1999).
Xing, X. et al. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat. Med. 6, 189–195 (2000).
Rastogi, A. et al. Functional antagonism of TMPRSS2-ERG splice variants in prostate cancer. Genes Cancer 5, 273–284. https://doi.org/10.18632/genesandcancer.25 (2014).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100. https://doi.org/10.1093/bioinformatics/bty191 (2018).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. https://doi.org/10.1093/bioinformatics/btp352 (2009).
Thorvaldsdóttir, H., Robinson, J. T. & Mesirov, J. P. Integrative genomics viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192. https://doi.org/10.1093/bib/bbs017 (2013).
Kiełbasa, S. M., Wan, R., Sato, K., Horton, P. & Frith, M. C. Adaptive seeds tame genomic sequence comparison. Genome Res. 21, 487–493. https://doi.org/10.1101/gr.113985.110 (2011).
Hamada, M., Ono, Y., Asai, K. & Frith, M. C. Training alignment parameters for arbitrary sequencers with LAST-TRAIN. Bioinformatics 33, 926–928. https://doi.org/10.1093/bioinformatics/btw742 (2017).
Broad Institute, C. U. https://igv.org.
Acknowledgements
This study has been funded in part by a start-up grant from the “Istituto Toscano Tumori” (MDA), by the institutional support from the Istituto per lo Studio, la Prevenzione e la Rete Oncologica (ISPRO), Florence, Tuscany, Italy (RN), and by the SLPI_PC grant awarded from Regione Toscana (bando Ricerca Salute 2018) to RN and MDA. We thank the “Fondazione ISPRO” (Florence, Italy) for the support. Part of the results are based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga.
Author information
Authors and Affiliations
Contributions
I.C., A.M. performed the experiments and participated in designing the research, analyzing data, and writing the paper; U.M. performed the nanopore sequencing and helped in analyzing sequencing data. S.D.G. performed the bioinformatic analysis. C.P., I.S. contributed in performing the experiments. S.G.C. designed the sequencing strategy, the script for sequencing data analysis and participated in the writing of the paper; R.N. and M.D.A. designed the research, analyzed the data, and wrote the paper. All authors reviewed the paper and ratified the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cosi, I., Moccia, A., Pescucci, C. et al. Identification and characterization of novel ETV4 splice variants in prostate cancer. Sci Rep 13, 5267 (2023). https://doi.org/10.1038/s41598-023-29484-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29484-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.