Abstract
The use of aromatase inhibitors (AIs) is associated with higher rates of cardiovascular events and lower endothelial function in breast cancer survivors. Psychosocial stress is associated with higher levels of inflammatory and aging markers, and lower endothelial function in otherwise healthy subjects. These associations among breast cancer survivors on AIs are not well defined. A cross-sectional study of 30 breast cancer survivors on AIs was performed to assess the associations between self-reported scores of psychosocial measures of depression, anxiety, and stress assessed by validated questionnaires with markers of inflammation (CRP; IL-6; IL-18), aging (p16INK4a), and endothelial function (ICAM-1, EndoPAT ratio). Significant positive correlations were observed between psychosocial measures and inflammatory markers including CRP, IL-6, and ICAM-1. However, no psychosocial scores were related to endothelial function or gene expression of the aging biomarker p16INK4a. Overall, survivors had endothelial dysfunction with reduced EndoPAT ratios. Psychosocial stress is associated with greater inflammation in breast cancer survivors on AIs, corroborating previous studies in cancer-free populations. The lack of association between psychosocial stress and either endothelial function or aging biomarkers could be due to the already low endothelial function and accelerated aging in our cohort of breast cancer survivors on AIs, though our small sample size limits conclusions. Further work in a larger and more diverse cohort of patients is needed to further understand the relationships among inflammation, aging and endothelial function in breast cancer survivors.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the world's leading cause of cancer mortality in women1. Approximately 1 in 8 women in the U.S. will be diagnosed with breast cancer in their lifetime. Although breast cancer is a leading cause of cancer mortality worldwide, the 5- and 10-year survival rates are 90% and 84%, respectively2. This is due in large part to early detection and improved treatment methods, including extended treatment with aromatase inhibitors (AIs), the primary adjuvant endocrine therapy for breast cancer survivors with estrogen positive tumors3. Although AIs reduce the risk of breast cancer recurrence, extended use of AIs is associated with increased risk of cardiovascular disease events. Aromatase catalyzes the final step in the biosynthesis of estrogen and reduced endogenous estrogen levels leading to a loss of estrogen-mediated protection from endothelial dysfunction and cardiovascular disease in postmenopausal women4,5. The vascular protective effects of estrogen are mediated via enhanced nitric oxide (NO) signaling6 and anti-inflammatory effects7, among others.Recent evidence suggests adverse changes in vascular function begin to occur almost as soon as AI treatment begins8.
Receiving a breast cancer diagnosis, subsequent treatments and symptom management can be a stressful and life-changing experience9. Indeed, the psychosocial impacts of breast cancer are well recognized, with higher rates of depression and anxiety observed among breast cancer survivors compared to the general female population, even 5 years post-diagnosis10,11. Depression and other indicators of both acute and chronic stress are associated with increased levels of circulating inflammatory and aging markers as well as changes in endothelial function in otherwise healthy subjects12,13,14. However, these associations among breast cancers survivors on extended AI treatments are not well understood.
To address this gap in the literature, the current study evaluated cross-sectional associations among three indicators of psychosocial stress (depression, anxiety, and perceived stress), markers of inflammation and aging, and endothelial function among a cohort of 31 breast cancer survivors on AI recruited to participate in a pilot randomized control trial (RCT) of mindfulness based stress reduction. Analyses reported herein were limited to the baseline data obtained as part of that RCT, prior to the intervention.
Methods
Subjects
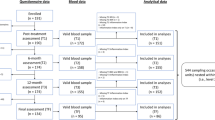
A cohort of 30 breast cancer survivors on AIs were recruited to participate in a pilot randomized control trial (RCT) of mindfulness-based stress reduction. Individuals with a diagnosis of hormone receptor positive HER2 negative breast cancer who were prescribed an aromatase inhibitor at the University of Minnesota Masonic Cancer Center were eligible. All cases had completed breast cancer treatment and were actively taking an AI. Subjects with a history of tobacco use, myocardial infarction, congestive heart failure, or cardiac catheterization requiring intervention were excluded. Medical record abstraction for cases confirmed diagnosis, stage at diagnosis, use of chemotherapy, use of radiation, and personal medical history including medications (including type of prescribed aromatase inhibitor). Participants completed surveys, laboratory and vascular testing. The protocol was approved by the University of Minnesota Institutional Review Board and the Masonic Cancer Center Review Committee. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent according to the Declaration of Helsinki.
Study recruitment
Potential cases were identified by their treating physician and were then invited to participate in the study between June and August 2020. For those who expressed interest, a screening phone call was placed to discuss the study and identify participants. Failure to answer the phone call or return the phone call was identified as passive refusal to participate in the study. Consented subjects were invited to undergo vascular assessment and biomarker assessment at baseline and completion of the study. Those consented subjects who completed the protocol received one $25 gift card. In total, 43 individuals were screened for the study, 33 met eligibility and enrolled, 30 had evaluable data. Most participants were diagnosed with stage 1 (n = 12; 40.0%) or stage 2 (n = 15; 50.0%) breast cancer, with just 3 participants (10.0%) diagnosed with stage 3. Among the study participants, 13 were on letrozole, 11 on anastrazole, and 6 on exemestane. Median time on AI was 33 months (range 12–76).
Vascular assessment
Vascular assessment was performed using the EndoPAT 2000 as previously described5,15,16. EndoPAT measures the dilatory response through pulsatile volume changes at the fingertips, known as Peripheral Artery Tone (PAT). EndoPAT technology is a non-invasive FDA-approved testing device for the diagnosis of endothelial dysfunction. The EndoPAT examination required the patient to be supine, at rest, in a quiet, air-conditioned room. Subjects were fasting at least 8 h before the study. Subjects were asked not to exercise, use caffeine, high-fat foods, or vitamin C for at least 4–6 h before the test. Low EndoPAT ratios (< 1.6) are indicative of worsening endothelial function and increased CV risk5.
All study assessments were conducted in a specifically designed research space for clinical vascular assessments. Blood collection and vascular functional measurements were conducted in the morning by a trained technician. All testing was completed by the same technician to reduce inter-technician variability. The EndoPAT assessments were performed in triplicate, and the mean (average of 3 values for each participant) indices were used in statistical analyses.
Biomarker assessment
Biomarkers were obtained using a fasting blood draw to evaluate the following inflammatory markers: C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-8 (IL-18). Prior research has shown that CRP, IL-6 and IL-18 are three key inflammatory biomarkers associated with inflammation in breast cancer survivors17,18. ICAM-1 is a cell surface glycoprotein expressed at low basal level in endothelial, epithelial, and immune cells, but is upregulated in response to inflammation19. ICAM-1 has been reliably used as a biomarker for endothelial dysfunction20. Cellular senescence was assessed by measuring the expression of CDKN2A, the gene encoding p16INK4a in CD3 + peripheral blood mononuclear cells (PBMCs) as a surrogate biomarker for human aging21,22. The Translational Therapy Laboratory (TTL) purified CD3 + PBMCs from bulk PBMCs isolates from whole blood samples. Total RNA was isolated from patient CD3 + PBMCs and CDKN2A/p16INK4a and 18S housekeeping gene expressions were measured by real-time quantitative polymerase chain reaction by TTL staff. Blood samples were collected first, prior to vascular functional assessments. All blood samples were appropriately processed and aliquoted for measurement of respective biomarkers.
Psychosocial measures
The following questionnaires were completed by participants at baseline: The 4-item CDC Healthy Days measure evaluating self-reported health and quality of life23, PHQ-9 Depression Scale24, Perceived Stress Scale25, and Generalized Anxiety Disorder26. The 4-item CDC Healthy Days measure assesses quality of life by asking about self-reported health (1 item; response options ranging from poor to excellent) and the number of days in the past month that physical health was poor, mental health was poor, and daily activities were impacted by either poor physical or mental health (3 items)23. The self-administered Patient Health Questionnaire (PHQ)-9 is a validated clinical and research tool to measure depression symptoms and severity24. Item responses range from “0” (not at all) to “3” (nearly every day) for a combined scoring of 0–27(scores of 5–9 are classified as mild depression; 10–14 as moderate depression; 15–19 as moderately severe depression; ≥ 20 as severe depression. The Perceived Stress Scale (PSS) measures the degree to which situations in one’s life are appraised as stressful25. The 10 item PSS is suggested for examining the role of nonspecific appraised stress and as an outcome measure of experienced levels of stress, with scores ranging from “0” (never) to “4” (very often). Scores ranging from 0–13, 14–26, 27–40 would be considered low, moderate, or high perceived stress, respectively. The GAD-7 is a clinical measure for assessing generalized anxiety disorder (GAD). The questionnaire consists of seven items that are summed to generate a global score26; cut points of 5, 10, and 15 can be interpreted as representing mild, moderate, and severe levels of anxiety.
Statistical methods
Analyses reported were limited to the baseline data obtained as part of the RCT, prior to the intervention, and thus represent a cross-sectional evaluation. Descriptive statistics were calculated across all subjects as mean (SD) for continuous variables and count (percentage) for categorical variables. Continuous variables that were highly skewed were log10 transformed before any statistical modeling was performed. One negative EndoPAT measurement was coded as NA and omitted from all analyses. Outcome measurements were assumed to be missing completely at random. All regression models used a complete-case analysis and subjects with a missing value for either the independent or dependent variable were removed. We estimated the mean increase in each inflammatory or senescence marker associated with a one point increase in each psychosocial score (GAD, PSS, and PHQ) through three independent simple regression models for each inflammatory or senescence marker. We reported point estimates of the associated average increase along with 95% confidence intervals. All significance testing was performed at the 5% level. No adjustments for multiple comparisons were made. All analyses were conducted using R v3.6.1 (R Core Team, 2019).
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Patient characteristics
The demographic characteristics of study participants (n = 30) are listed in Table 1. Of the participants, 46.7% (n = 14) had received both chemotherapy and radiation treatments, 33.3% (n = 10) had received only radiation treatment, 13.3% (n = 4) had received only chemotherapy treatment, and 6.7% (n = 2) had received neither chemotherapy nor radiation treatment. The average age of the subjects was 59.0 (SD = 10.5) years. Of the participants, 96.8% (n = 30) were white, and 93.5% (n = 29) had completed either college or graduate/professional training. All participants were postmenopausal women with estrogen positive breast cancer. They had a baseline cancer stage 1 (n = 12), stage 2 (n = 15), or stage 3 (n = 3).
Average EndoPAT ratio, psychosocial stress measure scores, inflammatory biomarker concentrations, and senescence biomarker concentrations for the study population as a whole are displayed in Table 2. The EndoPAT ratio (mean of 0.60) was low in all participants where low EndoPAT ratios (< 1.6) were previously associated with worsening endothelial function and increased CV risk5. Mean scores from the PHQ, GAD, and PSS assessments indicate that subjects had low levels of depression, anxiety, and stress. In our sample, we calculated Cronbach’s alpha values of 0.766 for the PHQ-9, 0.823 for the GAD-7, and 0.841 for the PSS. Values for all measures could not be obtained from all study participants and the number of subjects missing any one of these values is also shown in Table 2.
Associations between psychosocial stress scores and inflammatory marker concentrations are shown in Fig. 1. No subjects met the criteria for acute inflammation/infection. The highest CRP was < 10 mg/L. Higher GAD (β^ = 0.31 (0.00, 0.63); p = 0.05) and PHQ scores (β^ = 0.42 (0.17, 0.67); p < 0.01) were significantly and positively correlated with higher CRP levels as shown in Fig. 1A and B. A pattern of positive association between higher PSS scores and CRP levels was also noted, but the association was not statistically significant (β^ = 0.12 (− 0.02, 0.25); p = 0.09). Higher PHQ scores were significantly (p < 0.05) associated with increased IL-6 concentrations (β^ = 0.05 (0.01, 0.09); p = 0.02) (Fig. 1E). While a trend of positive association was observed between higher GAD scores (Fig. 1D; (β^ = 0.03 (− 0.02, 0.08); p = 0.21)) and PSS scores (Fig. 1F; (β^ = 0.01 (− 0.01, 0.03); p = 0.31)) and higher levels of IL-6, neither of these associations were statistically significant. There was no significant association between psychosocial stress scores and IL-18 levels (Supplemental Table 1).
Associations between psychosocial stress scores and endothelial dysfunction are shown in Fig. 2. Surface expression of ICAM-1, an intercellular adhesion molecule, is increased in states of inflammation and endothelial dysfunction and it can serve as a risk marker for future coronary events27. There were significant positive associations between ICAM-1 with higher GAD (β^ = 0.02 (0.00, 0.03); p = 0.01), PHQ (β^ = 0.02 (0.00, 0.03); p = 0.01), and PSS (β^ = 0.01 (0.00, 0.01); p = 0.05) scores as seen in Fig. 2A–C. However, actual measurements of endothelial dysfunction using EndoPAT did not translate into significant associations between EndoPAT ratios and higher psychosocial stress scores (Fig. 2D–F).
Activation of the CDKN2A gene by tissue damage or cellular stress induces the expression of p16, a common biomarker of cellular senescence21. The Ct (cycle threshold), as shown in Fig. 3, is the number of amplification cycles required to cross a fixed background level of fluorescence upon measuring gene expression by real-time PCR. The Ct values are inversely proportional to the gene expression (i.e. higher gene expression leads to lower Ct values). The Ct values reported in the figure were the measure of change in the count of CDKN2A normalized to those of 18S as a housekeeping gene. The associations between psychosocial stress scores and change in Ct were not statistically significant, indicating psychosocial stress scores were not associated with CDKN2A gene expression.
Discussion
Our findings in breast cancer patients on AIs demonstrate a significant positive correlation between psychosocial stress scores and several inflammatory markers, corroborating previous studies in cancer-free populations. Nevertheless, in contrast to studies in otherwise healthy subjects, there was no significant association between scores of perceived stress, depression, or anxiety and either endothelial function or aging biomarkers. In this cohort of breast cancer survivors on aromatase inhibitors, endothelial dysfunction was present, consistent with previous reports28.
Survivorship of breast cancer patients has remarkably improved over the past three decades, thanks to advancements in diagnostics, care models, and therapeutics including aromatase inhibitors (AIs). AIs are the standard treatment for postmenopausal patients with hormone receptor-positive breast cancer as both adjuvant and metastatic treatments29. It is estimated that 5 years of an AI compared with no endocrine therapy would reduce breast cancer recurrence by about two-thirds during treatment and by about one-third during years 5–930. Despite their superior efficacy over tamoxifen for hormone-sensitive breast cancer31, aromatase inhibitors may increase the risk of cardiovascular diseases, including hypertension, dyslipidemia, and ischemic heart disease32,33.
While the prognosis for breast cancer is generally positive, breast cancer survivors are affected by tremendous physical, functional, financial, emotional, and social challenges9, which creates significant psychosocial stress. The prevalence of depression is highest during the first year after a breast cancer diagnosis, and according to a systematic review, the frequency of depressive symptoms ranges from 9.4 to 66.1%10. Other studies have found a 42% rate of psychiatric disorders in recently treated breast cancer survivors and 35.7% of these women had depression, anxiety, or both11. Additional systematic reviews have suggested a higher prevalence of depression and anxiety among breast cancer survivors when compared to the general female population, even more than 5 years after diagnosis10. Nonetheless, we note that our patient sample had relatively low levels of depressive symptoms, anxiety and perceived stress at the time of their study enrollment. Our participants were, on average on AIs for 33 months suggesting they may have learned to cope well with their diagnosis and concomitant treatment. Additionally, psychosocial stress may be low in these women given that most of them had been diagnosed with early stage breast cancer, with excellent prognoses. Regardless, we did observe positive associations between psychosocial measures and most of our inflammatory biomarkers, suggesting a clear relationship between inflammation and psychosocial functioning.
Side effects of AIs such as vasomotor complaints, vaginal dryness, and diminished libido may also add to the higher stress levels in breast cancer survivors9,34. Breast cancer survivors taking AIs are more likely to report sexual problems than those taking tamoxifen35 or not having any endocrine therapy36.
Inflammatory pathways contribute to the pathophysiology of both cancer and cardiovascular disease37. Several studies have previously examined inflammatory markers as predictors of disease recurrence and death in cancer populations. Cancer survivors with persistent inflammation may have an elevated risk of recurrence as a result of the effects of inflammatory processes38. Three key inflammatory biomarkers associated with inflammation in breast cancer survivors are CRP, IL-6, and IL-18. CRP is a nonspecific protein produced by the liver in response to inflammation, infection, and tissue damage. CRP concentrations in women diagnosed with breast cancer are positively associated with death due to any cause, death due to breast cancer, and additional breast cancer events38. The association between IL-6 elevation and increased risk of cancer recurrence is less clear and may only apply to certain breast cancer subtypes such as HER2- tumors39. Studies have shown that breast cancer survivors with elevated IL-18 serum levels have shorter relapse-free survival durations than those with lower IL-18 levels40.
Higher levels of inflammatory cytokines were reported in the blood of breast cancer survivors by several groups38,41,42,43. Inflammatory cytokines increase to a varying degree following radiation44 and chemotherapy41,45. Significantly higher IL-6 among depressed cancer patients compared to healthy subjects, and higher levels of inflammatory markers were associated with fatigue and sleep disturbance41,44,46. Similarly, higher levels of IL-6 are associated with frailty in older breast cancer survivors47. Several studies have also reported the correlation between inflammatory mediators and measures of psychosocial stress in breast cancer survivors. The inflammatory mediators IL-6 and TNF-alpha were positively correlated with social anxiety and depressive symptoms, respectively in two studies of breast cancer survivors48,49. In breast cancer survivors one year post-radiotherapy, both depressive symptoms and higher inflammatory markers were independent risk factors of fatigue; however, the correlation between depressive symptoms and inflammatory markers was not statistically significant49,50. CRP was positively associated with depressive symptoms only among women who reported high levels of cancer-related stress in a longitudinal study of women with breast cancer51. Inflammation was positively correlated with depressive symptoms only in women who also reported high level of anxiety, perceived stress, negative affect, or sleep disturbance52. Counterintuitively, IL-6 was found to negatively correlate with anxiety symptoms in one study49. In agreement with the majority of these studies, in our cohort of breast cancer survivors on AIs, depressive symptoms were significantly and positively correlated with CRP and IL-6 with similar, albeit non-significant patterns of associations noted for anxiety and perceived stress. In contrast, IL-18 was not correlated to psychosocial measures in our study.
Several lines of evidence suggest that the associations between psychosocial stress and inflammation exist in cancer-free subjects as well12. Chronic psychosocial stress may induce heightened inflammatory activity through the hypothalamic–pituitary–adrenal axis (HPA) and the autonomic nervous system53. Psychosocial stress has been associated with higher circulating cytokines levels including TNF-alpha and IL-653. In women, high levels of job stress and burnout were associated with high levels of TNF-alpha13 and CRP14. A strong inverse correlation was found between socioeconomic status and levels of circulating CRP, TNF-alpha and IL-654,55. Loneliness was also associated with higher IL-656. In addition to the observational studies that show a strong association between psychosocial stress and inflammation, experimental studies demonstrate a bidirectional cause-effect relationship between stress and inflammation. Intriguingly, acute laboratory stress paradigms show that stress can cause an inflammatory response57,58, and inflammation can also provoke depressive symptoms59,60.
Aromatase inhibitors and ionizing radiation have been linked with reduced endothelial function in cancer survivors5,61. Endothelial dysfunction, which is an independent risk factor for cardiovascular events in the Framingham risk score, precedes and potentially mediates the development of several cardiovascular diseases15,62. Reactive hyperemia measured non-invasively by EndoPat is a consistent surrogate for endothelial function in clinical studies15. Using the EndoPat technique, we have previously demonstrated reduced endothelial function in postmenopausal breast cancer survivors on an AI in comparison to healthy postmenopausal women5. In the current study, the average EndoPat value in BC survivors on AIs was 0.6 similar to the value reported in our previous study5. The association between psychosocial stress and endothelial dysfunction has not been reported in breast cancer survivors. In the current small cohort of BC survivors on an AI, there was no significant correlation between parameters of psychosocial stress and EndoPat values.
Psychosocial stress is associated with impaired endothelial function in several populations, including young adults with major depressive disorder63. Mental stress induces endothelial dysfunction in healthy subjects64. The lack of association between psychosocial stress and low EndoPat values in the current study may be due to the already low values in a cohort of breast cancer survivors on AIs and/or the small sample size of our study. ICAM-1 is a biomarker for endothelial dysfunction that belongs to a family of adhesion molecules, which are mediators of cellular adhesion between endothelial cells and leukocytes20. ICAM-1 is an independent risk factor for atherosclerosis and developing coronary heart disease65. Higher chronic stress was associated with lower flow-mediated dilation and higher ICAM-166 and higher ICAM-1 was significantly higher in low socioeconomic status subjects67. In the current study, we observed a significant correlation between parameters of psychosocial stress and ICAM-1 in breast cancer survivors on AIs. The positive correlation between stress and ICAM-1 may be predictive of a later-onset decline in endothelial function if the stress continues.
Cancer survivors demonstrate clinical signs of premature aging and frailty68. In addition to the clinical evidence of premature aging, cancer survivors demonstrate high levels of aging biomarkers, such as p16INK4a a marker of cellular senescence that is encoded by the CDKN2a gene69. In breast cancer patients, chemotherapy significantly increased the expression of p16INK4a, comparable with 10–17 years increase of chronological age70,71. Anthracycline-based chemotherapy was associated with the largest increase in p16INK4a expression, comparable with 23–26 years of chronological age71. Intriguingly, aging-related functional limitations were reached 5–10 years earlier in breast cancer survivors than cancer-free women72. In the general population, chronic psychosocial stress was associated with increased expression of p16INK4a73. Nevertheless, the association between psychosocial stress and biomarkers of aging has not been specifically reported in breast cancer patients. In our cohort of breast cancer survivors on AIs, there was no significant correlation between psychosocial stress and p16INK4a.
The current study has a number of limitations that warrant discussion. First, the study lacks a comparison group of same-aged women without breast cancer. Therefore, we cannot infer whether the observed associations or the lack thereof is specific to breast cancer survivors on AIs or generalizable to an age-matched cancer-free population. Second, the small sample size may have hindered our ability to detect statistically significant correlations between multiples variables. Finally, our sample was predominantly white, well-educated women; thus, generalizability to other demographic groups could be limited.
In conclusion, our findings in breast cancer patients on AIs demonstrate a significant positive correlation between psychosocial stress scores and several inflammatory markers, corroborating previous studies in cancer-free populations. Nevertheless, in contrast to studies in otherwise healthy subjects, there was no significant association between psychosocial stress cores and either endothelial function or aging biomarkers. This observed lack of association may be due to the already low endothelial function and accelerated aging in our cohort of breast cancer survivors on AIs, or due to the small number of patients included in this study. Further work in a larger cohort of patients is needed to confirm or refute this lack of association.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Chiriac, V. F., Baban, A. & Dumitrascu, D. L. Psychological stress and breast cancer incidence: A systematic review. Clujul. Med. 91(1), 18–26 (2018).
Gradishar, W. J. et al. NCCN guidelines insights: Breast cancer, version 1.2017. J. Natl. Compr. Cancer Netw. 15(4), 433–451 (2017).
Dai, X., Xiang, L., Li, T. & Bai, Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J. Cancer 7(10), 1281–1294 (2016).
Sasaki, Y. et al. Estrogen plays a crucial role in Rab9-dependent mitochondrial autophagy, delaying arterial senescence. J. Am. Heart Assoc. 10(7), e019310 (2021).
Blaes, A. et al. Vascular function in breast cancer survivors on aromatase inhibitors: A pilot study. Breast Cancer Res. Treat. 166(2), 541–547 (2017).
Duckles, S. P. & Miller, V. M. Hormonal modulation of endothelial NO production. Pflugers Arch. 459(6), 841–851 (2010).
Straub, R. H. The complex role of estrogens in inflammation. Endocr. Rev. 28(5), 521–574 (2007).
Goldvaser, H. et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 110(1), 31–39 (2018).
Fallowfield, L. & Jenkins, V. Psychosocial/survivorship issues in breast cancer: Are we doing better?. J. Natl. Cancer Inst. 107(1), 335 (2015).
Maass, S. W., Roorda, C., Berendsen, A. J., Verhaak, P. F. & de Bock, G. H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 82(1), 100–108 (2015).
Tsaras, K. et al. Assessment of depression and anxiety in breast cancer patients: Prevalence and associated factors. Asian Pac. J. Cancer Prev. 19(6), 1661–1669 (2018).
Knight, E. L. et al. Perceived stress is linked to heightened biomarkers of inflammation via diurnal cortisol in a national sample of adults. Brain Behav. Immun. 93, 206–213 (2021).
Grossi, G., Perski, A., Evengard, B., Blomkvist, V. & Orth-Gomer, K. Physiological correlates of burnout among women. J. Psychosom. Res. 55(4), 309–316 (2003).
Toker, S., Shirom, A., Shapira, I., Berliner, S. & Melamed, S. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol. 10(4), 344–362 (2005).
Rubinshtein, R. et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 31(9), 1142–1148 (2010).
Blaes, A. H. et al. Arterial elasticity as a risk factor for early cardiovascular disease among testicular cancer survivors treated with platinum-based chemotherapy: A cross-sectional pilot study. Vasc. Health Risk Manag. 14, 205–211 (2018).
Zhou, Y., Jia, N., Ding, M. & Yuan, K. Effects of exercise on inflammatory factors and IGF system in breast cancer survivors: A meta-analysis. BMC Womens Health 22(1), 507 (2022).
Cozzolino, M. et al. A psychosocial genomics pilot study in oncology for verifying clinical, inflammatory and psychological effects of mind-body transformations-therapy (MBT-T) in breast cancer patients: Preliminary results. J. Clin. Med. 10(1), 136 (2021).
Hubbard, A. K. & Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 28(9), 1379–1386 (2000).
Mosevoll, K. A. et al. Cytokines, adhesion molecules, and matrix metalloproteases as predisposing, diagnostic, and prognostic factors in venous thrombosis. Front. Med. 5, 147 (2018).
Rayess, H., Wang, M. B. & Srivatsan, E. S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 130(8), 1715–1725 (2012).
Tuttle, C. S. L., Luesken, S. W. M., Waaijer, M. E. C. & Maier, A. B. Senescence in tissue samples of humans with age-related diseases: A systematic review. Ageing Res. Rev. 68, 101334 (2021).
CDC. Measuring Healthy Days: Population Assessment of Health-Related Quality of Life (CDC, 2000).
Kroenke, K., Spitzer, R. L. & Williams, J. B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 16(9), 606–613 (2001).
Cohen, S., Kamarck, T. & Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 24(4), 385–396 (1983).
Spitzer, R. L., Kroenke, K., Williams, J. B. & Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166(10), 1092–1097 (2006).
Luc, G. et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis 170(1), 169–176 (2003).
Blaes, A. et al. Vascular function in breast cancer survivors on aromatase inhibitors: A pilot study. Breast Cancer Res. Treat. 166, 541–547 (2017).
Chumsri, S., Howes, T., Bao, T., Sabnis, G. & Brodie, A. Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 125(1–2), 13–22 (2011).
Early Breast Cancer Trialists’ Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 386(10001), 1341–1352 (2015).
Cuzick, J. et al. investigators AL: Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 11(12), 1135–1141 (2010).
Chapman, J. A. et al. Competing risks of death in women treated with adjuvant aromatase inhibitors for early breast cancer on NCIC CTG MA27. Breast Cancer Res. Treat. 156(2), 343–349 (2016).
Khosrow-Khavar, F. et al. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 28(3), 487–496 (2017).
Cella, D. et al. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res. Treat. 100(3), 273–284 (2006).
Panjari, M., Bell, R. J. & Davis, S. R. Sexual function after breast cancer. J. Sex Med. 8(1), 294–302 (2011).
Perez, M. & Jeffe, D. B. A letter to the editor regarding the article by Panjari et al. J. Sex Med. 8(4), 1254 (2011).
Libby, P. & Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc. Res. 115(5), 824–829 (2019).
Villasenor, A. et al. Postdiagnosis C-reactive protein and breast cancer survivorship: Findings from the WHEL study. Cancer Epidemiol. Biomark. Prev. 23(1), 189–199 (2014).
Sparano, J. A. et al. Inflammatory cytokines and distant recurrence in HER2-negative early breast cancer. NPJ Breast Cancer 8(1), 16 (2022).
Inoue, N. et al. High serum levels of interleukin-18 are associated with worse outcomes in patients with breast cancer. Anticancer Res. 39(9), 5009–5018 (2019).
Liu, L. et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav. Immun. 26(5), 706–713 (2012).
Mills, P. J. et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav. Immun. 22(1), 98–104 (2008).
van der Willik, K. D. et al. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: A cohort study. Breast Cancer Res. 20(1), 135 (2018).
De Sanctis, V. et al. Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy. Biomed. Res. Int. 2014, 523568 (2014).
Paz, M. et al. Assessment of chemotherapy on various biochemical markers in breast cancer patients. J. Cell Biochem. 119(3), 2923–2928 (2018).
Bower, J. E. et al. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism?. J. Clin. Oncol. 29(26), 3517–3522 (2011).
Brouwers, B. et al. Biological ageing and frailty markers in breast cancer patients. Aging 7(5), 319–333 (2015).
Leschak, C. J. et al. Associations between psychosocial factors and circulating cytokines in breast cancer survivors. Psychol. Health 1, 1–15 (2021).
Perez-Tejada, J. et al. Distress, proinflammatory cytokines and self-esteem as predictors of quality of life in breast cancer survivors. Physiol. Behav. 230, 113297 (2021).
Xiao, C. et al. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol. Med. 47(10), 1733–1743 (2017).
Manigault, A. W. et al. Psychosocial resilience to inflammation-associated depression: A prospective study of breast-cancer survivors. Psychol. Sci. 33(8), 1328–1339 (2022).
Manigault, A. W. et al. Moderators of inflammation-related depression: A prospective study of breast cancer survivors. Transl. Psychiatry 11(1), 615 (2021).
Hansel, A., Hong, S., Camara, R. J. & von Kanel, R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 35(1), 115–121 (2010).
Nazmi, A. & Victora, C. G. Socioeconomic and racial/ethnic differentials of C-reactive protein levels: A systematic review of population-based studies. BMC Public Health 7, 212 (2007).
Koster, A. et al. Association of inflammatory markers with socioeconomic status. J. Gerontol. A 61(3), 284–290 (2006).
Loucks, E. B. et al. Social networks and inflammatory markers in the Framingham Heart Study. J. Biosoc. Sci. 38(6), 835–842 (2006).
Boyle, C. C., Stanton, A. L., Eisenberger, N. I., Seeman, T. E. & Bower, J. E. Effects of stress-induced inflammation on reward processing in healthy young women. Brain Behav. Immun. 83, 126–134 (2020).
Bobel, T. S. et al. Less immune activation following social stress in rural vs urban participants raised with regular or no animal contact, respectively. Proc. Natl. Acad. Sci. USA 115(20), 5259–5264 (2018).
O’Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14(5), 511–522 (2009).
O’Connor, J. C. et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J. Neurosci. 29(13), 4200–4209 (2009).
Baselet, B., Sonveaux, P., Baatout, S. & Aerts, A. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell. Mol. Life Sci. 76(4), 699–728 (2019).
Matsuzawa, Y. et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J. Am. Heart Assoc. 2(6), e000426 (2013).
Greaney, J. L., Koffer, R. E., Saunders, E. F. H., Almeida, D. M. & Alexander, L. M. Self-reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J. Am. Heart Assoc. 8(4), e010825 (2019).
Spieker, L. E. et al. Mental stress induces prolonged endothelial dysfunction via endothelin: A receptors. Circulation 105(24), 2817–2820 (2002).
Hwang, S. J. et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The atherosclerosis risk in communities (ARIC) study. Circulation 96(12), 4219–4225 (1997).
Kershaw, K. N., Lane-Cordova, A. D., Carnethon, M. R., Tindle, H. A. & Liu, K. Chronic stress and endothelial dysfunction: The multi-ethnic study of atherosclerosis (MESA). Am. J. Hypertens. 30(1), 75–80 (2017).
Hong, S., Nelesen, R. A., Krohn, P. L., Mills, P. J. & Dimsdale, J. E. The association of social status and blood pressure with markers of vascular inflammation. Psychosom. Med. 68(4), 517–523 (2006).
Blair, C. K. et al. Effects of cancer history on functional age and mortality. Cancer 125(23), 4303–4309 (2019).
Abdelgawad, I. Y. et al. Molecular mechanisms and cardiovascular implications of cancer therapy-induced senescence. Pharmacol. Ther. 221, 107751 (2021).
Sanoff, H. K. et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl. Cancer Inst. 106(4), 057 (2014).
Shachar, S. S. et al. Effects of breast cancer adjuvant chemotherapy regimens on expression of the aging biomarker, p16(INK4a). JNCI Cancer Spectr. 4(6), 082 (2020).
Zhu, J. et al. Accelerated aging in breast cancer survivors and its association with mortality and cancer recurrence. Breast Cancer Res. Treat. 180(2), 449–459 (2020).
Rentscher, K. E. et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinology 102, 139–148 (2019).
Funding
Funding was provided by Masonic Cancer Center, University of Minnesota.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr. A.H.B., J.W., and Dr. S.E.-R. and Dr. B.Z. The first draft of the manuscript was written by C.N. and Dr. A.H.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blaes, A.H., Nair, C., Everson-Rose, S. et al. Psychological measures of stress and biomarkers of inflammation, aging, and endothelial dysfunction in breast cancer survivors on aromatase inhibitors. Sci Rep 13, 1677 (2023). https://doi.org/10.1038/s41598-023-28895-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28895-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.