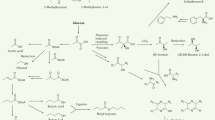
Abstract
The corpse plant (Amorphophallus titanum) is so named because it produces a pungent, foul odor when flowering. Little is known about how the emitted volatiles change throughout the two-day flowering period. In this study, the comprehensive monitoring of the presence and change in volatile molecules during the female and the male flowering phases of A. titanum was conducted, and the plant temperature was monitored. A total of 422 volatile features were detected over the entire sampling period, of which 118 features were statistically significantly different between the pre-flowering and both flowering phases, and an additional 304 features were found present throughout the flowering period. A total of 45 molecules could be assigned putative names. The volatile profile of A. titanum changes over the two-day flowering period, with the S-containing molecules and aldehydes dominant in the female flowering phase, and the alcohols and hydrocarbons dominant in the male flowering phase. The two-dimensional gas chromatography time-of-flight mass spectrometry (GC × GC-TOFMS) enabled us to identify 32 new molecules produced by A. titanum. Each of these molecules alone, and in combination, likely contribute to the different odors emitted during the flowering phase of A. titanum.
Similar content being viewed by others
Introduction
When a plant is unable to fertilize itself effectively, it uses another method, such as the wind, insects, or birds to help it reproduce. A plant can attract insects and birds via the use of flower shapes, flower color pattern, and emission of odors1. One fascinating example of the effective use of odor attraction for the purpose of using insects to fertilize flowers is by the Amorphophallus titanum, a native of Western Sumatra and Western Java. A. titanum has the largest unbranched inflorescence in the world2, which makes it public favorite in botanical gardens around the world. The spadix of A. titanum extends up to 2.5 m in height and is dull yellow in color. The spathe is about 3 m in circumference, about 1.6 m in height, and is pale green in color with white spots on the outside and purplish-wine lines on the inside3. A. titanum is economically important for food and medicinal purposes. The underground storage stem (tuber) of A. titanum contains more glucomannan than the other Amorphophallus species, which helps in reduction of cholesterol levels, obesity and diabetes3,4,5.
When flowering, A. titanum emits a decay-like stench during its two-day flowering period6. The pulsing waves of pungent odors produced by a flowering A. titanum has led to it being referred to as the “corpse plant”7. The most common odors describe it as smelling like a rotting animal, a dead mouse, foul, and sulfur-like during flowering. Though produced simultaneously, the individual volatile molecules emitted during female flowering include: dimethyl disulfide (garlic-like odor8), dimethyl trisulfide (foul odor9), methyl thioacetate (sulfurous odor10), and isovaleric acid (cheesy, sweaty odor10,11). The diffusion of volatile molecules from the flowers is enhanced by thermogenesis. The spadix thermogenesis period starts after the opening of the spathe on the first day, reaching 36 °C, in pulses, synchronizing with the waves of the carrion-like odor12. The thermogenesis of male flowers begins on the second day when pollens are being released, where the temperature of the florets can also reach up to 36 °C13. The flowering A. titanum draws insects that are typically attracted to carrion, including dung beetles and flesh flies9.
Little is known about the variety and abundance of the volatile molecules and odors emitted by A. titanum over time and publications appear to only focus on the female flowering phase8,10. In the work presented here, we undertake a comprehensive survey of the molecules produced by A. titanum before and during the female and the male flowering phases, an effort complimented by thermogenesis measurements.
Methods
Sampling of volatile molecules
Volatile molecules were sampled from an A. titanum grown in Dartmouth College’s Life Sciences Greenhouse during early November. Two samples were collected per day before the plant started flowering. When the spathe started to open and the odor became noticeable on the third day, samples were taken approximately every 2 h at a spot between the spadix and the top of the spathe. Each sample was drawn onto a thermal desorption tube (150 mL/min for 10 min; Carbopack Y, X, and Carboxen 1000 [Supelco, Bellefonte, PA])14. The thermal desorption tubes were hermetically sealed immediately after collection and stored at 4 °C for up to two weeks until analysis. A total of 16 samples were taken during this project.
Thermal imaging
Thermal images were taken at each volatile molecule sampling time point using a FLIR E60 thermal camera (FLIR, Wilsonville, OR) with a focal length of 18 mm and an exposure time of 1/59 s. For each image, the camera was placed at the same two locations. At each location, two angles of elevation were used, leading to four thermal images being taken at each time point.
Analytical instrumentation
The volatile molecules concentrated on the thermal desorption tubes were analyzed by a Pegasus 4D (LECO Corporation, St. Joseph, MI) GC × GC-TOFMS instrument with an Agilent 6890 GC equipped with a Gerstel Thermal Desorption Unit (TDU), a Gerstel CIS 4 cooled injection system, and a Multi-Purpose Sampler (Gerstel, Linthicum Heights, MD). The TDU desorption temperature was programmed to increase from 50 °C (held for 0.5 min) to 300 °C (held for 5 min) at 7 °C/min, and desorbed molecules were cryo-focused at −30 °C in the CIS inlet. Analytes were then introduced into the capillary column by ramping the CIS inlet temperature from −30 °C (held for 3 min) to 330 °C (held for 3 min) at 12 °C/s in splitless mode.
Chromatographic analysis was performed using a Rxi-624Sil (60 m × 250 μm × 1.4 μm) as the first dimension (1D)-GC column and a Stabilwax (1 m × 250 μm × 0.5 μm) as the second dimension (2D)-GC column, both purchased from Restek (Bellefonte, PA). High purity helium (Airgas, Radnor, PA) was used as the carrier gas with a flow rate at 3 mL/min. The primary oven was held at 40 °C for 2 min, and then increased to 235 °C (held for 3 min) at a rate of 3 °C/min. The temperature offsets were + 5 °C for the secondary oven and + 20 °C for the modulator. The modulation time was 2.5 s total, alternating 0.85 s hot and 0.40 s cold pulses. TOFMS was employed as a detector, with electron ionization at 70 eV, ion source maintained at 200 °C, and mass range of 40–400 m/z monitored at an acquisition rate of 150 spectra/s. Data acquisition and analysis were performed using ChromaTOF software, version 4.70.7.0 (LECO Corporation).
Processing and analysis of chromatographic data
Chromatographic data were processed and aligned using ChromaTOF. For peak finding, a signal-to-noise cutoff (S/N) was set at 100:1 (a minimum of three apexing masses) in at least one chromatogram and a minimum of 50:1 S/N in all others. The resulting peaks were identified by a forward search of the NIST 2011 library. For putative peak identification, a forward match score of ≥ 850 (of 1000) was required. For the alignment of peaks across the chromatograms, the maximum 1D and 2D retention time deviations were set at 7.5 s and 0.15 s, respectively, and the inter-chromatogram spectral match threshold was set at 600. The molecules eluting prior to 200 s in the first dimension and 0.4 s in the second dimension were removed prior to statistical analysis using the Classification feature in ChromaTOF15. A data cleaning step was performed to remove suspected chromatographic artifacts and common environmental contaminants, as defined previously16.
Statistical analyses
All statistical analyses were performed using R v3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). Prior to statistical analyses, the relative abundance of molecules across chromatograms was normalized using probabilistic quotient normalization (PQN)17 and peak intensities were log-transformed, mean-centered, and then unit-scaled. The Mann–Whitney U test18 was used to select volatile molecules that were statistically significantly different between the flowering phases, with a p-value of 0.05 as the threshold for statistical significance.
Ethics statement
We declare that the work reported here is consistent with the IUCN Policy Statement on Research Involving Species at Risk of Extinction.
Results and discussion
Observation of temperature change during flowering
The A. titanum (Fig. 1a–c) was sampled for temperature and volatile molecules over four days. Thermal profiling was used to help determine the onset and end of the female and male flowering periods12,13 (Fig. 1d, e). The highest female and male flowering temperatures were 35.6 °C and 33.2 °C, respectively. The maximum measured temperature during male flowering was 2.8 °C lower than expected13, either due to our 2 h sampling window being too coarse, causing us to miss the maxima, or due to a small window cut by the greenhouse staff to conduct hand pollination. The designation of the onset and end of the female and male flowering is projected onto Fig. 1f. The volatile molecules sampling was staged into three categories: pre-flowering, female flowering, and male flowering. All other samples were considered transition and not included in statistical analyses.
Temperature and odor changes during A. titanum flowering: Image of A. titanum (a) before flowering, (b) during flowering, and (c) male and female flowers; (d) thermal image of the spadix during the female flowering phase; (e) thermal image of the florets during the male flowering phase; (f) temperature profile of the spadix and the male flowers. The numbers 1–16 represent the samples collected at different time points.
Volatile profiles of A. titanum during the pre-flowering and the flowering phases
A total of 422 volatile features were detected in all 16 samples collected during the pre-flowering and the flowering phases of A. titanum. Of these, 118 features were statistically significantly different between the pre-flowering and the flowering phases (p ≤ 0.05), of which 26 features could be assigned putative names (Table 1).
Dominant volatile molecules exuded during the female flowering phase
Eight volatile features were statistically significantly different between the pre-flowering and the female flowering samples (p ≤ 0.05). Putative names could be assigned to six features (Table 1), of which one feature, identified as limonene, has been detected for the first time in A. titanum. The change in abundance of these six molecules over the entire sampling period is visualized using a heat map (Fig. 2), which shows five of the six being more abundant during female flowering, with sulfur-containing molecules dominating.
During the female flowering phase, dimethyl disulfide (DMDS) was present in the highest abundance. DMDS was also detected, albeit at a lower intensity and infrequently, in the pre-flowering and the male flowering phase (Fig. 2). DMDS has been previously reported as a dominant chemical component of A. titanum scent, with an odor generally described as garlic-like8,9,10,19. Dimethyl trisulfide (DMTS) was present in the second highest relative abundance, with peak emission during the female flowering phase and decreasing during the female to male transition. DMTS was not detected during the male flowering phase (Fig. 2). DMTS’s decayed cabbage, decayed meat and garlic smell is considered a main odor contributor for A. titanum8,10,19 with a very low odor threshold20.
Methyl thiolacetate was the third most abundant molecule, followed by methanethiol and 3-methylbutanal. The level of these three molecules was highest during the female flowering phase and was lower or undetected in the other phases (Fig. 2). Methyl thiolacetate has a cheesy, garlic smell21 and has been detected in the floral scent of A. titanum10. Methyl thiolacetate in combination with DMTS was attractive to adult carrion beetles (Necrophila americana and Oiceoptoma noveboracense)22. Methanethiol (sulfurous odor23) was released less than the other S-containing molecules (Table 1). Methanethiol is the precursor for biosynthesizing DMDS in plants24. 3-Methylbutanal was present in high abundance during the female flowering phase, and remained somewhat abundant thereafter (Fig. 2). 3-Methylbutanal (malty odor23) has been detected in the headspace of the fluid secreted from the spadix of A. titanum during female flowering10. Limonene was most abundant during the pre-flowering period, reaching its maximum just prior to the start of the female flowering phase and then dropping to barely detectable levels thereafter (Fig. 2). Although the citrus-scent of limonene has been detected in the floral scent of other Amorphophallus species, such as A. arnautovii, A. maximus and A. Zenkeri8,9, it is possible that the limonene monitored here came from a geranium flowering nearby at the time25.
One hundred features were statistically significantly different between the female and the male flowering phase samples (p ≤ 0.05). Of these, five features were overlapping with those found statistically significantly different between the pre-flowering and the female flowering samples, and 95 features were found new in this comparison. Of the 100 features, putative names could be assigned to 22 molecules (Table 1), among which seven (S-containing and aldehydes) molecules were found dominant in the female flowering phase (Fig. 2). Among the S-containing molecules, DMDS, DMTS, methyl thiolacetate and methanethiol, which were dominant in the female flowering phase in comparison to the pre-flowering phase, remained statistically significant and dominant in the female flowering phase in comparison to the male flowering phase, with the presence of dimethyl sulfide (cabbage-like odor23) being a new addition to this family. DMDS and methanethiol were present in lower abundances during the male flowering period but still likely contributing to the rotting/dead animals smell during that period as they have a low odor threshold. Aldehyde emission patterns were similar to the S-containing molecules, and had higher abundances in the female flowering phase. Nonanal (citrus and fatty odor26) and benzaldehyde (almond odor26) have been found previously in the appendix of A. titanum21. Benzaldehyde has been found attractive to Diabrotica virgifera beetle27.
Dominant volatile molecules exuded during the male flowering phase
Among the 22 putatively identified molecules, which were statistically significantly different between the female and the male flowering samples, 15 molecules were found dominant in the male flowering phase. Their abundance is visualized in Fig. 2, with alcohols and hydrocarbons dominating.
Within the alcohol group, 2-methyl-1-propanol, amylene hydrate, 2-butanol, 1-butanol and 3-methyl-3-buten-1-ol were the new volatiles identified in A. titanum during this study. 2-Methyl-1-propanol (isobutanol), which smells like sweet alcohol28, was present at the highest relative abundance, followed by amylene hydrate (tert-amyl alcohol; camphoraceous odor29), 3-methyl-3-buten-1-ol (isoprenol; fruity odor), 2-butanol (fruity odor) and 1-butanol (fruity odor). Various species of social wasps in the Vespula and Dolichovespula genera (yellow jackets) are attracted to 2-methyl-1-propanol, and the attraction extends to paper wasps (Polistes fuscatus) when 2-methyl-1-propanol is combined with acetic acid30. 3-Methyl-3-buten-1-ol (isoprenol) is a known metabolite of isopentenyl pyrophosphate, an isoprenoid precursor in the biosynthesis of terpenes and terpenoids in plants31,32. 2-Butanol and 1-butanol have been reported as the main constituents in the odor profile of A. pilosus and A. henryi, respectively20,33. 2-Butanol is a known aggregation pheromone of the male rhinoceros beetle, a coconut tree pest34. It has also been found as one of the attractants for two beetles, Parastasia bimaculata and Chaloenus schawalleri (Chrysomelidae)35. P. bimaculata is known to be attracted to heat-generating, odor-producing flowers, whereas C. schawalleri is less selective about the heat-generation. 1-Butanol is among the most abundant molecules in Petunia integrifolia and P. secreta pollens that attracts short-tongued bees of family Halictidae36.
The hydrocarbons are the second largest group among the named molecules. The long-chain n-alkanes (C13, C14, C25 to C28) have been reported as being produced by the female flowers of A. titanum previously37. This is, however, the first time the volatile presence of the branched chain hydrocarbons (Table 1) is being reported for A. titanum, which includes 2-methylhexane, 2,4-dimethylhexane, 2,4-dimethyl-1-heptene, 3-methylhexane, 2,4-dimethylheptane and 2-methyl-1-pentene. Some of these branched hydrocarbons have, however, been detected as volatiles produced by other plants38,39,40,41. 2-Methylhexane has been found in the volatile profile of a rose variety, Red Eagle40. 2,4-Dimethylhexane and 2,4-dimethyl-1-heptene have been identified in the volatile profiles of Gentiana asclepiadea leaves39 and flesh of Citrus grandis fruit38. 3-Methylhexane was also found, in lesser amount, in the pre-flowering phase. 3-Methylhexane is reported to be induced as a defense volatile molecule from an infested cotton plant (Gossypium hirsutum) against a green lacewing predator, Chrysoperla lucasina40.
Among ethers, ketones, esters and N-containing molecules, tetrahydro-2,2,5,5-tetramethylfuran, 2-methyl-2-(1-methylethoxy)propane, 2-methyl-1-propyl formate, 2-propenenitrile and 2-pentanone were the new molecules identified in A. titanum. Tetrahydro-2,2,5,5-tetramethylfuran is a known volatile molecule produced by the fungi Cladosporium cladosporioides that promotes plant growth42. 2-Propenenitrile has been detected as released by enophytic fungi, showing antifungal activity against Monilinia fructicola, a fungal plant pathogen43. It is possible that these two molecules monitored here were produced by microorganisms or perhaps produced by the plant itself. 2-Pentanone is the only ketone identified in the male flowering phase, and has been reported in the inflorescence odors of other Amorphophallus species, such as A. borneensis, A. eichleri and A. obscurus44.
The statistical analysis between the male flowering and the pre-flowering samples showed that 74 features were statistically significantly different (p ≤ 0.05). Of these, two features were overlapping with those found statistically significantly different between the pre-flowering and the female flowering samples, 57 features were overlapping with the statistically significantly different features between the female flowering and the male flowering samples, and 15 features were found new in this comparison. Of the 74 features, putative names could be assigned to seven molecules (Table 1) which were present at higher amounts in the male flowering phase, with alcohols and hydrocarbons dominating. Among these molecules, 2-butanol, 3-methylhexane, 2-methyl-2-(1-methylethoxy)propane, 3-methyl-3-buten-1-ol and 2,4-dimethylheptane were also found dominant in the male flowering phase during the comparison with the female flowering phase. 2-Methyl-1-pentene and 3-methyl-1-butanol were the additional molecules found dominant in the male flowering phase, and have been detected for the first time in the volatile profile of A. titanum. 2-Methyl-1-pentene was present at the highest relative abundance among all the hydrocarbons. 3-Methyl-1-butanol (isoamyl alcohol; fruity odor) has been reported previously in the inflorescence odors of A. borneensis, A. commutatus and A. konjac, as well as Aristolochia microstoma flowers44,45.
Some studies have shown that the inflorescence of a plant produces different smells when injured46. In this study, before sample 8 was collected, a small window was cut into the bottom of the spathe of A. titanum for the greenhouse staff to conduct hand pollinations (Fig. 1e). After the window was made, production of the S-containing molecules and aldehydes dropped sharply, though they increased subsequently. The intensity of most of the alcohols and hydrocarbons increased instead, before being reduced to much lower levels, and rising again during the male flowering phase. It was not clear whether the injury was the cause for the sudden change of volatiles in sample 10.
Other volatile features of interest
In addition, 304 other features were present throughout the flowering period of A. titanum, with hydrocarbons, aldehydes and ketones dominating. These statistically non-significant molecules, also likely contribute to the odors emitted during the pre-flowering and the flowering phases. Putative names could be assigned to 19 molecules in this group as shown in Table 2, of which 14 molecules were found for the first time in A. titanum during this study. These include, benzene, octane, 3-tridecene, butyl benzene, pentyl benzene, hexyl benzene, hexanal, octanal, pentanal, heptanal, acetone, 2,3-butanedione, 2-butanone and tetrahydrofuran.
The hydrocarbons mainly consisted of n-alkanes and alkylated benzenes (cyclic hydrocarbons), with toluene present at the highest abundance (Table 2). Toluene and heptane have been previously found in the scent of A. titanum inflorescence, with glue solvent-like and gasoline-like smell, respectively9. Toluene was also detected in the pollen aroma of Petunia integrifolia and Petunia secreta36. Benzene has been detected, in a low amount, in the floral scent of Gelsemium sempervirens47. Octane has been found among the aroma components of sweet cherry (Prunus avium L.) flower essential oils48. 3-Tridecene and pentylbenzene have been reported in the essential oil of Haplophyllum tuberculatum leaves and stem49. Butylbenzene has been detected in the volatile profiles of Gentiana asclepiadea flowers39. Pentylbenzene is reported as a component in the essential oil of aerial parts of Kellusia odoratissima (wild celery) ecotypes50.
Among the aldehydes, 2-propenal (acrolein) was present at the highest abundance (Table 2). It has been found as one of the indicators of A. titanum inflorescence blooming51. Hexanal (citrus, orange odor52) has been reported as a minor volatile molecule in A. pilosus33,44. Octanal, along with heptane, 3-methylhexane, nonanal, octanal and other volatiles, has been identified as a semiochemical produced in Gossypium hirsutum for protection against predatory Chrysoperla species41. Octanal has also been found in the essential oil of H. tuberculatum aerial parts, along with 3-tridecene and pentylbenzene49. Pentanal (valeraldehyde) has been found in ‘Fuego’ variety of rose40, along with hexanal, heptanal and octanal among other volatile molecules. Butanal (butyraldehyde; rancid, sweaty odor) has been reported in the floral odors from A. titanum23. Heptanal (citrus, orange odor52) is released in caterpillar-infested plant, Arabidopsis thaliana, along with 1-butanol, 2-pentanone, nonanal, heptanal and other volatiles, to attract Cotesia rubecula parasitoid in the defense mechanism against Pieris rapae caterpillars53.
Acetone was found at the highest abundance among all the statistically non-significant molecules (Table 2). It has been reported in various other Amorphophallus species, such as A. borneensis, A. commutatus, A. eburneus, A. erythrrorhachis, A. henryi, A. konjac, A. macrorhizus, A. plicatus and A. tinekeae33,44. 2-Butanone has been identified as a scent molecule in A. konjac33. 2,3-Butanedione (butter-like odor), along with heptanal and hexanal, has been found among the contributors to Symplocarpus renifolius floral scent during the female, bisexual, and male flowering phases52. Ethanol was the only alcohol identified throughout the flowering phase of A. titanum in the present study. It was previously found in the scent profile of other Amorphophallus species, such as A. cirrifer, A. konjac and A. obscurus33,44. Ethanol has also been detected among the major volatiles present in the pollen aroma of Petunia integrifolia and Petunia secreta36.
Study limitations
Samples in this study were from only one flowering A. titanum which was located in a greenhouse filled with other plants, a small number of which were concurrently flowering. In this case, isolating the plant from others was not possible, however, once the plant started flowering, we are reasonably confident that the sampling was dominated by A. titanum sources based on the proximity of the sampler and the natural convection from thermogenesis. We note also that the greenhouse was illuminated by a warm, strong (100 W) light bulb each night, which may have impacted the plant’s behavior. Importantly, while we reached level 2 of the naming convention outlined by the Metabolomics Standards Initiative (MSI)54,55, the molecules listed herein are only putative identities, which need to be verified using authentic standards.
Conclusion
The present study represents the first time series analysis of the volatile molecules emitted by A. titanum using GC × GC-TOFMS. By analyzing 16 samples collected over the flowering period, 422 volatile features were detected. Of these, 118 features were statistically significantly different between the pre-flowering phase and both flowering phases. A total of 26 of these molecules were assigned with putative identifications based on their mass spectral matching, of which 18 molecules are first time ever reported in A. titanum. The female flowering phase was dominated by the S-containing molecules and aldehydes, and the alcohols and hydrocarbons were dominant during the male flowering phase. While the S-containing molecules are the major contributors to the foul odor emitted during the female flowering phase, the aldehydes, alcohols, hydrocarbons and ketones also contribute to the unpleasant odor of the corpse flower. Some of these volatile molecules have been reported to play an important role in plant–insect interactions, and could be responsible for attracting the pollinators and inducing the defense mechanisms in A. titanum. The additional 304 features were found present during the entire flowering period, and 19 of these molecules were assigned putative names which also likely contribute to the putrid odor of the corpse flower. Of these 19 molecules, 14 molecules are the newly identified in A. titanum in this study. This study demonstrated that A. titanum not only emitted odorants in the female flowering phase, but also a large number and wide variety of volatile molecules in the male flowering phase.
Data availability
The datasets generated and/or analyzed during the current study are available on reasonable request to the corresponding author.
References
van der Kooi, C. J., Vallejo-Marin, M. & Leonhardt, S. D. Mutualisms and (A)symmetry in plant–pollinator interactions. Curr. Biol. 31, R91–R99 (2021).
Gandawijaja, D., Idris, S., Nasution, R., Nyman, L. P. & Arditti, J. Amorphophallus titanum Becc.: a historical review and some recent observations. Ann. Bot. 51, 269–278 (1983).
Latifah, D., Hartini, S. & Wawangningrum, H. Hand pollination of the giant corpse flower in the Bogor botanic gardens. Int. J. Conserv. Sci. 4, 1153–1160. https://doi.org/10.1093/oxfordjournals.aob.a086467 (2016).
Keithley, J. K. et al. Safety and efficacy of glucomannan for weight loss in overweight and moderately obese adults. J. Obes. 2013, 1–7. https://doi.org/10.1155/2013/610908 (2013).
Vuksan, V. et al. Konjac-Mannan (Glucomannan) improves glycemia and other associated risk factors for coronary heart disease in type 2 diabetes. Diabetes Care 22, 913–919. https://doi.org/10.2337/diacare.22.6.913 (1999).
Giordano, C. Observations on Amorphophallus titanum (Becc.) Becc. ex Arcangeli in the forest of Sumatra. Aroideana. 22, 10–19 (1999).
Attenborough, D. The Private Life of Plants (Princeton University Press, 1995).
Kite, G. C. & Hetterscheid, W. L. A. Inflorescence odours of Amorphophallus and Pseudodracontium (Araceae). Phytochem. 46, 71–75. https://doi.org/10.1016/S0031-9422(97)00221-5 (1997).
Kite, G. C. et al. Inflorescence odours and pollinators of Arum and Amorphophallus (Araceae). In Reproductive Biology (eds. Owens, S. J. & Rudall, P. J.) 295–315 (Royal Botanic Gardens, 1998).
Shirasu, M. et al. Chemical identity of a rotting animal-like odor emitted from the inflorescence of the titan arum (Amorphophallus titanum). Biosci. Biotechnol. Biochem. 74, 2550–2554. https://doi.org/10.1271/bbb.100692 (2010).
Amoore, J. E. Specific anosmia and the concept of primary odors. Chem. Senses. 2, 267–281. https://doi.org/10.1093/chemse/2.3.267 (1977).
Barthlott, W., Szarzynski, J., Vlek, P., Lobin, W. & Korotkova, N. A torch in the rain forest: thermogenesis of the Titan arum (Amorphophallus titanum). Plant Biol. 11, 499–505. https://doi.org/10.1111/j.1438-8677.2008.00147.x (2009).
Korotkova, N. & Barthlott, W. On the thermogenesis of the Titan arum (Amorphophallus titanum). Plant Signal. Behav. 4, 1096–1098. https://doi.org/10.4161/psb.4.11.9872 (2009).
Franchina, F. A., Purcaro, G., Burklund, A., Beccaria, M. & Hill, J. E. Evaluation of different adsorbent materials for the untargeted and targeted bacterial VOC analysis using GC×GC-MS. Anal. Chim. Acta. 1066, 146–153. https://doi.org/10.1016/j.aca.2019.03.027 (2019).
Bean, H. D., Hill, J. E. & Dimandja, J. D. Improving the quality of biomarker candidates in untargeted metabolomics via peak table-based alignment of comprehensive two-dimensional gas chromatography-mass spectrometry data. J. Chromatogr. A. 1394, 111–117. https://doi.org/10.1016/j.chroma.2015.03.001 (2015).
Mellors, T. R., Rees, C. A., Wieland-Alter, W. F., von Reyn, C. F. & Hill, J. E. The volatile molecule signature of four mycobacteria species. J. Breath Res. 11, 1–16. https://doi.org/10.1088/1752-7163/aa6e06 (2017).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290. https://doi.org/10.1021/ac051632c (2006).
Mann, H. B. & Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60 (1947).
Fujioka, K. et al. Objective display and discrimination of floral odors from Amorphophallus titanum, bloomed on different dates and at different locations, using an electronic nose. Sensors. 12, 2152–2161. https://doi.org/10.3390/s120202152 (2012).
Vazquez-Landaverde, P. A., Torres, J. A. & Qian, M. C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography–pulsed flame photometric detection. J. Dairy Sci. 89, 2919–2927. https://doi.org/10.3168/jds.S0022-0302(06)72564-4 (2006).
Chen, S., Sha, S., Qian, M. & Xu, Y. Characterization of volatile sulfur compounds in Moutai liquors by headspace solid-phase microextraction gas chromatography-pulsed flame photometric detection and odor activity value. J. Food Sci. 82, 2816–2822. https://doi.org/10.1111/1750-3841.13969 (2017).
Trumbo, S. T. & Dicapua, J. A. A synergism between dimethyl trisulfide and methyl thiolacetate in attracting carrion-frequenting beetles demonstrated by use of a chemically-supplemented minimal trap. EEB Articles. 46, 28. https://doi.org/10.1007/s00049-020-00330-4 (2020).
Milo, C. & Grosch, W. Detection of odor defects in boiled cod and trout by gas chromatography-olfactometry of headspace samples. J. Agric. Food Chem. 43, 459–462. https://doi.org/10.1021/jf00050a038 (1995).
Schmidt, A., Rennenberg, H., Wilson, L. G. & Filner, P. Formation of methanethiol from methionine by leaf tissue. Phytochem. 24, 1181–1185 (1985).
Ilić, M. D. et al. Chemical composition of volatiles of eight Geranium L. species from Vlasina plateau (South Eastern Serbia). Chem. Biodiversity. 17, 1–9. https://doi.org/10.1002/cbdv.201900544 (2020).
Yang, D. S., Shewfelt, R. L., Lee, K. S. & Kays, S. J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 56, 2780–2787. https://doi.org/10.1021/jf072685t (2008).
Cosse, A. A. & Baker, T. C. Electrophysiologically and behaviorally active volatiles of buffalo gourd root powder for corn rootworm beetles. J. Chem. Ecol. 25, 51–66. https://doi.org/10.1023/A:3A1020885032397 (1999).
Peinado, R. A., Moreno, J., Bueno, J. E., Moreno, J. A. & Mauricio, J. C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 84, 585–590. https://doi.org/10.1016/S0308-8146(03)00282-6 (2004).
Amoore, J. E. & Venstrom, D. Sensory analysis of odor qualities in terms of the stereochemical theory. J. Food Sci. 31, 118–128. https://doi.org/10.1111/j.1365-2621.1966.tb15424.x (1966).
Reed, H. C. & Landolt, P. J. Trap response of Michigan social wasps (Hymenoptera: Vespidae) to the feeding attractants acetic acid, isobutanol, and heptyl butyrate. Gt. Lakes Entomol. 35, 71–77 (2002).
Chang, W., Song, H., Liu, H. & Liu, P. Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 17, 571–579. https://doi.org/10.1016/j.cbpa.2013.06.020 (2013).
Ma, D. et al. Overexpression of a type-I isopentenyl pyrophosphate isomerase of Artemisia annua in the cytosol leads to high arteannuin B production and artemisinin increase. Plant J. 91, 466–479. https://doi.org/10.1111/tpj.13583 (2017).
Claudel, C. & Lev-Yadun, S. Odor polymorphism in deceptive Amorphophallus species – a review. Plant Signal. Behav. 16, e19917121. https://doi.org/10.1080/15592324.2021.1991712 (2021).
Rochat, D. et al. Activity of male pheromone of Melanesian Rhinoceros beetle Scapanes australis. J. Chem. Ecol. 28, 479–500. https://doi.org/10.1023/a:1014531810037 (2002).
Kumano-Nomura, Y. & Yamaoka, R. Beetle visitations, and associations with quantitative variation of attractants in floral odors of Homalomena propinqua (Araceae). J. Plant Res. 122, 183–192. https://doi.org/10.1007/s10265-008-0204-6 (2009).
Rodrigues, D. M. et al. Do we truly understand pollination syndromes in Petunia as much as we suppose?. AOB Plants. 10, 1–15. https://doi.org/10.1093/aobpla/ply057 (2018).
Raman, V., Tabanca, N., Demirci, B. & Khan, I. A. Studies on the floral anatomy and scent chemistry of titan arum (Amorphophallus titanum, Araceae). Turk. J. Bot. 41, 63–74. https://doi.org/10.3906/BOT-1604-34 (2017).
Chung, H. et al. Characterization of volatile aroma-active compounds in Dangyooja (Citrus grandis Osbeck). J. Korean Soc. Appl. Biol. Chem. 55, 133–136. https://doi.org/10.1007/s13765-012-0023-2 (2012).
Georgieva, E., Handjieva, N., Popov, S. & Evstatieva, L. Comparative analysis of the volatiles from flowers and leaves of three Gentiana species. Biochem. Syst. Ecol. 33, 938–947. https://doi.org/10.1016/j.bse.2005.01.002 (2005).
Gil, C. S. et al. Volatile content variation in the petals of cut roses during vase life. Sci. Hortic. 261, 1–6. https://doi.org/10.1016/j.scienta.2019.108960 (2020).
Hegde, M. et al. Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the Cotton Aphid, Aphis gossypii. J. Chem. Ecol. 37, 741–750. https://doi.org/10.1007/s10886-011-9980-x (2011).
Paul, D. & Park, K. S. Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth. Sensors. 13, 13969–13977. https://doi.org/10.3390/s131013969 (2013).
Pimenta, R. S., da Silva, J. F. M., Buyer, J. S. & Janisiewicz, W. J. Endophytic fungi from Plums (Prunus domestica) and their antifungal activity against Monilinia fructicola. J. Food Prot. 75, 1883–1889. https://doi.org/10.4315/0362-028x.jfp-12-156 (2012).
Kite, G. C. & Hetterscheid, W. L. A. Phylogenetic trends in the evolution of inflorescence odours in Amorphophallus. Phytochem. 142, 126–142. https://doi.org/10.1016/j.phytochem.2017.06.006 (2017).
Rupp, T. Flowers of deceptive Aristolochia microstoma are pollinated by Phorid flies and emit volatiles known from invertebrate Carrion. Front. Ecol. Evol. 9, 1–11. https://doi.org/10.3389/fevo.2021.658441 (2021).
Knutson, R. M. Plants in heat. Nat. Hist. 88, 42–47 (1979).
Johnson, O. B., Golonka, A. M., Blackwell, A., Vazquez, I. & Wolfram, N. Floral scent variation in the Heterostylous species Gelsemium sempervirens. Molecules 24, 1–16. https://doi.org/10.3390/molecules24152818 (2019).
Zhang, H., Yan, H., Li, Q., Lin, H. & Wen, X. Identification of VOCs in essential oils extracted using ultrasound- and microwave-assisted methods from sweet cherry flower. Sci. Rep. 11, 1–13. https://doi.org/10.1038/s41598-020-80891-0 (2021).
Hamdi, A. et al. In vitro antileishmanial and cytotoxicity activities of essential oils from Haplophyllum tuberculatum A. Juss leaves, stems and aerial parts. BMC Complement. Altern. Med. 18, 1–10. https://doi.org/10.1186/s12906-018-2128-6 (2018).
Shojaei, Z. A., Ebrahimi, A. & Salimi, M. Chemical composition of three ecotypes of wild celery (Kelussia odoratissima). 17, 62–68. https://doi.org/10.1080/10496475.2011.560089 (2011).
Latifah, D., Wawangningrum, H., Hartini, S. & Munawaroh, E. How to predict the blooming of the giant corpse inflorescence Amorphophallus titanum (Becc.) Becc. Ex Arcang. Berita Biologi 14, 111–120. https://doi.org/10.14203/BERITABIOLOGI.V14I2.1815 (2014).
Oguri, S., Sakamaki, K., Sakamoto, H. & Kubota, K. Compositional changes of the floral scent volatile emissions from Asian skunk cabbage (Symplocarpus renifolius, Araceae) over flowering sex phases. Phytochem. Anal. 30, 139–147. https://doi.org/10.1002/pca.2799 (2019).
Van Poecke, R. M. P., Posthumus, M. A. & Dicke, M. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 27, 1911–1928. https://doi.org/10.1023/a:1012213116515 (2001).
Salek, R. M., Steinbeck, C., Viant, M. R., Goodacre, R. & Dunn, W. B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience. 2, 1–3. https://doi.org/10.1186/2047-217X-2-13 (2013).
Fiehn, O. et al. The metabolomics standards initiative (MSI). Metabolomics 3, 175–178. https://doi.org/10.1007/s11306-007-0070-6 (2007).
Acknowledgements
We are grateful to the staff of the Darthmouth College’s Life Sciences Greenhouse, Theresa Barry and Kim DeLong, for giving us access to the greenhouse for sample collection.
Author information
Authors and Affiliations
Contributions
J.E.H. conceived the idea, designed the study, designed the experiments undertaken, and provided funding for all authors. J.E.H., K.W. and D.K. collected the plant samples, images and odor perception data. L.K. performed chemometrics and statistical analyses. L.K. and J.K. analyzed the data. J.E.H., L.K. and J.K. wrote the manuscript. All authors reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, L., Kaur, J., Winkeler, K. et al. How the volatile organic compounds emitted by corpse plant change through flowering. Sci Rep 13, 372 (2023). https://doi.org/10.1038/s41598-022-27108-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27108-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.