Abstract
Astaxanthin (AST), a super antioxidant with coloring and medical properties, renders it a beneficial feed additive for shrimp. This study conducted a white shrimp feeding trial of 3S, 3’S isoform AST, which was derived from metabolic-engineered Kluyveromyces marxianus fermented broth (TB) and its extract (TE) compared to sources from two chemically synthetic ASTs (Carophyll Pink [CP] and Lucantin Pink [LP]), which contain 3S, 3’S, 3R, 3’S (3S, 3’R) and 3R, 3’R isoforms ratio of 1:2:1. The effects on red coloration, immune parameters and resistance to Vibrio infection were evaluated. Four AST sources were incorporated into the diets at concentrations of 0 (control), 100 mg kg−1 (TB100, TE100, CP100, and LP100), and 200 mg kg−1 (TB200, TE200, CP200, and LP200). Results revealed that in week 4, shrimps that received AST-supplemented feeds, especially TB100, TB200, and TE200, significantly increased redness (a*) values. Immune responses including phagocytosis activity, superoxide-anion production, phenoloxidase activity, and immune-related genes were examined on days 0, 1, 2, 4, 7, 14, 21, and 28. Generally, shrimps that received AST-supplemented feeds exhibited higher immune responses on days 7 and 14 than the control feed. Gene expression levels of superoxide dismutase and glutathione peroxidase were significantly upregulated on days 7 and 14 in shrimps that received AST-supplemented feeds, while genes of penaeidins, antilipopolysaccharide factor, and lysozyme were upregulated on days 4, 7, and 14, especially received TB200 and TE200. Furthermore, shrimps that received TB100, TE100, CP100, and LP100 7 days were then challenged with Vibrio parahaemolyticus and the result demonstrated higher survival rates especially TB100 at 168 h than the control feed. In conclusion, incorporating AST into the diets enhanced shrimp red coloration, immune parameters, and resistance against V. parahaemolyticus infection. The K. marxianus-derived AST exhibited higher performance than did chemical AST to be a potential feed additive in shrimp aquaculture.
Similar content being viewed by others
Introduction
In recent years, white shrimp (Penaeus vannamei) has become one of the most valuable products in aquaculture because of its high protein with low fat content and commercial value. White shrimp can store high levels of the antioxidant astaxanthin (AST), leading to red body coloration when cooked1,2. Consumer purchasing power is affected by color, so the price of red shrimp will be higher3. The demand for AST in food, feed, cosmetics, and pharmaceutical applications is increasing because of its superior antioxidative and coloring properties. Aside from the use in animal health as an antioxidant through scavenging free radicals from cellular activity or oxidative stress4, AST is suggested as an essential growth factor in the early development of shrimp5. Dietary astaxanthin is useful for improving the growth performance by promoting metabolic-related pathways, such as pyruvate metabolism and glycolysis/gluconeogenesis pathway6. Shrimp innate immunity also can be modulated by treating AST via enhancing immune responses including hematocyte count, lysozyme, phagocytic activity, and phenoloxidase, etc.7. AST has been commercially produced through extraction from crustaceans, chemical synthesis, and microbial synthesis. Three representative microbes—namely the alga Haematococcus pluvialis, the yeast Xanthophyllomyces dendrorhous, and the bacterium Paracoccus carotinifaciens—are currently used for the commercial production of two natural stereoisomers of 3S, 3’S- and 3R, 3’R-AST forms, which 3S, 3’S-AST has the best antioxidant ability8. However, naturally produced AST is expensive, primarily because of its low productivity and limited sources. Chemically derived AST, which has three stereoisomers (3R, 3’R), (3R, 3’S; meso), and (3S, 3’S) of an approximate 1:2:1 ratio9, is commonly used as an aquaculture feed additive, but its synthetic AST of 3R, 3’S (3S, 3’R) forms may be toxic to humans and the production process may cause environmental pollution10.
AST production in microorganisms through the use of metabolic engineering a heterologous host and other new technologies such as atmospheric and room temperature plasma (ARTP) mutagenesis to promote astaxanthin production11 are desirable because of the increasing demand for sustainable and affordable AST yields. A study involving the probiotic yeast Kluveromyces marxianus achieved cell reprogramming toward the 3S, 3’S-AST production and demonstrated that the yeast produced up to 9.97 mg/g dry cell weight (DCW) of 3S, 3’S-AST in a 5 L bioreactor12. The use of probiotics in shrimp aquaculture has been under investigation for several years because probiotics are believed to improve shrimp value2. Probiotics, as “bio-friendly microorganisms”, are chosen to replace the antimicrobial agents and they act as natural immune enhancers, which stimulate the disease resistance in shrimp farm13. K. marxianus accounts the major yeast population in kefir14,15. Recently, its probiotic potential has been actively explored from several studies with a focus on its health benefits and safety including its adhesion to the intestinal epithelium, antagonism toward pathogenic bacteria, anti-microbial, and anti-inflammatory functions16,17,18.
Studies have reported that diets containing chemically derived AST can increase the red coloration of shrimp and growth performance of P. vannamei19,20,21, Marsupenaeus japonicu22, and P. monodon23. Feeding P. vannamei with algae-produced or chemically derived AST can improve its immune capacity20,24. Chemically derived AST has been observed to protect white shrimp against Vibrio parahaemolyticus infection25,26; this infection is the most concerning problem in shrimp aquaculture worldwide because it causes acute hepatopancreatic necrosis disease27. However, no study has investigated whether the administration of 3S, 3’S-AST derived from probiotic yeast results in resistance against V. parahaemolyticus infection in cultured shrimp.
This is the first study to compare the effects of dietary K. marxianus-produced and chemically derived AST on immune-related genes and in the red coloration of white shrimp; the study also analyzed the potential commercial uses of K. marxianus-produced 3S, 3’S-AST. To investigate whether shrimp fed a diet containing AST would exhibit enhanced nonspecific immune responses, this study conducted an in vivo assay to measure phagocytic activity, O2− production, PO activity, and immune-related gene expression. Furthermore, the study analyzed the effects of AST on resistance against V. parahaemolyticus infection in P. vannamei.
Methods
Sources of AST
Commercially available Carophyll Pink (CP) and Lucantin Pink (LP) with 10% AST content were purchased from DSM Nutrition (Tokyo, Japan) and BASF (Ludwigshafen, Germany), respectively. Dried powder of K. marxianus-produced AST fermented broth (TB) and its extract (TE) were obtained from Trade Wind Biotech Co. Ltd. (Taipei, Taiwan) and stored at 4 °C until use.
Experimental feed preparation
The basal diet obtained from Tairoun Products Co., Ltd. A basal feed with no AST was used as a control. The experimental feed was formulated and modified on the basis of the methods described by Ngo et al.28 (Table 1). Specifically, the experimental feed was divided into eight groups according to the concentration of AST used to supplement the basal feed: CP100, LP100, TB100, and TE100, formulated by supplementing the basal feed with 0.1 g/kg of AST; and CP200, LP200, TB200, and TE200, formulated by supplementing the basal feed with 0.2 g/kg of AST. For experimental feed preparation, dry ingredients were mixed with 1.5% fish oil and 30% water until a stiff dough was formed. Feed pellets of approximately 2 mm were prepared. The prepared pellets were dried in a drying cabinet equipped with an air blower at 40 °C and do not exceed 50 °C, which has been test the temperature didn’t destroy the AST (data not shown), until the moisture level dropped to approximately 10% overnight. The dried experimental feed pellets were analyzed AST by HPLC and stored in a sealed plastic box at 4 °C until use.
Experimental animals
White shrimp were obtained from the Aquatic Animal Center, National Taiwan Ocean University, Keelung, Taiwan. The shrimp were acclimatized to laboratory conditions in 10-ton tanks (5 m × 2 m × 1 m) and fed the commercial shrimp feed (Tairoun Products Co., Ltd.) at 5% body weight three times a day. Subsequently, the shrimp were moved indoors to tanks with a recirculating water system for the experiment. During the experiment, water temperature and salinity were maintained at 27 ± 1 °C and 33 ± 1‰, respectively.
High-pressure liquid chromatography
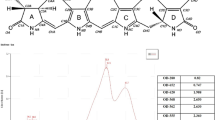
Carotenoid profiles were analyzed using high-pressure liquid chromatography (HPLC) in accordance with the method described by Lin et al.12. 50 mg of each experimental feeds together with basal feed (Control) per batch were measured the carotenoids amount with three replicates. Total carotenoids were extracted from the experimental feeds by using DMSO in three replicates and then homogenized using a PowerLyzer 24 Homogenizer (Qiagen, Düsseldorf, Germany); the supernatant was collected after centrifugation. A Nomura Chemical Develosil C30-UG Column (Interlink Scientific Services, Dartford, UK) was used along with two buffer types, namely buffer A and buffer B: buffer A contained methanol, methyl tert-butyl ether (MTBE), and water (81:15:4 vol/vol/vol), and buffer B contained methanol, MTBE, and water (7:90:3 vol/vol/vol). The flow rate of the mobile phase was 1 mL/min, and the solvent gradient was as follows: 100% to 0% of buffer A and 0% to 100% of buffer B from 0 to 25 min, followed by 0% to 100% of buffer A and 100% to 0% of buffer B from 26 to 30 min. Samples were monitored using a Jasco 870-UV Intelligent UV/Vis Detector (JASCO International, Tokyo, Japan). AST was identified through chromatography by using commercial compound as reference standard (Sigma-Aldrich, St. Louis, MO, USA). The reference standard was prepared through serial dilution with the following concentrations to produce standard curve: 3.125, 6.25, 12.5, 25, and 50 mg/L. The standard curve was used for quantitation in combination with the extinction coefficients.
Digital image acquisition and processing for coloration detection
A total of 180 white shrimp (9 ± 1 g) were randomly distributed into nine 149-L tanks (74 cm × 53 cm × 38 cm) for 9 groups (20 shrimp per group). The shrimp were maintained in a recirculating culture system with 50% water exchange per day. They were fed CP100, LP100, TB100, TE100, CP200, LP200, TB200, and TE200 or the control feed daily at 09:00, 15:00, and 21:00; the daily feeding rate was approximately 5% body weight. Body coloration were measured per the method of a previous study19,29 but with slight modifications. Five shrimp were collected from each tank on days 0, 7, 14, 21, and 28 and boiled at 100 °C for 3 min to observe their body coloration.
Shrimp body coloration was quantified using the three-dimensional CIE1976 (L*, a*, b*) color space system developed by the International Commission on Illumination, where L* represents lightness, a* represents redness, and b* represents yellowness. Adobe Photoshop 2020 software (Adobe, San Jose, CA, USA) was used for coloration index evaluation. Specifically, a captured image was first converted to the L*a*b* color space, after which gradation correction was executed to adjust the image’s white balance, as described by Wade et al.30. As illustrated in Fig. 1, five points were selected for color quantification; a color picking tool was used to obtain the color values at each point.
In vivo effect of AST on immune response
A total of 320 white shrimp (9 ± 1 g) were randomly distributed into nine 205-L tanks (90 cm × 60 cm × 38 cm) for 9 groups (35 shrimp per group). The remaining 5 shrimp were used as samples on day 0. The shrimp were maintained in a recirculating culture system with 50% water exchange per day. They were fed CP100, LP100, TB100, TE100, CP200, LP200, TB200, and TE200 or the control feed daily at 09.00, 15:00, and 21:00 at a feeding rate of approximately 5% body weight. Five shrimp were sampled from each tank on days 1, 2, 4, 7, 14, 21, and 28 to analyze the immune parameters and related gene expression.
The immune parameters assay according to published methods28 with a slight modification. Hemolymph was withdrawn from the ventral sinus using 1-mL sterile syringes and mix with an anticoagulant (30 mM trisodium citrate, 0.34 M sodium chloride, 10 mM ethylenediaminetetraacetic acid and 0.12 M glucose, pH 7.4) in a ratio of 1:9. The withdrawn hemolymph was divided into two tubes: one of the tubes was used to conduct an assay to determine immune parameters, and the other was used to perform an assay to evaluate immune-related gene expression. The density of hemocytes for the in vivo response immune assay was quantified as the total hemocyte count (THC) and then adjusted to 1 × 106 cells /mL using a hemocytometer and an inverted phase-contrast microscope (Leica DMIL, Leica Microsystems, Wetzlar, Germany). The hemolymph-anticoagulant mixture was centrifuged at 800 × g for 20 min at 4 °C, and then the supernatant was removed. Phenoloxidase (PO) activity, phagocytic rate (PR), and superoxide anion (O2−) production were measured as follows:
This study measured PO activity spectrophotometrically by recording the formation of dopachrome from L-dihydroxyphenylalanine (L-DOPA) by using the method described by Hernández-López et al.31, with slight modifications. Briefly, hemolymph (1 mL) was centrifuged at 800 × g at 4 °C for 20 min; subsequently, the supernatant was removed, and the cells were resuspended with 1 mL of cacodylate citrate buffer solution (0.01 M sodium cacodylate, 0.45 M sodium chloride, and 0.01 M trisodium citrate; pH 7.0). The tube was centrifuged, and the supernatant was removed; next, the pellet was resuspended with 200 μL of cacodylate buffer solution (0.01 M sodium cacodylate, 0.45 M sodium chloride, 0.01 M calcium chloride, and 0.26 M magnesium chloride; pH 7.0), and aliquots (cell suspension) were divided into two equal parts. For sample analysis, 100 μL of suspension was incubated with 50 μL of trypsin (1 mg/mL, Sigma-Aldrich) for 10 min at room temperature, which served as an activator for measuring total PO activity. For measuring background PO activity, 100 μL of cell suspension was incubated with 50 μL of cacodylate buffer for 10 min at room temperature, which served as a control. Subsequently, 50 μL of L-DOPA (3 mg/mL, Sigma-Aldrich) was added, and the solution was incubated for 15 min at room temperature. Next, 400 μL of cacodylate buffer was added to dilute the mixture. The sample was measured using a spectrophotometer (Model U-2000 Hitachi, Tokyo, Japan) at an optical density of 490 nm. PO activity was evaluated using the following equation:
Phagocytic activity was measured using the method described by a previous study28, with slight modifications. Briefly, hemolymph (100 μL) was dropped on a cover glass and incubated at 25 °C for 60 min for adherence. After incubation, the hemolymph was washed with modified complete Hank’s balanced salt solution (MCHBSS) (10 mM calcium chloride (Panreac), 3 mM magnesium chloride (Sigma-Aldrich) and 5 mM magnesium sulfate in HBSS (Gibco)); 100 μL of latex beads (0.8 μm; 3 × 107 beads/mL, Sigma-Aldrich) was then added, followed by incubation at room temperature for 60 min. Subsequently, the hemolymph was again washed with MCHBSS, fixated with methanol for 5 min, stained with Giemsa (6%) for 20 min, decolorized with distilled water, air dried, and observed under a light microscope (Olympus, Tokyo, Japan). The number of phagocytic hemocytes among 200 hemocytes was counted. Phagocytic activity was derived using the following equation:
O2− production was quantified by measuring the reduction of nitroblue tetrazolium (NBT) to formazan, as previously described28. Briefly, hemolymph (1 mL) was centrifuged at 800×g at 4 °C for 20 min, and the supernatant was removed. Zymosan (100 μL, 1 mg/mL, Sigma-Aldrich) or MCHBSS (100 μL) was added to 96-well plates for 30 min at room temperature to measure total or background O2− production, respectively. Zymosan was added to stimulate hemocytes. The supernatant was discarded, and the hemocytes were incubated with 100 μL of NBT solution (0.3%) for 30 min at room temperature. Subsequently, the NBT solution was removed, and 100 μL of methanol was added to stop the reaction. After 5 min, the methanol was removed. The 96-well plates were washed thrice with 70% methanol (100 μL) and air dried for 30 min. The insoluble formazan crystals that were formed were dissolved with the addition of 120 μL of 2 M potassium hydroxide and 140 μL of dimethyl sulfoxide and measured using an enzyme-linked immunosorbent assay microplate reader at an optical density of 630 nm. The amount of O2− produced was calculated using the following equation:
Gene expression
The method of gene expression as previously described32. Hemolymph was diluted with 1000 μL of anticoagulant buffer in 2-mL microcentrifuge tubes. The tubes were centrifuged at 800 ×g at 4 °C for 20 min. The supernatant was removed, and the hemocyte pellets were washed with 1000 mL of anticoagulant buffer. Subsequently, GENEzol reagent (Geneaid, New Taipei City, Taiwan) and chloroform (Thermo Fisher Scientific, Waltham, MA, USA) were added to each tube (5:1). The tubes were then vortexed for 15 s, incubated at room temperature for 5 min, and centrifuged at 12,000 ×g for 15 min at 4 °C. The colorless upper aqueous phase (0.4 mL) was transferred to a 1.5-mL tube containing equal volumes of isopropanol (Sigma-Aldrich) and incubated at room temperature for 10 min, followed by centrifugation at 12,000 ×g for 10 min at 4 °C. RNA pellets were washed twice with 1 mL of 75% ethanol and air dried; subsequently, they were dissolved in 13 μL of DEPC-treated water (Thermo Fisher Scientific). The quality and quantity of total RNA were measured using a SpectraMax QuickDrop Micro-Volume Spectrophotometer (Molecular Devices, San Jose, CA, USA).
First-strand synthesis was conducted using a HiScript I First Strand cDNA Synthesis Kit (Bio-Novas, Bremerton, WA, USA) in accordance with the manufacturer’s protocol. The reaction mixture (19 μL) contained DNase-treated RNA (7 μL), 2 × fast premix (10 μL), and primer oligo dT (2 μL). The cycling program was as follows: 65 °C for 5 min and 4 °C for 1 min. Subsequently, DNase-treated RNA was combined with HiScript Reverse Transcriptase and incubated at 42 °C for 30 min and then at 85 °C for 5 min; next, an inactivation step was performed at 95 °C for 1 min, and cDNA samples were further diluted 10 times in DEPC-treated water.
The primers listed in Table 2 were used to measure the expression levels of elongation factor-1α (EF-1α), serving as the reference gene; lysozyme (Lyz); penaeidin 2, 3, and 4 (Pen2, Pen3, and Pen4, respectively); superoxide dismutase (SOD); glutathione peroxidase (GPx); and Anti-LPS factor (ALF)33. A quantitative polymerase chain reaction (PCR) process (20 μL) involving 1 μL of cDNA, 10 μL of iQ SYBR Green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA, USA), 0.4 μL each of forward and reverse primers (100 μm), and 8.2 μL of DD water was performed using a QuantStudio1 Applied Biosystems Real-Time PCR machine (Thermo Fisher Scientific). The cycles were performed at 50 °C for 2 min and at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 min and at 60 °C for 1 min. Melting curves were derived for all samples for analysis. The gene expression levels of interest were normalized to the EF-1α expression level and are expressed herein as the fold change relative to the reference expression level at each time point. The PCR process was performed in accordance with the procedure reported by Livak and Schmittgen34.
Challenge test
A total of 198 white shrimp (9.67 ± 0.87 g) were randomly distributed into eighteen 103-L tanks (60 cm × 45 cm × 38 cm) for 6 groups in triplicate (33 shrimp per group). White shrimp were determined their resistance to V. parahaemolyticus infection, which pathogen was isolated from an acute hepatopancreas necrosis disease (AHPND) infected shrimp. Of these six tanks, two constituted the control groups (positive and negative groups), which were fed the control diet; the remaining four tanks constituted the experimental groups, which were fed the CP100, LP100, TB100, and TE 100 feeds (formulated by supplementing the basal feed with 0.1 g/kg of AST). These positive and experimental groups were challenged with V. parahaemolyticus on day 7 of the experimental period by injecting a 20-μL bacterial suspension (107 CFU/mL) while the negative group were injected phosphate-buffered saline solution into the ventral sinus of the cephalothorax. The shrimp were administered the experimental feeds at a feeding rate of approximately 5% body weight at 9:00, 15:00, and 21:00 for 7 days. Mortality was recorded at 6, 12, 24, 48, 72, 96, 120, 144 and 168 h post injection.
Data analysis
The experimental and control groups differed significantly with respect to PO activity, O2− production, phagocytic activity, body colorization, and challenge test results. The groups were compared using one-way analysis of variance and Tukey’s honestly significant difference test in SPSS Version 25.0 (IBM, Armonk NY, USA). P values of < 0.05 were considered significant.
Result
Quantification of AST concentration in experimental feeds
To ensure that the actual AST concentrations were consistent with the theoretical AST concentrations in the feeds, HPLC was used to quantify the total AST concentration in each feed groups The HPLC profiles are showed in Fig. 1 and the AST concentration are presented in Table 3. The qualified AST peaks of each experimental feeds were appeared at the retention time around 8.4 min as the reference standard, where the basic feed wasn’t detected. The quantified results indicated that the theoretical AST concentrations were not consistent with the actual CP and LP concentrations; therefore, the concentrations of the chemically-derived AST in the prepared feeds were increased by twofold. Finally, the actual AST concentrations in CP100, CP200, LP100, LP200, TE100, TE200, TB100, and TB200 were 97.26, 219.25, 106.82, 257.84, 94.03, 192.03, 106.57, and 229.57 mg/kg feed, respectively. Moreover, the concentrations of beta-carotene in TE100, TE200, TB100, and TB200 were 16.08, 71.89, 30.67, and 97.35 mg/kg feed, respectively.
Effects of dietary AST on shrimp body coloration
As mentioned, five points were selected for color quantification for each of the shrimps that received the various feed types, as shown in Fig. 2A. The body of each shrimp in each feed group was photographed in different sampling weeks, as depicted Fig. 2B. The results revealed that the L* (lightness) values derived for the shrimp that received all feed types ranged between 65 and 72 in week 1 and then decreased to 56 to 65 in the following sampling weeks (Fig. 3A, p < 0.05). However, the a* (redness) values derived for the shrimp that received all feed groups ranged between 35 and 55 in week 1 and then increased in the following sampling weeks for only the shrimp that received the AST-supplemented feeds (Fig. 3B). In weeks 1 and 2, the shrimp that received TE200 exhibited the highest a* value (p < 0.05). In week 4, the shrimp that received TB100 and TB200 had higher a* values (between 65 and 70) than did those that received the other feed groups (Fig. 3B). Furthermore, b* (yellowness) values derived for the shrimp that received all feed groups ranged between 45 and 56 in week 1 and then increased in the following sampling weeks (Fig. 3C). The shrimp that received TE200 exhibited the highest b* value (p < 0.05) in week 1. In week 4, those that received TE100 and TB200 had higher b* values (between 60 and 75) than did those that received the other feed groups, but the difference was not significant (p > 0.05).
The effect of astaxanthin on white shrimp body color parameter. (A) L* value (lightness), (B) a* value (redness), and (C) b* value (yellowness). One-way ANOVA and Tukey’s test were performed to compare the differences between groups at indicated time point. Significant differences (p < 0.05) between groups are indicated by different letters above bars. The data are expressed as mean ± standard deviation (n = 3).
Effects of dietary AST on immune response
Phagocytic activity
From days 2 to 28, the PR values of the shrimp that received the AST-supplemented feeds were higher than that of those that received the control feed (Fig. 4A). On day 7, the PR values of the shrimp that received all AST-supplemented feeds were significantly higher than that of those that received the control feed (p < 0.05); the shrimp that received TB100 exhibited the highest PR. The effect of TE100 on PR activity was highest on days 14 and 21 among did those that received the other feed groups. The effect of TB200 on PR activity was highest on day 28 among did those that received the other feed groups.
Effects of dietary AST on nonspecific immune response of white shrimp haemocyte. (A) Phagocytic rate, (B) superoxide anion production rate, and (C) phenoloxidase activity. One-way ANOVA and Tukey’s test were performed to compare the differences between groups at indicated time point. Significant differences (p < 0.05) between groups are indicated by different letters above bars. The data are expressed as mean ± standard deviation (n = 3).
O2 − production
From days 2 to 21, the shrimp that received all AST-supplemented feeds had higher O2− production rates than did those that received the control feed (Fig. 4B). On day 1, the O2− production rates in the shrimp that received LP200 and TB200 were significantly different from that in those that received the control feed (p < 0.05). On day 2, except for the O2− production rate in those that received CP100, the O2− production rates in the shrimp that received the other AST-supplemented feeds were significantly different from that in those that received the control feed (p < 0.05). On days 4, 7, 14, and 21, LP200 exhibited the highest effect on O2− production (p < 0.05) among did those that received the other feed groups. On day 28, the results revealed no significant difference in O2− production between the shrimp that received the AST-supplemented feeds and those that received the control feed (p > 0.05).
PO activity
From days 2 to 21, the shrimp that received the AST-supplemented feeds exhibited higher PO activity levels than did those that received the control feed (Fig. 4C). On day 2, the shrimp that received LP100, TE100, and TE200 groups exhibited significantly increased PO activity levels compared with those that received the control feed (p < 0.05). On days 4, 7, and 14, the shrimp that received the AST-supplemented feeds exhibited significantly higher PO activity levels than did those that received the control feed (p < 0.05). On day 21, the PO activity levels in the shrimp that received CP100, CP200, LP100, TE100, TB100, and TB200 increased significantly (p < 0.05). On day 28, the shrimp that received CP100, LP100, TE100, TE200, and TB200 demonstrated significantly enhanced PO activity levels compared with those that received the control feed (p < 0.05).
Immune-related gene expression
This study evaluated the expression of two antioxidant genes, namely SOD and GPx (Fig. 5). The shrimp that received the AST-supplemented feeds exhibited increased SOD (Fig. 5A) and GPx (Fig. 5B) expression on day 7, and the expression level peaked on day 14. Among the shrimp that received the AST-supplemented feeds, those that received TB200 registered the highest number of days with the highest SOD expression levels (days 1, 2, 4, and 7) and highest GPx expression levels (days 7 and 14) (p < 0.05).
Evaluated antioxidant genes expression of haemocytes in the white shrimp fed with AST contained feed. (A) SOD and (B) GPx. One-way ANOVA and Tukey’s test were performed to compare the differences between groups at indicated time point. Significant differences (p < 0.05) between groups are indicated by different letters above bars. The data are expressed as mean ± standard deviation (n = 3).
The study also evaluated the expression of five antimicrobial peptides, namely Pen2, Pen3, Pen4, ALF, and Lyz (Fig. 6). On days 1, 2, 4, and 14, Pen2 was significantly upregulated in the shrimp that received the AST-supplemented feeds compared with those that received the control feed (p < 0.05) (Fig. 6A). Among the shrimp that received the AST-supplemented feeds, those that received TE100 and TE200 exhibited the highest number of days with upregulated Pen2 expression (days 1 to 21) among did those that received the other feed groups. The results revealed no significant difference in peptide expression between the feed groups on day 28 (p > 0.05). On days 2, 4, 7, and 14, Pen3 was upregulated in the shrimp that received the AST-supplemented feeds compared with those that received the control feed (Fig. 6B). Pen3 expression was significantly upregulated in the shrimp that received TE100 compared with those that received the other AST-supplemented feeds on day 4 and 7 (p < 0.05); received TE200 compared with those that received the other AST-supplemented feeds on day 14 (p < 0.05). On day 7, Pen4 was significantly upregulated in the shrimp that received the AST feeds (p < 0.05) (Fig. 6C). The shrimp that received TB200 had the highest Pen4 expression level on day 7, which differed significantly from the expression levels in the shrimp that received the control feed, CP100, CP200, LP100, and LP200 (p < 0.05). The study revealed no significant difference in peptide expression between the various feed groups on days 1, 2, 4, and 21 (p > 0.05). The shrimp that received the AST-supplemented feeds exhibited the higher ALF expression on days 2, 4, 7, 14 (Fig. 6D). Subsequently, ALF expression gradually decreased until day 21 and 28 in the shrimp that received the AST feeds. Among the shrimp that received the AST-supplemented feeds, those that received TE200 exhibited the highest ALF expression levels on days 7 and 14, and those that received TB200 exhibited the highest ALF expression on days 1 and 2 (p < 0.05). On days 4, 7, and 21, Lyz expression was significantly upregulated in the shrimp that received the AST feeds compared with those that received the control feed (Fig. 6E). Among the shrimp that received the AST-supplemented feeds, those that received TE100 exhibited the highest number of days with upregulated Lyz expression (days 2, 4, 7, 14, 21, and 28). Overall, these results (Fig. 6) indicate that the AST feeds, especially in the TE200 and TB200 feeds, stimulated the expression of antimicrobial peptide genes.
Evaluated immune-related genes expression of haemocytes in the white shrimp fed with AST contained feed. (A) Pen2, (B) Pen3, (C) Pen4, (D) ALF, and (E) Lyz. One-way ANOVA and Tukey’s test were performed to compare the differences between groups at indicated time point. Significant differences (p < 0.05) between groups are indicated by different letters above bars. The data are expressed as mean ± standard deviation (n = 3).
Survival rates of dietary AST on white shrimp after challenged with V. parahaemolyticus
Figure 7 presents the survival rates of the shrimp that were fed the AST-supplemented feeds for 7 days and challenged with V. parahaemolyticus. No mortality was observed in the negative control group (PBS) over a 168-h period. However, mortality was observed in the positive control group (C) 12 h after injection with V. parahaemolyticus, and the survival rate decreased considerably 12 to 48 h after injection. The shrimp that received CP100, LP100, TE100, and TB100 had significantly higher survival rates (p < 0.05) 48 to 168 h after injection than did those in the C group. The survival rates of the shrimp in the PBS, C, CP100, LP100, TE100, and TB100 groups were 100.00 ± 0.00%, 66.67 ± 5.25%, 87.88 ± 5.25%, 84.85 ± 5.25%, 87.88 ± 5.25%, and 90.91 ± 0.00%, respectively.
Effects of dietary AST on white shrimp resistance to V. parahaemolyticus. Survival rate (%) of white shrimp fed diets containing AST for 7 days after injections with V. parahaemolyticus. One-way ANOVA and Tukey’s test were performed to compare the differences between groups at indicated time point. Significant differences (p < 0.05) between groups are indicated by different letters above the time points. The data are expressed as mean ± standard deviation (n = 3).
Discussion
Color plays an important role in the acceptance and market value of shrimp3,35. The body surface color of shrimp is affected by food, background color, and light35,36,37. Astaxanthin can promote the health of shrimp to increase the production of shrimp, and it can also significantly increase the red color of shrimp body surface to increase the selling price19. K. marxianus derived 3S, 3’S-AST has only one isomer. Carophyll Pink has 3 isomers (3S, 3’S), (3R, 3’R) and (3R, 3’S) in the ingredients and consisted the product forms in the ratio of 1:1:2. Therefore, this study explored the different proportions of AST isomers on shrimp. Previous studies have reported that AST has a positive effect on the intermediary metabolism of aquatic animals and might improve growth by enhancing nutrient utilization38,39. Ju et al. and Wang et al.19,22 have revealed that after being cooked, shrimp fed an AST-supplemented diet had a more pronounced red coloration than did those that received a control diet (light pink coloration). Their findings are consistent with our results. Specifically, our results reveal that redness (a*) and yellowness (b*) increased significantly in week 2 in all shrimp that received the AST-supplemented feeds compared with those that received the control feed; however, the results indicate a dose-independent difference in coloration between the shrimp that received the AST-supplemented feeds. Specifically, we observed that the shrimp that received TE200, which was supplemented with K. marxianus derived 3S, 3’S-AST, had significantly higher redness (a*) values (p < 0.05) in weeks 1 and 2 compared with those that received the other AST-supplemented feeds; however, those that received TB100 and TB200, which were supplemented with fermented broth derived from K. marxianus derived 3S, 3’S-AST, had the highest redness (a*) value (p < 0.05) in week 4. The red coloration of the shrimp that received TB100 and TB200 appeared at a later time point (week 4) because K. marxinus-produced fermented broth, which contains yeast cells, was heated to create a dry powder; the powder must be digested first before free-form AST could be released40,41. By contrast, K. marxianus derived 3S, 3’S-AST extract could be absorbed quickly in the digestive tract, resulting in a pronounced red coloration as early as week 1. Generally, in weeks 1 to 4, the shrimp that received K. marxianus derived 3S, 3’S-AST (TE100, TE200, TB100, and TB200) exhibited higher yellowness (b*) values than did those that received chemically-derived AST (CP100, CP200, LP100, and LP200). This can be attributed to the presence of beta-carotene in both the fermented broth and extract forms of K. marxianus derived 3S, 3’S-AST. Díaz-Jiménez et al. found that an increase in the level of β-carotene in the diet can increase the accumulation of astaxanthin in the tissue42. They suggested that because the oxidisation of the positions 3, 3ʹ, 4, 4ʹ of β-ionone rings of β-carotene occurs43, allowing a gradual increase relative to the dietary concentration, which can be readily integrated into metabolic processes44.
Several reports have shown that immune parameters such as total hemocyte count (THC), cell viability, PR, phagocytic index (PI), PO, O2−, and antibacterial activity can be considered reliable indicators of the health status and physiological conditions of shrimp45,46. Wang et al.22 demonstrated that AST supplementation, especially with approximately 400 mg/kg dietary AST, enhances the pigmentation of juvenile kuruma shrimp, in addition to enhancing their immune response, stress resistance response, growth performance, and fatty acid content. Amar et al.47 also revealed that dietary carotenoids from both the marine green algae Dunaliella salina (beta carotene) and the red yeast Phaffia rhodozyma (astaxanthin) can modulate some of the innate defense mechanisms of shrimp. In the present study, hemocytes were used at several sampling time points to evaluate immune response after AST administration. Phagocytosis can be triggered by activating the components of the putative prophenoloxidase (proPO) system, where proPO is activated by PO when reacting with zymosan, lipopolysaccharide, calcium ions, and trypsin48. PO can adhere to the surfaces of various pathogens, such as fungi, as a sticky protein49 to enhance phagocytosis50,51, which is commonly used as an indicator of shrimp immunity. Chuchird et al. found that Pacific white shrimp fed with diets containing 50 ppm AST for a 90-day trial had PO activity significantly higher than the control25. Wang et al. showed that fed L. vannamei with 80 mg/kg astaxanthin supplemented diet for 4 weeks significantly affects the serum PO activity compared to the control24. Feeding L. vannamei with distinct AST-containing diets including 25, 50, 100, and 200 mg/kg diet for 8 weeks was associated with significant increases in the PO value7. The results of our in vivo assay indicated that the AST-supplemented feeds resulted in a significantly high PR on days 7 and 14 compared with the control feed (p < 0.05). These immune response results are similar to the observed activation of PO in the shrimp after the administration of the AST-supplemented feeds, especially in the TE and TB groups. Yowaphui et al. reported that immune parameters including THC, Phagocytosis (%), PO, and SOD activity of shrimp fed a diet containing 50 ppm β-carotene were significantly improved52. Previous research showed that AST was better than β-carotene either as dietary pigment or as dietary antioxidant in the commercial diet of P. monodon53, but dietary β-carotene could provide a cheaper source of carotenoid alternative54. Fawzy et al. studied that dietary supplementation of β-carotene could improve growth performance and survival rate, and achieve the desired coloration at the optimum β-carotene level ranged from 265.2 to 341.28 mg/kg diet for Litopenaeus vannamei. Besides, the combination of β-carotene and AST seemed to exert a synergistic effect54. Taken together, K. marxianus derived 3S, 3’S-AST in either TE or TB groups contains additional β-carotene benefits may refer to its efficiency in improving the overall performance.
During phagocytosis, antimicrobial substances such as O2− are produced to eliminate bacteria. This study observed that on days 4, 7, 14, and 21, the shrimp that received the AST-supplemented feeds exhibited relatively high O2− production rates compared with those that received the control feed. However, excessive O2− production may lead to cell damage due to the possible oxidative stress55. Wu et al.56 reported that SOD activation is based on O2− production. The SOD enzyme can reduce excessive O2− production to prevent the formation of hydrogen peroxide (H2O2). If H2O2 is present, it can be degraded by GPx or catalase during phagocytosis. These mechanisms constitute an antioxidant self-protection system57,58. As a result of the respiratory burst post-phagocytic event, ROS will be released, such as superoxide anion then hydroxyl radicals will occur. Similar results were observed for SOD and GPx expression to exhibit significantly higher anti superoxide anion ability. SOD and GPx upregulation started on day 7 and peaked on day 14 in the shrimp that received the AST-supplemented feeds compared with those that received the control feed indicating the ability to scavenge superoxide anion was improved after feeding with AST supplement24. Eldessouki et al. also showed that the dietary AST enhanced (P < 0.05) the activities of serum SOD, catalase, GPx, and increased levels of total antioxidant capacity especially in the 200 mg AST/kg feed treatment in L. vannamei7. In our result, different sources of AST can increase the immune response, but only K. marxianus derived 3S, 3’S-AST can increase more antioxidant ability.
Regarding humoral parameters, the antibacterial activity of plasma is another indicator of health status; it can be considered an immune indicator in shrimp. Antimicrobial peptides can act against a broad range of pathogens, including gram-positive and gram-negative bacteria, yeast, fungi, parasites, enveloped viruses, and even tumor cells59. Penaeidins, which are unique antimicrobial peptides generated from shrimp, can be classified into three types: Pen2, Pen3, and Pen4. These peptides are distinguished by the N-terminal proline-rich domain and the C-terminal cysteine-rich domain60. ALF can be incorporated to inhibit infections induced by filamentous fungi and gram-positive and gram-negative bacteria; this is highly beneficial against drug-resistant vibrio species61. Lyz is efficient against gram-positive bacterial infections due to its ability to cleave the cell wall composition of β-1,4 glycosidic bonds between N-acetylmuramic acid and N-acetylglucosamine in bacteria62,63. In the present study, the shrimp that received the AST-supplemented feeds generally exhibited higher Pen2, Pen3, Pen4, ALF, and Lys expression levels on days 4, 7, and 14 than did those that received the control feed, demonstrating that AST supplementation can potentially prevent infections induced by fungi and gram-positive and gram-negative bacteria. Similar result was showed by Liu et al. that dietary supplementation of AST at 250 mg kg−1 diet for 4 weeks feeding trial significantly increased the relative expression of crustins, Pen-3α, SOD and LZM compared to the control diet64.
Furthermore, K. marxianus derived 3S, 3’S-AST exhibited higher gene expression of antibacterial peptides than chemically derived AST. In this study, we observed higher levels of immune response and immune-related gene expressions on days 7 and 14. Shrimp were fed AST at a concentration of 100 mg per feed for 7 days, which was sufficient to induce an immune response; subsequently, they were challenged with V. parahaemolyticus. The results reveal that 168 h after injection with V. parahaemolyticus, the shrimp that received TB100 had the highest survival rate. No significant difference in survival was observed between the shrimp that received the AST-supplemented feeds, but the survival rates of these shrimp differed significantly from that of those that received the control feed. These results are similar to those obtained from the assessment of in vivo antibacterial activity, especially the shrimp received K. marxinus-produced AST (TE100, TE200, and TB200), which consist of 3S, 3’S-AST can stimulate higher antibacterial peptides expression. Therefore, groups receiving the 3S, 3’S AST-supplemented feeds had the highest ability against to V. parahaemolyticus infection on white shrimp. Churchird et al. also demonstrated the effect of dietary astaxanthin on the survival of Pacific white shrimp and their resistance to V. parahaemolyticus25. Our results are consistent with those of previous studies that have reported that AST administration can enhance the survival rate of V. parahaemolyticus-infected shrimp25,26.
Our findings suggest the wide range of physiological benefits that K. marxianus derived 3S, 3’S-AST confers to as a potential feed supplement in aquatic animals, which these animals may be presented various improvements in survival, pigmentation, stress tolerance, disease resistance and immune-related gene expression.
Conclusion
Both K. marxianus derived 3S, 3’S-AST and chemically-derived AST successfully enhanced red body coloration in white shrimp, especially those that received TE200, TB100, and TB200. K. marxianus derived 3S, 3’S-AST administration improved multiple immune parameters and prevented V. parahaemolyticus infection in the shrimp. The results of this study suggest that K. marxianus derived 3S, 3’S-AST has the potential to serve as an alternative aquaculture feed additive.
Data availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zokaeifar, H. et al. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 33, 683–689 (2012).
Nguyen, T. T. et al. Cooperative improvement in growth rate, red-colour score and astaxanthin level of white-leg shrimp by Bacillus strains originating from shrimp gut. J. Appl. Microbiol. 129, 51–62 (2020).
Erickson, M. et al. Consumer differentiation, acceptance, and demographic patterns to consumption of six varieties of shrimp. Food Prod. Technol. 15, 35–51 (2007).
Dose, J. et al. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 17(1), 103 (2016).
Dall, W. Carotenoids versus retinoids (vitamin A) as essential growth factors in penaeid prawns (Penaeus semisulcatus). Mar. Biol. 124, 209–213 (1995).
Wang, Y. et al. Comparative transcriptome analysis reveals the potential influencing mechanism of dietary astaxanthin on growth and metabolism in Litopenaeus vannamei. Aquac. Rep. 16, 100259 (2020).
Eldessouki, E. A. A. et al. Dietary astaxanthin modulated the performance, gastrointestinal histology, and antioxidant and immune responses and enhanced the resistance of Litopenaeus vannamei against Vibrio harveyi infection. Aquac. Int. 30, 1869–1887 (2022).
Wan, X. et al. Reprogramming microorganisms for the biosynthesis of astaxanthin via metabolic engineering. Prog. Lipid Res. 81, 101083 (2020).
Hu, I. C. Biofuels from Algae 345–358 (Elsevier, 2019).
Capelli, B., Bagchi, D. & Cysewski, G. R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 12, 145–152 (2013).
Jin, J. et al. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 11, 230 (2018).
Lin, Y. J. et al. Metabolic engineering a yeast to produce astaxanthin. Bioresour. Technol. 245, 899–905 (2017).
Madhana, S., Kanimozhi, G. & Panneerselvam, A. Advances in Probiotics 309–325 (Academic Press, 2021).
Kim, D. H. et al. Detection and enumeration of lactic acid bacteria, acetic acid bacteria and yeast in kefir grain and milk using quantitative real-time PCR. J. Food Saf. 35, 102–107 (2015).
Kim, D. H., Kim, H. & Seo, K. H. Microbial composition of Korean kefir and antimicrobial activity of Acetobacter fabarum DH1801. J. Food Saf. 40, e12728 (2020).
Lane, M. M. & Morrissey, J. P. Kluyveromyces marxianus: A yeast emerging from its sister’s shadow. Fungal Biol. Rev. 24, 17–26 (2010).
Liu, H. et al. Effect and mechanism of cholesterol-lowering by Kluyveromyces from Tibetan kefir. Adv. Mater. Res. 343-344, 1290–1298 (2011).
Romanin, D. E. et al. Probiotic yeast Kluyveromyces marxianus CIDCA 8154 shows anti-inflammatory and anti-oxidative stress properties in in vivo models. Benef. Microbes 7, 83–93 (2016).
Ju, Z. Y., Deng, D. F., Dominy, W. & Forster, I. Pigmentation of Pacific white shrimp, Litopenaeus vannamei, by dietary astaxanthin extracted from Haematococcus pluvialis. J World Aquac. Soc. 42, 633 (2011).
Zhang, J. et al. Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquac. Nutr. 19, 917–927 (2013).
Niu, J. et al. Effect of dietary Astaxanthin on growth, survival, and stress tolerance of postlarval shrimp Litopenaeus vannamei. J. World Aquac. Soc. 40, 795–802 (2009).
Wang, W. et al. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp Marsupenaeus japonicus. Aquaculture 491, 197–204 (2018).
Wade, N. et al. Dietary astaxanthin levels affect colour, growth, carotenoid digestibility and the accumulation of specific carotenoid esters in the Giant Tiger Shrimp Penaeus monodon. Aquac. Res. 48, 395–406 (2015).
Wang, H., Dai, A., Liu, F. & Guan, Y. Effects of dietary astaxanthin on the immune response, resistance to white spot syndrome virus and transcription of antioxidant enzyme genes in Pacific white shrimp Litopenaeus vannamei. IFRO 14, 699–718 (2015).
Chuchird, N., Rorkwiree, P. & Rairat, T. Effect of dietary formic acid and astaxanthin on the survival and growth of Pacific white shrimp (Litopenaeus vannamei) and their resistance to Vibrio parahaemolyticus. Springerplus 4, 440 (2015).
Liu, X., Wang, B., Li, Y., Wang, L. & Liu, J. Effects of dietary botanical and synthetic astaxanthin on E/Z and R/S isomer composition, growth performance, and antioxidant capacity of white shrimp, Litopenaeus vannamei, in the nursery phase. Invertebr. Surviv. J. 15, 131–140 (2018).
Nguyen, T. V., Alfaro, A., Arroyo, B. B., Leon, J. A. R. & Sonnenholzner, S. Metabolic responses of penaeid shrimp to acute hepatopancreatic necrosis disease caused by Vibrio parahaemolyticus. Aquaculture 533, 736174 (2021).
Ngo, H. V. T. et al. Effects of Phyllanthus amarus extract on nonspecific immune responses, growth, and resistance to Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish Shellfish Immun. 107, 1–8 (2020).
Pavlidis, M., Papandroulakis, N. & Divanach, P. A method for the comparison of chromaticity parameters in fish skin: Preliminary results for coloration pattern of red skin Sparidae. Aquaculture 258, 211–219 (2006).
Wade, N. M. et al. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 80, 64–74 (2019).
HernandezLopez, J., GollasGalvan, T. & VargasAlbores, F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp. Biochem. Phys. C 113, 61–66 (1996).
Huang, H. T. et al. Effects of Bidens alba and Plectranthus amboinicus dietary supplements on nonspecific immune responses, growth, and resistance to Vibrio alginolyticus in white leg shrimp (Penaeus vannamei). Aquaculture 546, 737306 (2022).
Abidin, Z. et al. Moringa oleifera leaves’ extract enhances nonspecific immune responses, resistance against Vibrio alginolyticus, and growth in whiteleg shrimp (Penaeus vannamei). Animals 12, 42 (2022).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 (2001).
Rodríguez, C. E. B. et al. The color of marine shrimps and its role in the aquaculture. Int. J. aquac. 3, 062–065 (2017).
Parisenti, J. et al. Effect of background color on shrimp pigmentation. Bol. Inst. Pesca. 37, 177–182 (2011).
Calvo, N. S. et al. Reflected-light influences the coloration of the peppermint shrimp, Lysmata boggessi (Decapoda: Caridea). J World Aquacult. Soc. 47, 701–711 (2016).
Segner, H., Arend, P., Von Poeppinghausen, K. & Schmidt, H. The effect of feeding astaxanthin to Oreochromis niloticus and Colisa labiosa on the histology of the liver. Aquaculture 79, 381–390 (1989).
Amar, E., Kiron, V., Satoh, S. & Watanabe, T. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 32, 162–173 (2002).
Mimoun-Benarroch, M., Hugot, C., Rhazi, L., Niamba, C. N. & Depeint, F. The bioavailability of astaxanthin is dependent on both the source and the isomeric variants of the molecule. Bull. Univ. Agric. Sci. Vet. Med. Cluj. Napoca. 73, 61–69 (2016).
Coral-Hinostroza, G. N., Ytrestøyl, T., Ruyter, B. & Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3’ R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 139, 99–110 (2004).
Díaz-Jiménez, L., Hernández-Vergara, M. P., Pérez-Rostro, C. I. & Ortega-Clemente, L. A. The effect of astaxanthin and β-carotene inclusion in diets for growth, reproduction and pigmentation of the peppermint shrimp Lysmata wurdemanni. Lat. Am. J. Aquat. Res. 47, 559–567 (2019).
Tapia-Salazar, M., Ricque-Marie, D., Nieto-López, M. & Cruz-Suárez, L. E. Uso de Pigmentos de Flor de Cempasúchil (Tagetes erecta) como Aditivos en Alimentos para Camarón L. vannamei. Avances en Nutrición Acuicola (2008).
Chien, Y. H. & Jeng, S. C. Pigmentation of kuruma prawn, Penaeus japonicus Bate, by various pigment sources and levels and feeding regimes. Aquaculture 102, 333–346 (1992).
Itami, T. et al. Enhancement of disease resistance of kuruma shrimp, Penaeus japonicus, after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture 164, 277–288 (1998).
Dewi, N. R. et al. Guava (Psidium guajava) leaf extract enhances immunity, growth, and resistance against Vibrio parahaemolyticus in white shrimp Penaeus vannamei. Fish Shellfish Immunol. 118, 1–10 (2021).
Amar, E., Kiron, V., Satoh, S. & Watanabe, T. Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish shellfish Immunol. 16, 527–537 (2004).
Söderhäll, K. & Cerenius, L. Crustacean immunity. Annu. Rev. Fish Dis. 2, 3–23 (1992).
Söderhäll, K., Häll, L., Unestam, T. & Nyhlén, L. Attachment of phenoloxidase to fungal cell walls in arthropod immunity. J. Invertebr. Pathol. 34, 285–294 (1979).
Leonard, C., Ratcliffe, N. A. & Rowley, A. F. The role of prophenoloxidase activation in non-self recognition and phagocytosis by insect blood cells. J. Insect Physiol. 31, 789–799 (1985).
Holmblad, T. & Söderhäll, K. Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture 172, 111–123 (1999).
Yowaphui, N., Rairat, T. & Chuchird, N. Effect of formic acid, β-carotene and vitamin E on growth, survival and prevention to Vibrio parahaemolyticus in rearing of pacific white shrimp (Litopenaeus vannamei). J. Fish. Environ. 40, 1–14 (2016).
Niu, J. et al. Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture 422, 8–17 (2014).
Fawzy, S. et al. Can dietary β-carotene supplementation provide an alternative to astaxanthin on the performance of growth, pigmentation, biochemical, and immuno-physiological parameters of Litopenaeus vannamei?. Aquac. Res. 23, 101054 (2022).
Chiu, C. H., Guu, Y. K., Liu, C. H., Pan, T. M. & Cheng, W. Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol. 23, 364–377 (2007).
Wu, Y. S. et al. Effects of traditional medical herbs “minor bupleurum decoction” on the non-specific immune responses of white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 64, 218–225 (2017).
Chen, Y. Y. et al. Shrimp that have received carrageenan via immersion and diet exhibit immunocompetence in phagocytosis despite a post-plateau in immune parameters. Fish Shellfish Immunol. 36, 352–366 (2014).
Nordberg, J. & Arnér, E. S. J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system1. Free Radic. Biol. Med. 31, 1287–1312 (2001).
Tassanakajon, A., Amparyup, P., Somboonwiwat, K. & Supungul, P. Cationic antimicrobial peptides in penaeid shrimp. Mar. Biotechnol. 13, 639–657 (2011).
O’Leary, N. A. & Gross, P. S. Genomic structure and transcriptional regulation of the penaeidin gene family from Litopenaeus vannamei. Gene 371, 75–83 (2006).
Somboonwiwat, K. et al. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 29, 841–851 (2005).
Wu, T. et al. What is new in lysozyme research and its application in food industry? A review. Food Chem. 274, 698–709 (2019).
Simser, J. A., Macaluso, K. R., Mulenga, A. & Azad, A. F. Immune-responsive lysozymes from hemocytes of the American dog tick, Dermacentor variabilis and an embryonic cell line of the Rocky Mountain wood tick D. andersoni. Insect Biochem. Mol. Biol. 34, 1235–1246 (2004).
Liu, L. et al. Dietary supplementation of astaxanthin is superior to its combination with Lactococcus lactis in improving the growth performance, antioxidant capacity, immunity and disease resistance of white shrimp (Litopenaeus vannamei). Aquac. Rep. 24, 101124 (2022).
Acknowledgements
We thank Animal Aquatic Center, National Taiwan Ocean University, for preparing the shrimp which was used in this experiment. This study was supported financially by the Council of Agriculture [111AS-6.1.6-FA-F1], China Medical University [CMU110-MF-97], National Science and Technology Council [MOST 110-2221-E-039-002-MY3, MOST 110-2313-B-005-003 and MOST 111-2313-B-005-037].
Author information
Authors and Affiliations
Contributions
Writing original draft by Y.J.L. Methodology by Y.J.L., J.J.C., H.T.H. and C.P.L; Formal analysis by Y.J.L., H.T.H., C.P.L., Y.F.H. and M.L.W.; Investigation by C.Y.H.; Supervision by F.H.N. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, YJ., Chang, JJ., Huang, HT. et al. Improving red-color performance, immune response and resistance to Vibrio parahaemolyticus on white shrimp Penaeus vannamei by an engineered astaxanthin yeast. Sci Rep 13, 2248 (2023). https://doi.org/10.1038/s41598-023-29225-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29225-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.