Abstract
Certain cut-off points for sarcopenia screening and diagnosis are arbitrary and based on European populations, with normative references often obtained from healthy young adults. Although respiratory skeletal muscle strength tests represent low-cost clinical measures commonly performed in clinical practice by health professionals, a gap remains regarding whether respiratory skeletal muscle strength tests are adequate and sensitive measures for sarcopenia screening. This study aimed to verify the value of handgrip and respiratory muscle strength as possible discriminators to identify sarcopenia and to establish cut-off points for sarcopenia screening in community-dwelling, Brazilian women. In a cross-sectional study, 154 community-dwelling, Brazilian women (65–96 years) were assessed for appendicular skeletal muscle mass, handgrip (HGS), and respiratory muscular strength, including maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). The data were analyzed using the ROC curve and the Youden Index determined cut-off points. Statistical significance was set at 5%. 88 participants (57%) were sarcopenic. MEP (OR 0.98 [95%CI 0.97, 1.00], p = 0.023) and HGS (OR 0.82 [95% CI 0.75, 0.90], p < 0.001) were independent factors for sarcopenia in older. The optimal cut-off points for identifying sarcopenia were ≤ 77 cmH2O for MEP (AUC = 0.72), and ≤ 20 kg for HGS (AUC = 0.80). Simple muscular strength tests, including HGS and MEP, may be considered in the identification of sarcopenia in older, community-dwelling, Brazilian women. Future work is still needed to assess external validation of the proposed cut-offs before the clinical application.
Similar content being viewed by others
Introduction
Sarcopenia is a progressive and generalized skeletal muscle disorder associated with increased likelihood of adverse outcomes, including falls, fractures, functional disability, physical performance and skeletal muscle strength impairment, mortality, and others1. In financial terms for healthcare systems, the presence of sarcopenia increases the risk of hospitalization and the subsequent cost of care during hospitalization2. In recent decades, there has been a dramatic increase in the prevalence of sarcopenia due to the aging population. In Brazil, sarcopenia prevalence in community-dwelling and hospitalized older people is estimated to be around 13.9–21.8%3,4,5.
There is a wide variety of tests and tools available for sarcopenia stratifications in clinical practice and in research6,7. According to the European Working Group on Sarcopenia in Older People (EWGSOP2), sarcopenia diagnosis is based on parameters of skeletal muscle mass and strength, and physical performance1. In clinical practice, for sarcopenia screening and diagnosis, EWGSOP2 recommends the use of muscle strength tests such as handgrip strength (HGS) to identify probable presence of sarcopenia8. HGS is an easy measure with practical applicability and is associated with lower limb strength and disability9. The use of dual-energy X-ray absorptiometry (DXA) is recommended to detect low skeletal muscle quantity10. In addition, physical performance measures are often used to determine sarcopenia severity1.
As certain cut-off points for sarcopenia screening and diagnosis are arbitrary and based on European populations, with the normative references often being obtained from healthy young adults, studies have recommended caution in adopting cut-off points for populations with different genotypic and phenotypic characteristics11,12,13. Thus, normative data obtained from appropriate reference populations should be used to determine specific cut-off points14,15. Therefore, to identify older adults at risk of sarcopenia15,16, it is crucial to use appropriate, specific cut-off points for the target population.
HGS measurement has been commonly used to indirectly screen and classify the presence of sarcopenia8. However, in the screening and classification of the presence of sarcopenia in women, a wide range of values from 16 to 20 kg have been adopted as cut-off point8,17,18,19,20. In this sense, there is, no known study evaluating the effectiveness of HGS in identifying low muscle mass for screening and diagnosis of sarcopenia in older, community-dwelling, Brazilian women. Therefore, a gap remains regarding the cut-off point for sarcopenia screening in the aforementioned population.
Other strength tests have also been suggested to discriminate sarcopenia, such as maximal respiratory pressures. As in the case of HGS, they were previously related to peripheral muscle strength21 and sarcopenia indicators22,23,24. Thus, maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) may represent additional tools to HGS in the screening and diagnosis of sarcopenia.
Although respiratory skeletal muscle strength tests represent low-cost clinical measures commonly performed in clinical practice by health professionals, a gap remains regarding whether respiratory skeletal muscle strength tests are adequate and sensitive measures for sarcopenia screening and diagnosis. Hence, the aim of this research was to evaluate the association of respiratory muscle strength and HGS with the presence of sarcopenia in older, community-dwelling, Brazilian women and to establish cut-off points for sarcopenia screening, thereby facilitating early detection and better management of sarcopenia in clinical practice.
Methods
Patients and methods
This was a cross-sectional study involving participants evaluated at the Laboratório de Fisiologia do Exercício (Exercise Physiology Laboratory) (LAFIEX) at the Universidade Federal dos Vales do Jequitinhonha e Mucuri (Federal University of the Jequitinhonha and Mucuri Valleys) (UFVJM), from June 2016 to June 2017. The research was approved by the Institutional Ethics Committee (UFVJM; identification number 1.461.306) and carried out according to the Declaration of Helsinki25. All patients gave their written informed consent before participating in the study and were evaluated consecutively. The present study was edited following the guidelines of the STARD (Standards for Reporting of Diagnostic Accuracy Studies) statement26, when applicable.
Older, community-dwelling adults were recruited in their homes and invited to participate in the study. Following application of the inclusion and exclusion criteria, the eligible volunteers were subjected to previously scheduled assessments at LAFIEX, including body composition analyses and muscular strength tests (HGS, MIP, and MEP).
The inclusion criteria were older women, aged 65 or over, regardless of race or social class and who were willing to participate in the research. The exclusion criteria were cognitive impairment, detected by the Mini-Mental State Examination, according to schooling; being unable to walk independently without the aid of a walking device; having been hospitalized or having suffered fractures in the last 3 months; using the medication digoxin, due to its positive influence on respiratory strength27; inflammatory disease in the acute phase; thyroid dysfunction; neoplasm in activity in the last 5 years; being in palliative care; using anti-inflammatory drugs or drugs that act on the immune system; performing physical activity on a regular basis (at least three times a week); severe visual and auditory impairment; and acute cardiorespiratory diseases.
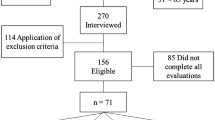
The sample size for determining a suitable cut-off value was obtained considering 1.96 as the percentage for the normal distribution, area under the curve of 0.8, confidence interval of 0.05, and a ratio of 1 between number of participants with and without sarcopenia28. Thus, the sample size was estimated at 154 participants. In addition, at least 77 participants should be sarcopenic in the estimated sample.
Measurements
Body composition and diagnosis of sarcopenia
Body mass and height were measured using scales with a stadiometer. Body mass index (BMI) was calculated by body weight (kg) divided by height squared (m2). Assessment of lean mass, fat mass, and bone mineral density, among others, was performed using dual-energy X-ray absorptiometry—DXA (Lunar Radiation Corporation, Madison, Wisconsin, USA, model DPX), which is considered a suitable and ideal measure in the research setting, clinical practice, and primary health care for confirming sarcopenia diagnosis9. Thus, DXA was the reference standard. The diagnosis of sarcopenia was made considering the cut-off points of Appendicular Skeletal Muscle Mass (ASM), measured as the sum of the non-bone and non-fat mass of the four limbs10,19. The reference value of 15 kg, relative to ASM, was used as a cut-off point for the detection of sarcopenia and participants were classified as non-sarcopenic and sarcopenic1,29.
Handgrip strength
Handgrip strength was used as the index test and evaluated using the Jamar® dynamometer, measured in kg, by means of an isometric contraction applied over its loops, according to the American Society of Hand Therapists (2002)30. Three measurements were performed with the dominant hand and their average was used for analysis. An interval of one minute was given between each measurement31.
Respiratory muscle strength.
Respiratory muscle strength was assessed by measuring the maximal inspiratory pressure (MIP) and the maximal expiratory pressure (MEP) using a hand vacuum pump, model MV-150/300, manufactured by Ger-Ar Comércio e Equipamentos Ltda®. Each volunteer was seated with their feet supported and their nose occluded with a nasal clip. The maneuvers were repeated up to five times. Three acceptable maneuvers were collected and the maximal respiratory efforts sustained for at least 2 s. The measurements considered acceptable were those without air leaks and with a variation ≤ 10% from the highest value detected32. The sequence of MIP and MEP measurements was random, and the highest measurement was selected for analysis. The MIP was measured based on the residual volume, and the MEP on total lung capacity33. An interval of at least 1 min was established between each MIP and MEP measurement to allow the participant to recover. For precision in the interval between MIP and MEP collection, normalization of O2 saturation and the return of systemic blood pressure to basal levels were observed34.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences version 22.0 (SPSS Statistics; IBM, Armonk, NY) and MedCalc Statistical Software version 13.1 (MedCalc Software, Ostend, Belgium). The data distribution was verified using the Kolmogorov–Smirnov test. Continuous variables were shown as mean and standard deviation (normal distribution) or median and interquartile range (non-normal distribution). Comparisons between groups were performed through independent t-test and Mann–Whitney’s test. Univariate and multivariate logistic regression analyses were used to examine the association between muscular strength tests (MIP, MEP, HGS) and sarcopenia. The receiver operating characteristic (ROC) curves were used to test the sensitivity and specificity of MIP, MEP, and HGS tests in identifying sarcopenia. The area under the ROC curve (AUC) and 95% confidence interval (CI) were calculated for all tests and optimal cut-offs were determined using the Youden Index. An AUC greater than 0.7 was considered acceptable, while an area greater than 0.8 was considered excellent for the propose the cut-offs points35. Statistical significance was set at 5%.
Ethics approval and consent to participate
Ethics approval has been granted by the Institutional Ethics Committee (Universidade Federal dos Vales do Jequitinhonha e Mucuri, Diamantina, Brazil; identification number 1.461.306). Informed consent was obtained from all study participants.
Results
Characteristics of subjects
Four hundred and forty-one patients were initially recruited for participation. After applying the exclusion criteria, a 154 elderly community residents were enrolled in the study (Fig. 1).
Characteristics of the participants are presented in Table 1. There were differences in age, as well anthropometric and body composition variables between the groups. Thus, the sarcopenic group were older, with lower BMI, lean mas, fat mass, and BMD. There was also a statistical difference in strength tests (MIP, MEP, and HGS) between groups, with lower muscle strength in the sarcopenic group.
Logistic regression analysis between MIP, MEP, HGS, and sarcopenia
While all the variables being significantly associated with sarcopenia in the univariate analysis [age (OR 1.09; 95% CI 1.04–1.15, p = 0.001), MIP (OR 0.99; 95% CI 0.98–1.00, p = 0.004), MEP (OR 0.97; 95% CI 0.96–0.99, p < 0.001), and HGS (OR 0.79; 95% CI 0.72–0.86, < 0.001)], in the multiple logistic regression with all the variables, only only MEP [OR 0.98; 95% CI 0.97–1.00, p = 0.047] and HGS [OR 0.82; 95% CI 0.75–0.90, p < 0.001] were significantly and inversely associated with sarcopenia (protective impact) (Table 2). Of note, when the data were adjusted for age, the results were the same [OR 1.02; 95% CI 0.96–1.08; p = 0.515].
ROC curves for MIP, MEP and HGS
The area under the ROC curve (AUC) to exclude sarcopenia in elderly women using strength tests were MIP [AUC = 0.65 (0.56–0.73)], B). MEP [AUC = 0.72 (0.64–0.80)], and C). HGS [AUC = 0.80 (0.72–0.87)]. The MIP showed low discriminatory power while MEP had acceptable accuracy and HGS was excellent for sarcopenia screening (Fig. 2). Table 3 shows properties of the cut-off points with the best combination of sensitivity and specificity, as well negative and positive predictive value of muscle strength tests to screening sarcopenia.
Discussion
To the best of our knowledge, the present study was the first to demonstrate the accuracy of HGS and MEP in identifying older, community-dwelling, Brazilian women with sarcopenia. The main data revealed that sarcopenic, older, community-dwelling women are more likely to have (1) lower respiratory muscle strength, that is, lower MEP and MIP and reduced HGS compared with older community-dwelling women with low muscle mass or sarcopenia. In addition, (2) muscle strength test cut-off points for HGS and MEP appear to be complementary tools for screening sarcopenia in this population. These results are clinically relevant, as simple, low-cost tests have potential value in screening and identifying older, community-dwelling women with sarcopenia, especially when DXA is not available.
The general characteristics of the participants showed differences between the groups regarding anthropometric variables and body composition due to sarcopenia. These findings are consistent with previous studies on aging sarcopenia36,37. Regarding muscle strength, both handgrip strength and respiratory muscle strength were lower in individuals with sarcopenia when compared to the non-sarcopenic elderly. Thus, the data of the present study is in accordance with the findings of previous studies that identified lower MIP, MEP, and HGS in older adults with sarcopenia1,8,22,24,38.
According to the EWGSOP2, the determination of low muscle strength is essential and the most important component in the diagnosis of sarcopenia1. One of the muscle strength tests recommended by the EWGSOP2 with normative reference for healthy, British women is HGS. Thus, the cut-off for healthy British women adults is > 16 kg, based on -2.5 standard deviations from the normative reference mean, that is, healthy British women1,8.
In this study, the cut-off point for sarcopenia in elderly, community-dwelling, Brazilian women was 20 kg. A systematic review with HGS reference values in different countries8 found that the magnitude of HGS if often related to the level of development of the country. This suggests that the cut-off points from British normative data may not be specific enough in developing regions like Brazil. Nonetheless, the EWGSOP2 recommend the use of regional normative populations when available, given that cut-off values for parameters related to sarcopenia may differ between populations due to ethnicity, body size, lifestyles, and cultural origins1.
A few Brazilian studies with different aims have proposed cut-off points for HGS. Sampaio et al.39 investigated HGS cut-off values in relation to fear of falling among older, Brazilian adults and the cut-off for women (69.4 ± 6.6 years) was 21.7 kg (AUC = 0.56; 95% CI 0.51–0.62; p = 0.02). De Souza et al.40 also showed the cut-off point of HGS to identify mobility limitation in older, community-dwelling people (73.4 ± 6,4 years) and found values of ≤ 17.4 kg (AUC = 0.68; 95% CI 0–64-0.71). These findings are similar to those of the present study since they found higher HGS cut-off points for Brazilian women, but they differ in terms of the specific values and outcomes of interest assessed. Although such clinical outcomes are related to sarcopenia, it should be noted that the AUC had low discriminatory power in both studies (AUC < 0.7).
Of the analyzed tests, only MEP and HGS presented a satisfactory area under curve that enabled the ROC curve to be calculated. The cut-off point for MEP was 77 cmH2O (sensitivity: 77.3%; specificity: 62.1%; AUC: 0.72) and the HGS was 20 kg (sensitivity: 70.5%; specificity: 77.3%; AUC: 0.80). As in the EWGSOP2, we opted to use rounded figures because, despite minimally reducing the accuracy, it makes their use easier. Therefore, we propose the values of ≤ 77 cmH2O for MEP and ≤ 20 kg for HGS for identifying sarcopenia in older, community-dwelling, Brazilian women.
Some studies have already investigated the relationship between respiratory muscle strength and sarcopenia. Shin et al.23 verified the relationship between MIP and MEP and sarcopenia indicators (skeletal muscle mass index, HGS, gait speed, and SPPB) among 65 elderly people with a mean age of 69.90 ± 7.63 years. Both MIP and MEP were positively related to skeletal muscle mass index and HGS. Izawa et al.22 also proposed a cut-off point for MIP as a discriminator of sarcopenia, with a value of 55.6 cmH2O (sensitivity: 0.76; specificity: 0.37; AUC: 0.70). However, the individuals included in the analysis were elderly male patients with heart disease, so sex-related differences could not be evaluated. This limits the use of this cut-off point with females and individuals without heart disease41,42.
Ohara et al.38 also developed cut-off values of MIP and MEP for the diagnosis of sarcopenia in elderly Brazilians22,38. In this study, the cut-off points ≤ 45 cmH2O for MIP (sensitivity: 0.73; specificity: 0.64; AUC: 0.73) and ≤ 55 cmH2O for MEP (sensitivity: 0.73; specificity: 0.58; AUC: 0.71) were discriminators of sarcopenia in elderly women. However, the authors evaluated the muscle mass component based on the total muscle mass estimated by the equation proposed by Lee et al.43. In this regard, although the equation proposed by Lee is validated for the European population, it is not a gold standard to measure muscle mass, especially for the Brazilian population.
Another recent study demonstrated that frail and pre-frail older adults present significantly lower MIP and MEP compared to non-frail older people44, indicating that respiratory muscle strength may be useful for discriminating frailty34. This reinforces the importance of considering respiratory muscle strength to assess frailty in older adult populations. Together, these results demonstrate the importance of the relationship between respiratory muscle strength and sarcopenia indicators.
Although the present study evaluated MIP and MEP, only MEP was considered an acceptable clinical test for identifying sarcopenia. A possible explanation could be that expiratory muscles seem to be more vulnerable to the aging process than inspiratory muscles45,46,47. Black and Hyatt48 observed that respiratory muscle strength declines at a rate between 0.25 and 0.79 cmH2O a year for MIP and between 1.14 and 2.33 cmH2O a year for MEP in both men and women, respectively. Enright et al.21 also found similar age-related decrements in both MIP and MEP with a rate of decline in MIP of about 1 cmH2O a year and for MEP of about 2 to 3 cmH2O a year for individuals between 65 and 85 years of age.
Our findings suggest that respiratory muscle strength is a relevant component in the clinical evaluation of the elderly due to its association with sarcopenia. In addition, MEP and HGS are easily accessible measures performed in clinical practice and can be useful to provide information about the health status of the elderly population. Advances in research are needed for external validation of the cut-off points proposed by the present study.
This study has several strengths that are worth highlighting. First, we used DXA, a measure considered the gold standard for measuring muscle mass and which is recommended in research and clinical practice by the EWGSOP2 to confirm a sarcopenia diagnosis1. Furthermore, this was the first study to establish cut-off points for HGS and one of the first for MEP in identifying sarcopenia in older, community-dwelling, Brazilian women. It is also noteworthy that the fact elderly women from different locations were recruited enabled us to obtain data that more reliably portray the diversity of the residents of this community.
The Southeast Region of Brazil is a region with multiple local realities, including physical, social, and economic characteristics, therefore the results of the present study should be read with caution and cannot be extrapolated to all Brazilian elderly women. In addition, results cannot be extrapolated to elderly women who engage in physical activity on a regular basis because we included only sedentary elderly in our study. Limitations of this cross-sectional study include the inclusion of female subjects only, so gender-related differences could not be assessed. Future studies evaluating factors such as MEP and HGS in longitudinal settings, performed for longer periods, will be required to determine the risk of developing sarcopenia.
Conclusion
MEP and HGS are probably valuable tools with potential value in the screening of sarcopenia in older, community-dwelling, Brazilian women. Cut-off points of ≤ 77 cmH2O for MEP and ≤ 20 kg for HGS seem to be useful and should be considered for the identification of sarcopenia in the studied population. Future work is still needed to assess external validation of the proposed cut-offs before the clinical application.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due the maintenance of confidentiality of our participants and declarations within the written information which participants had agreed on, but are available from the corresponding author on reasonable request.
References
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48(1), 16–31 (2019).
Cawthon, P. M. et al. Clinical definitions of sarcopenia and risk of hospitalization in community-dwelling older men: The osteoporotic fractures in men study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 72(10), 1383–1389 (2017).
Alexandre, T. et al. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J. Nutr. Health Aging 18, 8 (2014).
Barbosa-Silva, T. G. et al. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: Results of the COMO VAI? study. J. Cachexia. Sarcopenia Muscle 7(2), 136–143 (2016).
Martinez, B. P. et al. Frequency of sarcopenia and associated factors among hospitalized elderly patients. BMC Musculoskelet. Disord. 16(1), 1–7 (2015).
Mijnarends, D. M. et al. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: A systematic review. J. Am. Med. Dir. Assoc. 14(3), 170–178 (2013).
Reginster, J.-Y. et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin. Exp. Res. 28(1), 47–58 (2016).
Dodds, R. M. et al. Grip strength across the life course: Normative data from twelve British studies. PLoS One 9(12), e113637 (2014).
Beaudart, C. et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 16(1), 170 (2016).
Buckinx, F. et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 9(2), 269–278 (2018).
Lourenço, R. A. et al. Performance of the European Working Group on Sarcopenia in Older People algorithm in screening older adults for muscle mass assessment. Age Ageing 44(2), 334–338 (2015).
Van Ancum, J. M. et al. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 90, 104125 (2020).
Stuck, A. K. et al. Performance of the EWGSOP2 cut-points of low grip strength for identifying sarcopenia and frailty phenotype: A cross-sectional study in older inpatients. Int. J. Environ. Res. Public Health 18(7), 3498 (2021).
Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing. 40(4), 423–429 (2011).
Bahat, G. et al. Comparison of standard versus population-specific handgrip strength cut-off points in the detection of probable sarcopenia after launch of EWGSOP2. Aging Male 23(5), 1564–1569 (2020).
Savas, S. et al. A cross-sectional study on sarcopenia using EWGSOP1 and EWGSOP2 criteria with regional thresholds and different adjustments in a specific geriatric outpatient clinic. Eur. Geriatr. Med. 11(2), 239–246 (2020).
Lauretani, F. et al. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. (Bethesda, Md.: 1985) 95(5), 1851–1860 (2003).
Anjum, S. N. et al. Comparative evaluation of grip and pinch strength in an Asian and European population. Hand Ther. 17(1), 11–14 (2012).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69(5), 547–558 (2014).
Blanquet, M. et al. Handgrip strength as a valid practical tool to screen early-onset sarcopenia in acute care wards: A first evaluation. Eur. J. Clin. Nutr. 20, 25 (2021).
Enright, P. L. et al. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am. J. Respir. Crit. Care Med. 149(2), 430–438 (1994).
Izawa, K. P. et al. Respiratory muscle strength in relation to sarcopenia in elderly cardiac patients. Aging Clin. Exp. Res. 28(6), 1143–1148 (2016).
Shin, H. I. et al. Relation between respiratory muscle strength and skeletal muscle mass and hand grip strength in the healthy elderly. Ann. Rehabil. Med. 41(4), 686–692 (2017).
Deniz, O. et al. Diaphragmatic muscle thickness in older people with and without sarcopenia. Aging Clin. Exp. Res. 33(3), 573–580 (2021).
Association GAotWM. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 81(3), 14–18 (2014).
Bossuyt, P. M. et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Clin. Chem. 61(12), 1446–1452 (2015).
Siafakas, N. et al. Effect of digoxin on global respiratory muscle strength after cholecystectomy: A double blind study. Thorax 55(6), 497–501 (2000).
Zhou, X.-H., McClish, D. K. & Obuchowski, N. A. Statistical Methods in Diagnostic Medicine Vol 569 (Wiley, 2009).
Studenski, S. A. et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. Ser. A 69(5), 547–558 (2014).
ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care Med. 166(4), 518–624 (2002).
Dias, J. A. et al. Força de preensão palmar: Métodos de avaliação e fatores que influenciam a medida. Rev. Bras. Cineantropometria Desempenho Humano 12(3), 209–216 (2010).
Costa, D. et al. Novos valores de referência para pressões respiratórias máximas na população brasileira. J. Bras. Pneumol. 36, 306–312 (2010).
Spruit, M. A. et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 188(8), e13–e64 (2013).
Parentoni, A. N. et al. Comparison of respiratory muscle strength between fragility subgroups in community elderly. Fisioterapia Pesquisa 20(4), 361–366 (2013).
Hajian-Tilaki, K. Receiver operating characteristic (ROC) Curve analysis for medical diagnostic test evaluation. Caspian J. Internal Med. Spring 4(2), 627–635 (2013).
Kim, H. et al. Sarcopenia: Prevalence and associated factors based on different suggested definitions in community-dwelling older adults. Geriatr. Gerontol. Int. 16(S1), 110–122 (2016).
Kitamura, M. et al. Prevalence and related factors of sarcopenia in community-dwelling elderly with long-term care insurance. Rev. Recent Clin. Trials 16(3), 335–340 (2021).
Ohara, D. G. et al. Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: A cross-sectional study. J. Nutr. Health Aging 22(8), 952–958 (2018).
Sampaio, R. A. C. et al. Cutoff values for appendicular skeletal muscle mass and strength in relation to fear of falling among Brazilian older adults: Cross-sectional study. Sao Paulo Med. J. 135, 434–443 (2017).
de Souza Vasconcelos, K. S. et al. Handgrip strength cutoff points to identify mobility limitation in community-dwelling older people and associated factors. J. Nutr. Health Aging 20(3), 306–315 (2016).
Chen, H.-I. & Kuo, C.-S. Relationship between respiratory muscle function and age, sex, and other factors. J. Appl. Physiol. 66(2), 943–948 (1989).
Nagano, A. et al. Respiratory sarcopenia and sarcopenic respiratory disability: Concepts, diagnosis, and treatment. J. Nutr. Health Aging 25(4), 507–515 (2021).
Lee, R. C. et al. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 72(3), 796–803 (2000).
Vidal, M. B. et al. Respiratory muscle strength for discriminating frailty in community-dwelling elderly: A cross-sectional study. Arch. Gerontol. Geriatr. 89, 104082 (2020).
Kim, J. & Sapienza, C. M. Implications of expiratory muscle strength training for rehabilitation of the elderly: Tutorial. J. Rehabil. Res. Dev. 42(2), 211–224 (2005).
Mizuno, M. Human respiratory muscles: Fibre morphology and capillary supply. Eur. Respir. J. 4(5), 587–601 (1991).
Kim, J. & Sapienza, C. M. Implications of expiratory muscle strength training for rehabilitation of the elderly: Tutorial. J. Rehabil. Res. Dev. 42, 2 (2005).
Black, L. F. & Hyatt, R. E. Maximal respiratory pressures: Normal values and relationship to age and sex. Am. Rev. Respir. Dis. 99(5), 696–702 (1969).
Acknowledgements
We would like to thank the Universidade Federal dos Vales do Jequitinhonha e Mucuri for institutional support, CNPq (402574/2021-4), CAPES, and FAPEMIG for support and scholarships.
Author information
Authors and Affiliations
Contributions
L.A.S.: conception and design, analysis and interpretation of the data, writing—review and editing—original draft. L.P.L.: analysis and interpretation of the data, writing—review and editing—original draft. A.C.N.P.: analysis and interpretation of the data, writing—review and editing—original draft. A.N.R.: analysis and interpretation of the data, writing—review and editing—original draft. L.A.C.T.: conception and design, data curation, methodology, writing—review and editing—original draft. T.C.D: conception and design, data curation, methodology, writing—review and editing—original draft. J.M.S.: writing—review and editing—original draft. V.K.S.L.: writing—review and editing—original draft. F.A.P.: writing—review and editing—original draft. H.S.C.: data curation, methodology, writing—review and editing—original draft. P.H.S.F.: writing—review and editing—original draft. V.M.T.L.A.: writing—review and editing—original draft. N.S.A.: writing—review and editing—original draft. S.P.C.: writing—review and editing—original draft. F.P.B.: writing—review and editing—original draft. R.R.L.: writing—review and editing—original draft. R.L.T.: writing—review and editing—original draft. L.S.M.P.: conception and design, writing—review and editing—original draft. F.S.M.P.: writing—review and editing—original draft. A.N.P.: conception and design, data curation, methodology, writing—review and editing—original draft. N.C.P.V.: writing—review and editing—original draft. A.A.O.L.: writing—review and editing—original draft. V.A.M.: conception and design, data curation, methodology, writing—review and editing—original draft. A.C.R.L.: conception and design, data curation, methodology, writing—review and editing—original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soares, L.A., Lima, L.P., Prates, A.C.N. et al. Accuracy of handgrip and respiratory muscle strength in identifying sarcopenia in older, community-dwelling, Brazilian women. Sci Rep 13, 1553 (2023). https://doi.org/10.1038/s41598-023-28549-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28549-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.