Abstract
Though triazole antifungals are the first choice for preventing and treating invasive fungal infections, periostitis caused by voriconazole has been described in emerging case reports; however, no studies exist on this association in real-world clinical settings. Our study aimed to identify the association between periostitis and triazole antifungals by analyzing data from the FDA Adverse Event Reporting System (FAERS). We extracted and analyzed reports on the association between periostitis and triazole antifungals in FAERS from the first quarter of 2004 to the second quarter of 2022 using OpenVigil 2.1. Disproportionality analysis was performed to evaluate the association between periostitis and triazole antifungals, and chi-squared (χ2), relative reporting ratio (RRR), reporting odds ratio (ROR), proportional reporting ratio (PRR), and Bayesian confidence propagation neural networks (BCPNN) of information components (IC) were reported. In total, 143 patients experienced periostitis while using voriconazole. Disproportionality analysis identified an association between periostitis and voriconazole (χ2 = 82,689.0, RRR = 583.6, 95%CI [472.4, 721.1], PRR = 1808.9, 95%CI [1356.0, 2412.9], ROR = 1831.7, 95%CI [1371.6, 2446.3], IC = 9.2, 95%CI [8.6, 9.8]). However, no safety signals were observed between periostitis and other triazole antifungals. When stratified by sex and age, disproportionality analysis identified positive signals between periostitis and voriconazole. The possible association between periostitis and voriconazole should attract sufficient attention in clinical practice. Alternative treatment with other triazole antifungals can be considered, and causality needs to be verified in further prospective studies.
Similar content being viewed by others
Introduction
Triazole antifungals are the first choice for preventing and treating invasive fungal infections. In addition, they are recommended for prophylactic treatment or general antifungal prophylaxis in patients undergoing solid organ and hematopoietic stem cell transplantation1. Generally, triazole antifungals must be taken for long periods (weeks to months), followed by long-term suppressive therapy2. However, the long-term use of triazole antifungals has raised concerns about cost, development of resistance, and treatment-related adverse effects. Meanwhile, with the increasing use frequency of triazole antifungal drugs, their safety is receiving more and more attention. Common adverse events with voriconazole include visual impairment, fever, rash, nausea, vomiting, diarrhea, headache, septicemia, peripheral edema, abdominal pain, and respiratory dysfunction. Periostitis caused by voriconazole therapy has been described in various case reports3,4,5,6,7,8,9,10,11,12. Symptoms of periostitis typically include myalgia or diffuse pain in one or more skeletal areas, often involving the tibia, orbital bone, or root bone. A case report published in The New England Journal of Medicine revealed that a patient with voriconazole-induced periostitis had reduced pain at a 7-month follow-up after discontinuation of voriconazole, but the range of motion limitations and bone swelling persisted in the hand13. In cases of infectious periostitis, sepsis may occur and involve all body organs14. In addition, it has been suggested that periostitis is mainly caused by the fluoride in voriconazole, and alternative therapy with other triazole antifungals can be clinically considered to avoid periostitis15. However, there have been no studies on the association between periostitis and triazole antifungals in a large population, including signal mining studies based on the adverse event reporting database. Therefore, it is unclear whether there is an association between periostitis and triazole antifungals in real-world clinical settings.
The FDA Adverse Event Reporting System (FAERS) is a database utilized for collecting information on spontaneously reported adverse drug events. Due to the large amount of data, diversity of data information, and free access to the public, it is often used in studies of adverse drug event signal mining16. FAERS receives approximately 1.5 million reports of adverse events about drugs and medical devices every year and can reflect adverse events in real-world clinical settings. In addition, data mining algorithms have already been developed for signal detection in this database; for example, a “positive signal” refers to a statistical association between an adverse event and a drug17. Thus, FAERS is now widely used to detect potential drug-associated adverse events18,19.
Therefore, in this study, we identified the possible association between periostitis and triazole antifungals based on the FAERS database. This study can provide research hypotheses on the causality between triazole antifungal drugs and periostitis for further studies, and can also provide evidence for optimizing treatment, prevention, and response to adverse drug events.
Results
Characteristics of patients taking triazole antifungals and experiencing periostitis
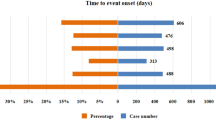
We found 143 patients taking voriconazole and experiencing periostitis. No relevant reports on fluconazole, itraconazole, posaconazole, and ravuconazole were available. Detailed characteristics of the patients are shown in Table 1. For voriconazole, 46.85% of the patients with periostitis were male, 41.26% were female, and 11.89% were unknown. There were 4 patients (2.80%) aged less than 18 years, 87 patients (60.84%) aged 18–65 years, and 31 patients (21.68%) aged more than 65 years. The largest number of patients was reported between 2019 and 2022, with 62 patients (43.36%). The United States had the highest number of reports among all reporting countries, with 89 patients (62.24%). Among all outcomes, other outcomes (46.15%) accounted for the largest proportion, followed by hospitalization (24.48%), unknown (20.28%), disability (5.59%), death (2.10%), and life-threatening (1.40%). Infection accounted for the largest proportion (36.36%) of all the indications. In addition, for fluconazole and itraconazole, the three patients that emerged were included in the 143 patients taking voriconazole at the same time. Therefore, except for voriconazole, triazole antifungals have no data on the occurrence of periostitis.
The association between periostitis and triazole antifungals
For the 143 patients with voriconazole-related periostitis, χ2 was 82,689.0, relative reporting ratio (RRR) was 583.6, 95% confidence interval (CI) [472.4, 721.1]; proportional reporting ratio (PRR) was 1808.9, 95% CI [1356.0, 2412.9]; reporting odds ratio (ROR) was 1831.7, 95% CI [1371.6, 2446.3]; and Bayesian confidence propagation neural networks (BCPNN) of information components (IC) was 9.2, 95%CI [8.6, 9.8] (Table 2), which met the criteria for positive signals in disproportionality analysis. Therefore, there may be an association between periostitis and voriconazole. No association was observed between periostitis and other triazole antifungals.
The association between periostitis and voriconazole in different sexes
When stratified by sex, the number of voriconazole-related periostitis reports in males was 67, χ2 was 29,713.6, RRR was 451.4, 95% CI [327.8, 621.6]; PRR was 2226.4, 95% CI [1308.3, 3788.9]; and ROR was 2251.9, 95% CI [1321.5, 3836.5], while the number of voriconazole-related periostitis reports in females was 59, χ2 was 45,751.2, RRR was 790.2, 95% CI [574.7, 1086.5]; PRR was 1848.4, 95% CI [1252.6, 2727.6]; and ROR was 1877.7, 95% CI [1269.1, 2778.1] (Table 3). Thus, there may be an association between periostitis and voriconazole, regardless of sex.
The association between periostitis and voriconazole in different age groups
When stratified by age, the number of voriconazole-related periostitis reports in patients less than 18 years was 4, χ2 was 582.0, RRR was 191.7, 95% CI [56.2, 653.8]; PRR was 446.1, 95% CI [100.0, 1990.0]; and ROR was 448.1, 95% CI [100.1, 2005.4]. The number of voriconazole-related periostitis reports in patients aged 18–65 years was 87, χ2 was 39,262.2, RRR was 457.8, 95% CI [348.7, 601.0]; PRR was 1503.7, 95% CI [1028.3, 2199.0]; and ROR was 1531.7, 95% CI [1045.1, 2244.6]. Finally, the number of voriconazole-related periostitis reports in patients more than 65 years was 31, χ2 was 14,057.9, RRR was 469.7, 95% CI [292.7, 753.7]; PRR was 2545.2, 95% CI [1121.7, 5775.4]; and ROR, 2573.1, 95% CI [1132.1, 5848.2] (Table 4). Thus, there may be an association between periostitis and voriconazole at different ages.
Discussion
To our knowledge, this is the first study to investigate the association between periostitis and triazole antifungals by analyzing FAERS data. Our study observed 143 patients with adverse event reports of voriconazole-related periostitis, whereas no reports were found between periostitis and other triazole antifungals. The results of the subgroup analysis suggested that voriconazole may be associated with periostitis, regardless of sex and age. The reason for the large values of RRR, PRR, ROR may be that the proportion of voriconazole-associated periostitis cases were much higher than that of other drugs. This also indicated that voriconazole is probably associated with periostitis. The wide confidence intervals of PRR, RRR, and ROR is likely because of the extremely low proportion of reports with periostitis.
Recently, emerging case reports have raised awareness of periostitis, a potentially fatal complication associated with voriconazole12,20. No case reports have described periostitis caused by other triazole antifungals15. Our study validated the findings of these case reports by identifying an association between periostitis and voriconazole. Voriconazole, a trifluoride antifungal, is believed to cause periostitis by increasing circulating levels of fluoride following voriconazole metabolism or by direct effects of the fluoride component of voriconazole11,14,21,22,23. Voriconazole-related periostitis usually occurs after high-dose (median 600 mg/day) or prolonged (median 5.6 months) voriconazole therapy during transplantation. The main features of the diagnosis include diffuse bone pain, white spots on the teeth, elevated serum alkaline phosphatase and plasma fluoride levels, positive nuclear bone scans, and radiological findings. Under the circumstances, discontinuing voriconazole is the primary method of resolving bone pain. Case studies have shown that patients with periostitis who were switched from voriconazole to itraconazole experienced rapid relief of bone pain6,11. Previous studies have also suggested that periostitis may be related to the fluoride in voriconazole. Therefore, due to the absence of fluoride in itraconazole and the low fluoride content in posaconazole or itraconazole, voriconazole can be replaced by these triazole antifungals if clinically required15.
Our study has several strengths. First, this is the first study to detect the signals between periostitis and triazole antifungals using FAERS data, which could provide valuable evidence for further studies and clinical practice in this field. Second, the risks of periostitis in different sex and age groups are unclear, and subgroup analyses were conducted to identify sex-specific and age-specific adverse events associated with voriconazole. Third, FAERS includes all reports of adverse events submitted to the FDA, with a sample size large enough to identify rare adverse events that are difficult to detect in traditional epidemiological studies25.
However, several limitations of the present study must be considered when interpreting the results. First, the FAERS database is a spontaneous reporting adverse event database, which is limited by the initiative, accuracy, and timeliness of reporting adverse reactions by physicians, patients, and other healthcare providers; therefore, there may be the possibility of under-reporting and misreporting. Second, there are problems with non-standard reports and missing data in FAERS, such as age and sex. Third, most reporters come from the United States, the United Kingdom, and other countries. Thus, there are limitations in the generalization of conclusions among Asians. Fourth, the causality between periostitis and voriconazole is difficult to determine and needs to be verified in future prospective studies.
There may be an association between periostitis and voriconazole, which should attract sufficient attention in clinical practice. Alternative treatment with other triazole antifungals, such as posaconazole and itraconazole, can be considered clinically, and the causality between periostitis and voriconazole needs to be verified in future prospective studies.
Methods
Data source
FAERS is a spontaneous reporting adverse event database submitted by physicians, pharmacists, manufacturers, patients, and other healthcare providers that provides information on adverse events and medicine error reports24. The FAERS data included patient demographics, administrative information, drug treatment information, adverse events, and patient outcomes25. OpenVigil 2.1 is a pharmacovigilance data extraction, cleaning, mining, and analysis tool for the FAERS database26. Relevant information was extracted from FAERS from the first quarter of 2004 to the second quarter of 2022 using OpenVigil 2.1 software.
Query construction
OpenVigil 2.1 mapped drug names (generic names, brand names, and abbreviations) to unique drug names using Drugbank and Drugs@FDA27. First, we identified the target drugs in the triazole antifungal family (fluconazole, itraconazole, voriconazole, posaconazole, and ravuconazole). We then extracted adverse event reports that included one or more triazole antifungals. The sex and age of patients' data were limited in the subgroup analysis.
Definition of periostitis-related adverse events
In FAERS, adverse events are coded using preferred terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA) terminology. We ultimately selected periostitis as an adverse event based on the structural hierarchy of the MedDRA terminology.
Statistical analysis
Descriptive analysis was performed to characterize the features of patients taking triazole antifungals and experiencing periostitis as an adverse event. Disproportionality analyses were performed by calculating RRR, ROR, PRR, PRR, and BCPNN of IC with their 95% CIs to evaluate the association between adverse events and drugs24,28. Disproportionality analysis is commonly used by the World Health Organization for pharmacovigilance when analyzing post-marketing surveillance databases to evaluate the association between adverse events and drugs29,30,31. Moreover, disproportionality analysis is based on a four-fold table to detect potential adverse drug reaction signals by comparing the proportion of target events occurring for the target drug with the proportion of target events occurring for all other drugs (Table 5). According to the criteria outlined by Evans et al.32, a positive signal was defined as more than three patients with chi-squared (χ2) greater than four and PRR greater than two. First, we performed a disproportionality analysis to evaluate the association between periostitis and triazole antifungals. Next, considering that the risks of periostitis in different sex and age groups were unclear, we performed subgroup analyses by sex (male and female) and age groups (< 18 years old, 18–65 years old, and > 65 years old)33,34,35,36.
“a” is the number of reports with adverse events of interest of the drugs of interest, “b” is the number of reports with adverse events of interest of all other drugs, “c” is the number of reports with all other adverse events of the drugs of interest, and “d” is the number of reports with all other adverse events of all other drugs.
(1) \(\upchi 2 = \frac{{(ad-bc)}^{2}(a+b+c+d)}{(a+b)(c+d)(a+c)(b+d)}\) (2) \(\mathrm{RRR}=\frac{\mathrm{a}/(\mathrm{a}+\mathrm{b})}{\mathrm{c}/(\mathrm{c}+\mathrm{d})}\) (3) \(\mathrm{PRR}=\frac{\mathrm{a}/(\mathrm{a}+\mathrm{c})}{\mathrm{b}/(\mathrm{b}+\mathrm{d})}\) (4) \(\mathrm{ROR}=\frac{\mathrm{a}/\mathrm{c}}{\mathrm{b}/\mathrm{d}}\) (5) \(\mathrm{IC}={log}_{2}\left(\mathrm{RRR}\right)\).
Ethics statement
The data sets are de-identified. There was no direct human participation in this study. Institutional Review Board requirements do not apply under 45 CFR 46.102. All experiments were performed in accordance with relevant guidelines and regulations.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Patterson, T. F. et al. Executive summary: Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 63, 433–442 (2016).
Husain, S. & Camargo, J. F. Invasive aspergillosis in solid-organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 33, e13544 (2019).
Tailor, T. D. & Richardson, M. L. Case 215: Voriconazole-induced periostitis. Radiology 274, 930–935 (2015).
Glushko, T. & Colmegna, I. Voriconazole-induced periostitis. CMAJ 187, 1075 (2015).
Baird, J. H., Birnbaum, B. K., Porter, D. L. & Frey, N. V. Voriconazole-induced periostitis after allogeneic stem cell transplantation. Am. J. Hematol. 90, 574–575 (2015).
Hirota, K., Yasoda, A., Fujii, T. & Inagaki, N. Voriconazole-induced periostitis in a patient with overlap syndromes. BMJ Case Rep. https://doi.org/10.1136/bcr-2013-203485 (2014).
Rossier, C. et al. Voriconazole-induced periostitis. Eur. J. Nucl. Med. Mol. Imaging 39, 375–376 (2012).
Becce, F., Malghem, J., Lecouvet, F. E., Vande Berg, B. C. & Omoumi, P. Clinical images: Voriconazole-induced periostitis deformans. Arthritis Rheum. 64, 3490 (2012).
Wise, S. M. & Wilson, M. A. A case of periostitis secondary to voriconazole therapy in a heart transplant recipient. Clin. Nucl. Med. 36, 242–244 (2011).
Skiles, J. L., Imel, E. A., Christenson, J. C., Bell, J. E. & Hulbert, M. L. Fluorosis because of prolonged voriconazole therapy in a teenager with acute myelogenous leukemia. J. Clin. Oncol. 29, e779–e782 (2011).
Lustenberger, D. P., Granata, J. D. & Scharschmidt, T. J. Periostitis secondary to prolonged voriconazole therapy in a lung transplant recipient. Orthopedics 34, e793–e796 (2011).
Ayub, A., Kenney, C. V. & McKiernan, F. E. Multifocal nodular periostitis associated with prolonged voriconazole therapy in a lung transplant recipient. J. Clin. Rheumatol. 17, 73–75 (2011).
Hedrick, J. & Droz, N. Voriconazole-induced periostitis. N. Engl. J. Med. 381, e30 (2019).
Moon, W. J. et al. Plasma fluoride level as a predictor of voriconazole-induced periostitis in patients with skeletal pain. Clin. Infect. Dis. 59, 1237–1245 (2014).
Guarascio, A. J., Bhanot, N. & Min, Z. Voriconazole-associated periostitis: Pathophysiology, risk factors, clinical manifestations, diagnosis, and management. World J. Transplant. 11, 356–371 (2021).
Subeesh, V., Maheswari, E., Singh, H., Beulah, T. E. & Swaroop, A. M. Novel adverse events of iloperidone: A disproportionality analysis in US food and drug administration adverse event reporting system (FAERS) database. Curr. Drug Saf. 14, 21–26 (2019).
Duggirala, H. J. et al. Use of data mining at the food and drug administration. J. Am. Med. Inform. Assoc. 23, 428–434 (2016).
Huang, J. et al. Safety profile of epidermal growth factor receptor tyrosine kinase inhibitors: A disproportionality analysis of FDA adverse event reporting system. Sci. Rep. 10, 4803 (2020).
Han, N., Oh, J. M. & Kim, I. W. Assessment of adverse events related to anti-influenza neuraminidase inhibitors using the FDA adverse event reporting system and online patient reviews. Sci. Rep. 10, 3116 (2020).
Jakobsen, D. M., Justad, B. A., Helweg-Larsen, J. & Katzenstein, T. L. Voriconazole-induced periostitis. Ugeskr. laeger 181, 1–3 (2019).
Gerber, B. et al. Reversible skeletal disease and high fluoride serum levels in hematologic patients receiving voriconazole. Blood 120, 2390–2394 (2012).
Wermers, R. A. et al. Fluoride excess and periostitis in transplant patients receiving long-term voriconazole therapy. Clin. Infect. Dis. 52, 604–611 (2011).
Chen, L. & Mulligan, M. E. Medication-induced periostitis in lung transplant patients: Periostitis deformans revisited. Skelet. Rad. 40, 143–148 (2011).
Noguchi, Y., Tachi, T. & Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 22(6), bbab347 (2021).
FDA Adverse Event Reporting System (FAERS) Public Dashboard. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reportingsystem-faers-public-dashboard (2018). Accessed 1 December 2022.
Böhm, R., Höcker, J., Cascorbi, I. & Herdegen, T. OpenVigil–Free eyeballs on AERS pharmacovigilance data. Nat. Biotechnol. 30, 137–138 (2012).
Bröhan, R. & Steglich, M. OpenVigil 2 tutorial. https://openvigil.sourceforge.net/doc/OpenVigil2-Tutorial.pdf (2015). Accessed 1 December 2022.
Khadem, T. M., van Manen, R. P. & Brown, J. How safe are recently FDA-approved antimicrobials? A review of the FDA Adverse Event Reporting System Database. Pharmacotherapy 34, 1324–1329 (2014).
Böhm, R. et al. Pharmacovigilance-based drug repurposing: The search for inverse signals via OpenVigil identifies putative drugs against viral respiratory infections. Br. J. Clin. Pharmacol. 87, 4421–4431 (2021).
Duron, D., Blaise, S., Cracowski, J. L., Roustit, M. & Khouri, C. Drug-induced skin ulcers: A disproportionality analysis from the WHO pharmacovigilance database. J. Am. Acad. Dermatol. 85, 229–232 (2021).
Khouri, C. et al. Investigating the association between ALK receptor tyrosine kinase inhibitors and pulmonary arterial hypertension: A disproportionality analysis from the WHO pharmacovigilance database. Eur. Respir. J. 58, 2101576 (2021).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486 (2001).
Gastaldon, C., Raschi, E., Kane, J. M., Barbui, C. & Schoretsanitis, G. Post-marketing safety concerns with esketamine: A disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Psychother. Psychosom. 90, 41–48 (2021).
Morris, R. et al. Bradycardia due to donepezil in adults: Systematic analysis of FDA adverse event reporting system. J. Alzheimers Dis. 81, 297–307. https://doi.org/10.1038/nbt.2113 (2021).
Jiao, X. F. et al. Ovary and uterus related adverse events associated with statin use: An analysis of the FDA adverse event reporting system. Sci. Rep. 10, 11955 (2020).
Guo, M. et al. Evaluation of rivaroxaban-, apixaban- and dabigatran-associated hemorrhagic events using the FDA adverse event reporting system (FAERS) database. Int. J. Clin. Pharm. 43, 1508–1515 (2021).
Funding
This study was supported by the National Natural Science Foundation for Young Scholars of China (72004151), and the Science and Technology Plan Project of Sichuan Province (2020YFS0035).
Author information
Authors and Affiliations
Contributions
H.L., W.Z., and Li.Z. designed the study; H.L., M.Z., X.J., and Le.Z. performed the data analysis; H.L., W.Z., and Li.Z. managed and checked all the data. All authors contributed equally to results interpretation and manuscript writing. All authors read, checked, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Zhang, M., Jiao, X. et al. Using disproportionality analysis to explore the association between periostitis and triazole antifungals in the FDA Adverse Event Reporting System Database. Sci Rep 13, 4475 (2023). https://doi.org/10.1038/s41598-023-27687-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27687-0
This article is cited by
-
Voriconazole, risk of periostitis
Reactions Weekly (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.