Abstract
Congenital Diaphragmatic Hernia (CDH) is a diaphragm defect associated with lung hypoplasia and ventilation inhomogeneity (VI). The affected neonates are usually born with respiratory failure and require mechanical ventilation after birth. However, significant interindividual VI differences make ventilation difficult. So far, there are no clinical methods of VI assessment that could be applied to optimize ventilation at the bedside. A new VI index is a ratio of time constants T1/T2 of gas flows in both lungs. Pressure-controlled ventilation simulations were conducted using an infant hybrid (numerical-physical) respiratory simulator connected to a ventilator. The parameters of the respiratory system model and ventilator settings were based on retrospective clinical data taken from three neonates (2, 2.6, 3.6 kg) treated in the Paediatric Teaching Clinical Hospital of the Medical University of Warsaw. We searched for relationships between respiratory system impedance (Z) and ventilation parameters: work of breathing (WOB), peak inspiratory pressure (PIP), and mean airway pressure (MAP). The study showed the increased VI described by the T1/T2 index value highly correlated with elevated Z, WOB, PIP and MAP (0.8–0.9, the Spearman correlation coefficients were significant at P < 0.001). It indicates that the T1/T2 index may help to improve the ventilation therapy of CDH neonates.
Similar content being viewed by others
Introduction
Congenital diaphragmatic hernia (CDH) occurs in 1 of 2500–3000 live births and has a high mortality of 22–62% in severe forms1,2. The vast majority of CDH newborns require immediate mechanical ventilation. The main goal of the therapy is to establish gentle, lung-protective, yet effective ventilation. As the degree of lung hypoplasia and ventilation inhomogeneity (VI) is highly variable in particular patients, ventilation therapy requires an individualized approach.
VI can be defined as non-uniform, uneven ventilation of both lungs or different lung compartments. It may result from asymmetrical airway structure causing different airway resistances and/or lung compliances, non-uniform pulmonary tissue leading to disturbances in convective and diffusive gas transport, as well as ventilation/perfusion mismatch. In minor lung hypoplasia and VI, ventilation may not present any difficulties. In the most severe cases with only one functional lung (often underdeveloped), achieving acceptable gas exchange is extremely challenging. In CDH, the compressed, ipsilateral lung is smaller than expected, with less developed respiratory structures and blood vessels3. Both lungs are hypoplastic and differ in resistive-compliance properties that causes VI. Too aggressive ventilation may lead to ventilatory-induced lung injury (VILI) and increased morbidity and mortality; particularly pneumothorax is an independent risk factor of mortality4,5,6. Because of that, protective lung ventilation with limited peak inspiratory pressure (PIP) up to 25 ± 2 cmH2O, positive end-expiratory pressure (PEEP) of 3–5 cmH2O, high respiratory rates (RR) of 40–60 bpm, permissive hypercapnia (pCO2: 50–70 mmHg) and acceptance of pre-ductal SaO2 targets of 85–95% reduces the risk of VILI and improves survival in this group of patients6,7.
Despite these general recommendations there are no accurate and objective clinical methods of assessment of lung hypoplasia and VI. Therefore, the treating physician has very limited tools to adjust ventilator settings to the individual patient’s lung pathology8.
In our previous pilot study9, we proposed a new index of VI—T1/T2 that is a ratio of time constants of gas flows in both lungs (R1·C1/R2·C2), and we put forward some ideas on how it can be used in clinical conditions. The T1/T2 index pertains to the two-compartments of the RLC (R—resistance, L—inertance, C—compliance) respiratory system model10, numerically implemented in the Infant Hybrid Respiratory Simulator (IHRS)10,11.
This study aimed to assess the application of the T1/T2 index as a tool to optimize ventilation therapy of CDH neonates under laboratory conditions and based on retrospective clinical data obtained from three babies treated in the Paediatric Intensive Care Unit (PICU) of the Department of Paediatric Anaesthesiology and Intensive Therapy, in the Paediatric Teaching Clinical Hospital of the Medical University of Warsaw. The relationships between respiratory system impedance (Z), work of breathing (WOBvt), peak inspiratory pressure (PIP) and mean airway pressure (MAP) obtained during conventional mechanical ventilation (CMV), and the T1/T2 index for different respiratory system parameters and ventilation settings, were examined.
The study hypotheses were as follows:
-
there is a significant correlation between respiratory system impedance (Z) and VI degree (T1/T2)
-
there is a significant correlation between WOB, PIP, MAP and VI degree (T1/T2)
-
the correlation coefficients depend on respiratory rate (RR)
-
the correlation coefficients depend on the ratio Cw/CL between chest wall compliance (Cw) and lung compliance (CL).
Study design
The simulation study using the Infant Hybrid Respiratory Simulator (IHRS) was conducted at the Nalecz Institute of Biocybernetics and Biomedical Engineering, Polish Academy of Sciences (IBBE PAS), Warsaw, Poland.
The ventilation parameters of the three enrolled neonates were collected in the Paediatric Intensive Care Unit (PICU) of the Department of Paediatric Anaesthesiology and Intensive Therapy at the Paediatric Teaching Clinical Hospital of the Medical University of Warsaw, Poland. Following the current Polish law and the Declaration of Helsinki, the study was registered by the Bioethics Committee of the Medical University of Warsaw (AKBE/141/22).
Statement on Bioethics Committee approval and informed consent
Ethical aproval was requested from the Bioethics Committee of the Medical University of Warsaw. However, it was judged by the committee as not required as the data collection on ventilation parameters was retrospective. The committee decided the study did not fulfil the criteria of the medical experiment. As a result, also the informed consent of the parents of ventilated babies was waived by the Bioethics Committee.
Study population
The demographic and clinical characteristics of the three enrolled patients with isolated CDH were included in Table 1.
Children involved in the study were assessed for eligibility criteria.
The inclusion criteria are listed below:
Newborns with isolated (one-side) congenital diaphragmatic hernia;
Patients undergoing ventilator therapy using conventional ventilation;
Patients with a planned hernia repair.
The exclusion criteria are listed below:
Patients undergoing High Frequency Ventilation (HFV);
Patients undergoing ECMO therapy.
In the PICU of the Department of Paediatric Anaesthesiology and Intensive Therapy at the Paediatric Teaching Clinical Hospital of the Medical University of Warsaw, when conventional ventilation fails children are switched to HFV, if HFV also fails we consider ECMO. The study was focused on the CMV that is the recommended first-choice therapy in CDH neonates6,7.
Study outcomes
The parameters of the respiratory system and mechanical ventilation were collected by the anesthesiologist (one of the researchers), from the ventilator monitor at the bedside, in the Paediatric Intensive Care Unit (PICU).
The simulation data of the study were collected in the laboratory of IBBE PAS, where the received data sets were graphically presented and statistically analyzed. The final results were discussed by researchers from both centres.
Outcome measures
The clinical respiratory parameters of the three enrolled neonates were assessed in the stage of patient stabilization, before hernia repair. There were the parameters usually set and measured during conventional ventilation, obtained using Fabian ventilator SW 3.4 (ACUTRONIC Med. Sys. AG, Hirzel, Switzerland).
During laboratory simulations, the same as in clinic respiratory parameters were received by using Puritan Bennet 840 Ventilator, and some additional parameters—using NICO 7300 respiratory monitor, e.g. WOBvt, total R and C of the respiratory system.
The assessed parameters:
-
BW—body weight, in kg;
-
R—total dynamic resistance of the respiratory system, in cmH2O s/l;
-
C—total dynamic compliance of the respiratory system, in ml/cmH2O;
-
MV—minute ventilation, in l;
-
RR—respiratory rate, in bpm;
-
PIP—peak inspiratory pressure, in cmH2O;
-
MAP—mean airway pressure, in cmH2O;
-
PEEP—positive end-expiratory pressure, in cmH2O;
-
I:E—inspiration to expiration time ratio;
-
WOBvt—work of breathing done by a ventilator, in J/l;
-
Z—respiratory system impedance, in cmH2O⋅s/l;
-
Spearman correlations coefficients (Rs) for dependencies: Z vs T1/T2, WOBvt vs T1/T2, PIP vs T1/T2, MAP vs T1/T2, WOBvt vs Z, PIP vs Z, MAP vs Z, WOBvt vs PIP, WOBvt vs MAP;
-
The equations of the curves for these dependencies and determination coefficients (R2).
Laboratory set-up
The laboratory study was carried out using Infant Hybrid Respiratory Simulator (IBBE, PAS, Warsaw, Poland), the Puritan Bennett 840 Ventilator (Medtronic, Fridley, MI, USA) and NICO 7300 respiratory monitor (Respironics Corp., Murrysville, PA, USA) (Fig. 1).
IHRS is a measurement-control platform with LabVIEW Real-Time Professional Development System 2013 (National Instruments Co., Austin, TX, USA) that coordinates the work of the numerical and physical part of the simulator and its interaction with a ventilator (Fig. 1).
A numerical part of the IHRS is based on the equations set of the lung model shown in Fig. 210. Using this model, it is possible to simulate different lung pathologies associated with peripheral and central airway obstruction/ restriction, the changes in Cw and VI10,11.
The physical part of IHRS is the Impedance Transformer of a cylinder-piston construction. It uses AD and DA converters that linearly transform back and forth digital signals of gas pressure and flow, obtained from lung model equations, into real physical signals of pressure and flow.
Simulation study protocol
Based on clinical data (Table 1), the simulations of pressure-controlled ventilation (PCV)—the mode of conventional mechanical ventilation, for different T1/T2 index values (1, 1.5, 2, 3, 4, 5, 7, 10, 100) describing lung inhomogeneity, were carried out with the following ventilator settings: inspiration to expiration ratio (I:E) of 1:1.5–1:2, respiratory rate (RR) of 35, 40, 45, 50 and 55 bpm, and positive end-expiratory pressure (PEEP) equal to 4–6 cmH2O. Minute ventilation (MV) of 0.8 ± 0.1 l was kept constant for different combination settings, for each patient (P1, P2, P3). In Table 2, there are clinical data that were used for ventilator settings.
WOBvt, PIP and MAP were measured using a NICO monitor fitted with the mainstream sensor, neonatal flow adapter and a probe placed between the simulator and Y-piece of the ventilation circuit (Fig. 1). The impedance of the respiratory system (Z) was calculated basing on the total airway resistance and total compliance of the respiratory system measured by NICO.
Statistical analysis
For the dependencies analysed in the study, Spearman correlation coefficients (Rs) were calculated together with their P-levels. The differences between them were tested to check the influence of respiratory rate (RR) and the Cw/CL ratio on the Rs value. All analyses were conducted using the Statistica software (StatSoft, Inc. (2011). STATISTICA (data analysis software system), version 10; www.statsoft.com). A P-value < 0.05 was considered to be statistically significant.
Results
The simulation results are presented in Figs. 3, 4 and 5.
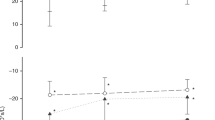
Respiratory system impedance (Z) in relation to VI degree (T1/T2) (a), work of breathing (WOBvt) vs T1/T2 (b), peak inspiratory pressure (PIP) vs T1/T2 (c), and mean airway pressure (MAP) vs T1/T2 (d) for patient P3 (R = 205 cmH2O·s/l; RC = 131 cmH2O·s/l, R1 = R2 = 88 cmH2O s/l; C = 1.14 ml/cmH2O; Cw/CL = 5).
Relationship between work of breathing (WOBvt) and respiratory rate (RR) depending on the T1/T2 index and the Cw/CL ratio in patient P1 (R = 244 cmH2O·s/l, C = 0.8 ml/cmH2O, Cw/CL = 15) (a), P2 (R = 188 cmH2O·s/l, C = 1.26 ml/cmH2O, Cw/CL = 12) (b) and P3 (R = 205 cmH2O·s/l, C = 1.14 ml/cmH2O) with Cw/CL = 5 (c), Cw/CL = 8 (d), and Cw/CL = 12 (e).
Z, WOBvt, PIP and MAP as a function of the T1/T2 index, describing VI, were presented in Fig. 3. As the relationships: Z vs T1/T2, WOBvt vs T1/T2, PIP vs. T1/T2, and MAP vs T1/T2 were highly influenced by the RR values (P < 0.001), separate characteristics for different RR were made. The experimental data were fitted with a second or third-degree polynomial function. The course of the mentioned characteristics was acknowledged by corresponding measurements, performed at the hypothetical value of T1/T2 = 100.
Correlation coefficients for the dependencies: Z vs T1/T2, WOBvt vs T1/T2, PIP vs T1/T2, and MAP vs T1/T2 ranged from 0.7 to 0.9 (P < 0.001). For example, in the patient P3 (R = 205 ml/cmH2O, C = 1.14 ml/cmH2O, see Table 1) at the assumed Cw/CL = 12 and ventilated with RR = 45 bpm, correlation coefficients were as follows: 0.762 (Z vs T1/T2), 0.889 (WOBvt vs. T1/T2), 0.821 (PIP vs T1/T2), 0.756 (MAP vs T1/T2), P < 0.001 (see Supplementary Tables S3–S5 online). Then, at the assumed Cw/CL = 5, correlation coefficients were as follows: 0.948 (Z vs T1/T2), 0.861 (WOBvt vs T1/T2), 0.88 (PIP vs T1/T2), 0.754 (MAP vs T1/T2), P < 0.001 (Fig. 3).
Generally, the T1/T2 index is positively correlated with Z, WOBvt, PIP and MAP, whereas RR is negatively correlated with these ventilation indices. For example, according to our results, when ventilating a patient (R = 205 ml/cmH2O, C = 1.14 ml/cmH2O, Cw/CL = 5.3) with homogenous lungs (T1/T2 = 1) at RR = 50 bpm, the set PIP should amount about 24.4 cmH2O, and required WOBvt—1.58 J/l. However, when T1/T2 raises from 1 to 10, PIP elevates to 27.6 cmH2O (13%), and WOBvt to 1.88 J/l (19%), (P < 0.001), i.e. a significant elevation of PIP and WOBvt is forced by the increase of VI degree.
On the other hand, when changing RR from 50 to 40 bpm, in a patient (R = 205 ml/cmH2O, C = 1.14 ml/cmH2O, Cw/CL = 5) with homogenous lungs (T1/T2 = 1), PIP will increase to 26.5 cmH2O (8.6%), and WOBvt—to 1.73 J/l (9.5%), whereas in a patient with inhomogeneous lungs (T1/T2 = 10), PIP will elevate to 28.8 cmH2O (4.3%) and WOBvt to 1.98 J/l (5.3%).
Strong and significant correlations were received for relationships between WOBvt and PIP (P1: 0.93, P2: 0.95, P3: 0.98, P < 0.001) and WOBvt vs MAP (P1: 0.68, P2: 0.95, P3: 0.73; P < 0.001); see Supplementary Fig. S1and Tables S1–S5 online.
The dependencies between WOBvt, PIP, MAP vs Z for different respiratory rates (RR) were presented in the form of characteristics in Fig. 4. They show that the WOBvt, PIP and MAP are highly correlated with Z. Correlation coefficients ranged from 0.7 to 0.9, e.g. in P3 (Cw/CL = 5) at RR = 45 bpm, there are as follows: 0.849 (WOBvt vs Z), 0.833 (PIP vs Z), 0.769 (MAP vs Z), P < 0.001 (see also Supplementary Tables S1–S5 online).
Figure 5 shows the relationship between WOBvt and RR depending on VI degree (T1/T2) and the Cw/CL ratio (5, 8, 12) for the patient P3 (R = 208 cmH2O s/l and C = 1.14 ml/cmH2O). WOBvt increases with the rise of both—the T1/T2 index and Cw/CL ratio, whereas it decreases with the elevation of RR.
General Spearman correlation coefficients of Z, WOB, PIP, MAP vs T1/T2 obtained for patient P3 were 0.527, 0.491, 0.388 and 0.47 (P < 0.001). The coefficients increased significantly when they were calculated separately for different RR (0.828 ± 0.09; 0.722 ± 0.157; 0.621 ± 0.149; 0.792 ± 0.068), or for a given parameter pair: RR—Cw/CL (0.963 ± 0.017; 0.916 ± 0.046; 0.916 ± 0.031; 0.849 ± 0.121). Therefore, the data in Fig. 5 are presented in the form of the characteristics of RR (35, 40, 45, 50, 55 bpm) assigned to three values of Cw/CL (5, 8, 12).
Discussion
Neonates with inhomogeneous lungs are much more vulnerable to adverse effects of ventilation and VILI than neonates with homogeneous lungs. In the cases with considerable VI, gentle, lung protective ventilation, with adequate tidal volumes and optimal PEEP as well as PIP levels, is difficult to achieve because some lung regions (having short time constants) are rapidly aerated and overdistended, whereas others, having long time constants, are insufficiently aerated and may even collapse (atelectasis)12,13.
The optimal PEEP and PIP should be the minimal pressure created by a ventilator at maximum inspiration and end-expiration delivered to the patient, which is required to reach acceptable levels of arterial partial pressures of O2 and CO2 and with less overdistension and less lung de-recruitment.
Evaluation of lung inhomogeneity can be accomplished using Multi Breath Washout (MBW), Electrical Impedance Tomography (EIT) or Hyperpolarized 3-Helium Magnetic Resonance Imaging (HP 3-He MRI). However, they are hardly accessible in daily clinical practice due to high costs, extensive workload and the problems with standardization9,14,15,16,17,18,19,20.
Kallio et al.21 evaluated the ventilation of neonates suffering from a Respiratory Distress Syndrome (RDS). They noticed that the risk of alveolar overdistention and pneumothorax in neonates with inhomogeneous lungs can be reduced by continuous monitoring of aeration and distribution of ventilation using EIT. They suggested that a steep increase of regional end-expiratory lung impedance (EELZ) rapidly signalises the risk of pneumothorax.
In another study22 we showed adverse phenomena taking place inside the inhomogeneous lungs of CDH infants. We observed high PIP in the more hypoplastic lung (short time constant), and high auto-PEEP in the healthier lung (long time constant) that can lead to air-trapping. The higher the difference between the time constants the higher the PIP (the risk of VILI) and the auto-PEEP.
The results of Pulletz et al.23, in adults with healthy lungs and ARDS, indicated that ARDS patients exhibited shorter time constants regarding the “fast and slow” regions, compared to the healthy controls. Moreover, the dorsal and ventral time constants differed and were significantly influenced by PEEP levels; both decreased with PEEP increase.
Depta, Čandik et al.24,25,26 presented a strategy based on recurrent changes in PEEP levels, enabling recruitment of lung area of long time constants and protecting the lung area of short time constants from overextension and injury. They also proposed the concept of optimal breath frequency, based on time constant measurement24. Their studies in ARDS patients showed that applying this technique led to a significant rise in respiratory system compliance, and improvement in CO2 washout, due to the increase of gas exchange area (lung recruitment)25, in comparison to the conventional mechanical ventilation.
However, Guevorkian et al.27 observed in CDH infants that respiratory system compliance (C) decreased by 30% when PEEP increased from 2 to 5 cmH2O. It is known, that the lower C, the higher values of PIP and MAP are required for effective ventilation. The authors indicated that hypoplastic lungs in CDH are prone to overdistension and have poor tolerance to distending pressures.
The problems with high-value local strains influencing inhomogeneous lungs were reported before by Mead28 and Rausch29. They assessed that the strain forces are much higher in inhomogeneous lungs. From the point of view of a neonatal ventilation, the ratio between chest wall compliance and lung compliance (Cw/CL) is unfavourable, comparing to the older population. It amounts up to 3–6 in infants with healthy lungs30,31,32, whereas it is about 1 in healthy adults. As a result, an increase in tidal volume requires higher pressures in neonates than in adults. This fact, together with lung tissue immaturity, make neonates more vulnerable to VILI that can lead to chronic lung disease (CLD), also called bronchopulmonary dysplasia (BPD) in premature babies.
According to our knowledge, there are no clinical data on the Cw/CL ratio in CDH infants in the literature. Lungs of neonates with CDH are hypoplastic, smaller, with a reduced number of alveoli and compressed by herniated organs occupying the intrathoracic space27. These conditions influence respiratory system compliance (C) that can be several times lower compared to babies with healthy lungs31,32,33,34. Therefore, it might suggest that the Cw/CL ratio should be higher than in healthy neonatal lungs. However, our study did not prove this. On the contrary, the results suggest that the Cw/CL ratio, especially before hernia repair, can be similar or lower than in neonates with healthy lungs (3–6)7,31,32,33,34. It is an important issue that should be studied and explained in the future, especially in the context of the C decrease after operation. The decreased respiratory system compliance (C) is an adverse phenomenon commonly observed after hernia repair35. Currently, it is unclear why this happens. Probably, the herniated organs occupying the thorax before hernia repair, compress not only the lungs, but also push the chest wall outside, limiting the thorax movements and making its muscles stiffer (Cw↓). The distribution of the forces acting within the thorax changes, after the herniated organs are removed from the thoracic to the abdominal cavity. These forces, resulting from the direct pressure of the herniated organs on the lungs and chest wall, stop to act. On the other hand, they may exert strain on the diaphragm from the bottom, indirectly affecting the lungs and the chest wall; however, this influence is limited by the physical barrier created by the diaphragm. The effect may be variable, depending on many factors, such as the initial thorax content (stomach, liver, intestines), the properties of the diaphragmatic muscle itself (anatomy, saturation, the size and exact location of the hole), the method of hernia repairing (stitching or patching with artificial material, e.g. Gore-Tex).
It is known, that liver herniation significantly increases the risk of mortality in CDH infants3,7,34,35. The higher percentage of the liver in the thorax (%LH), the higher the risk of therapy failure. Probably, the higher the %LH, the greater decline of C as a result of the operation can be expected. Sakai35 showed that a higher than 50% decrease in C was associated with mortality. According to Takayasu et al.36, who enrolled 49 children treated for CDH within two decades in one institution, liver-up and patch repair were the main risk factors for long-term complications (it concerned 50% of the patients with liver-up and 36% with patch-repair). In the study conducted by Brandt et al.37, covering the treatment of 66 CDH infants within 16 years, a liver-up position was found in 51% of survivors as opposed to 80% of non-survivors (P < 0.05)38.
Jancelewicz et al.7, based on their long-term cohort study, observed that the patch repair was the most strongly and independently associated parameter with subsequent surgical complications. As shown by Lally et al.38, large hernia size (that requires artificial patch repair) strongly reduces overall survival as well as increases the risk of multiple adverse outcomes.
CDH neonates belong to one of the most complex and demanding patients treated in NICU/PICU (Neonatal/ Paediatric Intensive Care Units). According to the last CDH Euro Consortium and APSA OEBP committee27 gentle ventilation with limited PIP up to 25 ± 2 cmH2O, PEEP of 3–5 cmH2O, with permissive hypercapnia strategy6 is recommended during ventilation of CDH neonates. As a first-line strategy CMV is preferred over HFV.
The results of our study simulating PCV mode in CDH newborns seem to indicate that strong relationships exist between the ratio of time constants of the right and left lung (T1/T2) and ventilation parameters: WOBvt, PIP, and MAP. Increased T1/T2 results in increase in respiratory system impedance (Z), work of breathing (WOBvt) and the airway pressures (PIP and MAP) in the patient-ventilator system. All the above indicate that the T1/T2 index can serve as a lung inhomogeneity measure. Secondly, this index could probably be useful in the prediction of optimal PIP and MAP values at the bedside, corresponding to the degree of lung ventilation inhomogeneity. Thirdly, in selected cases, the use of the above measurements could possibly help to decide on changing ventilation strategy from CMV to HFV; for example using algorithms similar to that proposed by us9. However, further extensive studies are necessary.
Potential clinical implementation of the method in the future
Below, we present graphically how our method could possibly be used in clinical practice. Figure 6 shows two examples of the clinical application of the VI assessment based on the T1/T2 index at the bedside with the use of respiratory parameters available from the ventilator connected to the patient’s respiratory system. First, at the set RR, the R and C measured by the ventilator are found (step 1). Then, using R, C and RR, respiratory system impedance Z is calculated (step 2). Using the Z value, the T1/T2 value is obtained for set RR from the dependence Z = f (T1/T2), in a graphical way (Fig. 6a) or solving the equation Z = f (T1/T2) (Fig. 6b) (step 3). Based on the T1/T2 value, PIP, MAP and WOBvt are assessed, graphically (Fig. 6a) or using relevant equations MAP = f (T1/T2) and WOBvt = f (T1/T2) (Fig. 6b) (step 4). As a result, ventilator settings are adjusted to the VI degree (T1/T2), i.e. adequate PIP is set on the ventilator. If predicted PIP is higher than recommended in CDH, the early, elective change to HFO can be made (step 5).
Examples of clinical application of the VI assessment using T1/T2 index to correct settings of PIP at PCV mode of conventional mechanical ventilation (CMV): method 1 (a), method 2 (b). Inertance (L) was abandoned in the calculation of impedance (step 2) for simplicity; its significance is low at RR < 60 bpm (< 1 Hz).
CDH is a rare neonatal disease, and the collection of a large group of patients is a long-term task, but we are in the course of acquiring more data. Another option we might consider is to conduct an animal model study. However, performing a prospective study in an animal model depends on many factors (economic costs, ethical approval, implementation of an animal CDH-model, etc.). The difficulties in transforming animal study results into a clinical method implementation in humans should be considered carefully. From our point of view, laboratory simulations based on retrospective clinical data taken from CDH neonates seem to be a better solution, although analyzing data from more patients is indispensable. It is warranted to create more data within laboratory research prior to the implementation of the method in the daily practice.
Limitations
The simulations were based on some theoretical assumptions that would require verification in the future investigations. The assumptions concerned value distribution of lung model parameters, between central and peripheral airways (Cw/CL, Rc/R). Clinical data on the topic are hardly available in the literature32,33. Based on our study results, we hypothesize that in CDH infants, before hernia repair, the chest wall is stiffer than in healthy infants. It means that the Cw/CL ratio may be lower than it has been believed, so far. Further studies are necessary to prove actual Cw/CL relation, to explain the reasons for decreased compliance C after hernia repair and to move the research from the simulation level to the clinical use.
Summary
The main study results confirm the pilot study results9 and indicate the following:
-
there are strong and significant correlations between respiratory system impedance (Z), ventilation parameters (WOBvt, PIP, MAP) and ventilation inhomogeneity degree, described by the T1/T2 index (0.8–0.9, P < 0.001); the correlations are dependent on respiratory rate (RR) and the ratio between chest wall compliance and lung compliance (CW/CL); these dependencies can be expressed in the form of characteristics consisted of several curves of polynomial functions (Fig. 3, Supplementary Tables S1–S5 online);
-
the work of breathing (WOBvt), PIP, and MAP highly correlate with respiratory system impedance (Z) (0.8–09, P < 0.001) (Fig. 4); the correlation is nearly-linear and dependent on respiratory rate (RR) and the ratio between chest wall compliance and lung compliance (CW/CL) (Fig. 5, Supplementary Tables S1-S5 online);
-
the work of breathing (WOBvt) highly correlates with PIP and MAP (0.9, P < 0.001), in the patient-ventilator system; the correlation is linear and independent of RR and the CW/CL ratio (Supplementary Fig. S1 online).
These results may indicate that the T1/T2 index could potentially serve as a measure of ventilation inhomogeneity degree in the future. It could be clinically useful to optimize ventilation settings based on objective, measurable parameters, i.e. to determine appropriate levels of PIP and MAP and choose the correct strategy of ventilation. If the predicted PIP and MAP appeared to be too high to continue CMV, due to the risk of lung injury, HFV might be implemented earlier compared to the existing procedures.
Data availability
The datasets used and/ or analyzed in the study are available from the corresponding author for reasonable request.
References
Snoek, K. G. et al. Congenital diaphragmatic hernia: 10-year evaluation of survival, extracorporeal membrane oxygenation and foetoscopic endotracheal occlusion in four high-volume centres. Neonatology 113, 63–68. https://doi.org/10.1159/000480451 (2018).
Coughlin, M. A. et al. Prenatally diagnosed severe CDH: Mortality and morbidity remain high. J. Pediatr. Surg. 51, 1091–1095. https://doi.org/10.1016/j.jpedsurg.2015.10.082 (2016).
Baerg, J., Thirumoorthi, A. & Hazboun, R. Congenital diaphragmatic hernia. In Hernia (ed. Derbel, F.) 153–183 (IntechOpen, 2017).
van den Hout, L. et al. The VICI-trial: High frequency oscillation versus conventional mechanical ventilation in newborns with congenital diaphragmatic hernia: An international multicentre randomized controlled trial. BMC Pediatr. 11, 98. https://doi.org/10.1186/1471-2431-11-98 (2011).
Anagnostopoulou, P. et al. Multiple breath washout analysis in infants: Quality assessment and recommendations for improvement. Physiol. Meas. 37, L1–L15. https://doi.org/10.1088/09673334/37/3/L1/data (2016).
Snoek, K. G., Reiss, I. K. M., Greenough, A. & Capolupo, I. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: The CDH EURO consortium consensus: 2015 update. Neonatology 110, 66–74. https://doi.org/10.1159/000444210 (2016).
Jancelewicz, T. et al. Toward standardized management of congenital diaphragmatic hernia: An analysis of practice guidelines. J. Surg. Res. 243, 229–235. https://doi.org/10.1016/j.jss.2019.05.007 (2019).
Robinson, P. D. et al. Consensus statement for inert gas washout measurement using multiple- and single-breath tests. Eur. Respir. J. 41, 507–522. https://doi.org/10.1183/09031936.00069712 (2013).
Stankiewicz, B. et al. Ventilation inhomogeneity in CDH infants – a new attitude within a simulation study. Biocybern. Biomed. Eng. 41, 1378–1389. https://doi.org/10.1016/j.bbe.2021.08.002 (2021).
Stankiewicz, B., Pałko, K. J., Darowski, M., Zieliński, K. & Kozarski, M. A new infant hybrid respiratory simulator: Preliminary evaluation based on clinical data. Med. Biol. Eng. Comput. 55, 1937–1948. https://doi.org/10.1007/s11517-017-1635-9 (2017).
Stankiewicz, B., Darowski, M. & Pałko, K. J. Influence of preterm birth, BPD and lung inhomogeneity on respiratory system impedance—model studies. In Recent Developments and Achievements in Biocybernetics and Biomedical Engineering. Advances in Intelligent Systems and Computing Vol. 647 (eds Augustyniak, P. et al.) 68–83 (Springer, 2017).
Miedema, M., de Jongh, F. H., Frerichs, I., van Veenendaal, M. B. & van Kaam, A. H. Regional respiratory time constants during lung recruitment in high-frequency oscillatory ventilated preterm infants. Intensive Care Med. 38, 294–299. https://doi.org/10.1007/s00134-011-2410-2 (2012).
Riedel, T., Kyburz, M., Latzin, P., Thamrin, C. & Frey, U. Regional and overall ventilation inhomogeneities in preterm and term-born infants. Intensive Care Med. 35, 144–151. https://doi.org/10.1007/s00134-008-1299-x (2009).
Landolfo, F. et al. Functional residual capacity (FRC) and lung clearance index (LCI) in mechanically ventilated infants: Application in the newborn with congenital diaphragmatic hernia (CDH). J. Pediatr. Surg. 48, 1459–1462. https://doi.org/10.1016/j.jpedsurg.2012.12.047 (2013).
Bikker, I. G., Holland, W., Specht, P., Ince, C. & Gommers, D. Assessment of ventilation inhomogeneity during mechanical ventilation using a rapid-response oxygen sensor-based oxygen washout method. ICMx 2, 14 (2014).
Latzin, P., Thamrin, C. & Kraemer, R. Ventilation inhomogeneities assessed by the multibreath washout (MBW) technique. Thorax 63, 98–99. https://doi.org/10.1136/thx.2007.085332 (2008).
Zhao, Z., Pulletz, S., Frerichs, I., Müller-Lisse, U. & Möller, K. The EIT-based global inhomogeneity index is highly correlated with regional lung opening in patients with acute respiratory distress syndrome. BMC Res. Notes 7, 82. https://doi.org/10.1186/1756-0500-7-82 (2014).
Frerichs, I. et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 72, 83–93. https://doi.org/10.1136/thoraxjnl-2016-208357 (2017).
Durlak, W., Klimek, M. & Kwinta, P. Regional lung ventilation pattern in preschool children with bronchopulmonary dysplasia is modified by bronchodilator response. Pediatr. Pulmonol. 52, 353–359. https://doi.org/10.1002/ppul.23540 (2017).
Brandt, J. B., Mahlknecht, A., Werther, T., Ullrich, R. & Hermon, M. Comparing ventilation modes by electrical impedance segmentography in ventilated children. J. Clin. Monit. Comput. 36, 1795–1803. https://doi.org/10.1007/s10877-022-00828-y (2022).
Kallio, M. et al. Electrical impedance tomography reveals pathophysiology of neonatal pneumothorax during NAVA. Clin. Case Rep. 8, 1574–1578. https://doi.org/10.1002/ccr3.2944 (2020).
Stankiewicz, B., Pałko, K. J., Darowski, M., Kozarski, M. & Górczyńska, K. Challenges with conventional ventilation of infants with inhomogeneous lungs. In Current Trends in Biomedical Engineering and Bioimages Analysis. PCBEE 2019. Advances in Intelligent Systems and Computing Vol. 1033 (eds Korbicz, J. et al.) 234–244 (Springer, 2020).
Pulletz, S. et al. Dynamics of regional lung aeration determined by electrical impedance tomography in patients with acute respiratory distress syndrome. Multidiscip. Respir. Med. 7, 44 (2012).
Depta, F., Török, P., Reeves, V. & Gentile, M. Programmed multi-level ventilation: A strategy for ventilating non-homogenous lungs. Med. Devices 14, 277–285. https://doi.org/10.2147/MDER.S329352 (2021).
Čandik, P. et al. Relationship between dynamic expiratory time constant τedyn and parameters of breathing cycle in pressure support ventilation mode. Physiol. Res. 67, 875–879. https://doi.org/10.33549/physiolres.933750 (2018).
Čandik, P. et al. Use of programmed multilevel ventilation as a superior method for lung recruitment in heart surgery. Int. J. Crit. Care Emerg. Med. 5, 067. https://doi.org/10.23937/2474-3674/1510067 (2019).
Guevorkian, D. et al. Lower distending pressure improves respiratory mechanics in congenital diaphragmatic hernia complicated by persistent pulmonary hypertension. J. Pediatr. 200, 38–43. https://doi.org/10.1016/j.peds.2018.04.027 (2018).
Mead, J., Takishima, T. & Leit, D. Stress distribution in lungs: A model of pulmonary elasticity. J. Appl. Physiol. 28, 596–608. https://doi.org/10.1152/jappl.1970.28.5.596 (1970).
Rausch, S. M. K., Haberthür, D., Stampanoni, M., Schittny, J. C. & Wall, W. A. Local strain distribution in real three-dimensional alveolar geometries. Ann. Biomed. Eng. 39, 2835–2843. https://doi.org/10.1007/s10439-011-0328-z (2011).
Tibby, S. M. Monitoring the critically ill or injured child. In Pediatric Critical Care Medicine (eds Wheeler, D. S. et al.) 519–542 (Springer-Verlag, 2014).
Clement, K. C. et al. Respiratory mechanics in the mechanically ventilated patient. In Paediatric and Neonatal Mechanical Ventilation from Basics to Practice 293–371 (Springer, 2015).
Morini, F., Capolupo, I., van Weteringen, W. & Reiss, I. Ventilation modalities in infants with congenital diaphragmatic hernia. Semin. Pediatr. Surg. 26, 159–165. https://doi.org/10.1053/j.sempedsurg.2017.04.003 (2017).
Mortola, J. P. How to breathe? Respiratory mechanics and breathing pattern. Respir. Physiol. Neurobiol. 261, 48–54. https://doi.org/10.1016/j.resp.2018.12.005 (2019).
Papastamelos, C., Panitch, H. B., England, S. E. & Allen, J. L. Developmental changes in chest wall compliance in infancy and early childhood. J. Appl. Physiol. 78, 179–184. https://doi.org/10.1152/jappl.1995.78.1.179 (1995).
Sakai, H. et al. Effect of surgical repair on respiratory mechanics in congenital diaphragmatic hernia. J. Pediatr. 111, 432–438. https://doi.org/10.1016/S0022-3476(87)80475-4 (1987).
Takayasu, H. et al. Analysis of risk factors of long-term complications in congenital diaphragmatic hernia: A single institution’s experience. Asian J. Surg. 40, 1–5. https://doi.org/10.1016/j.asjsur.2015.02.005 (2017).
Brandt, J. B. et al. Risk factors for mortality in infants with congenital diaphragmatic hernia: A single center experience. Wien Klin Wochenschr 133, 674–679. https://doi.org/10.1007/s00508-021-01843-w (2021).
Lally, K. P. & Engle, W. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics 121, 627–632. https://doi.org/10.1542/peds.2007-3282 (2008).
Acknowledgements
This study was supported by grant no FBW/4.6/22 of Nalecz Institute of Biocybernetics and Biomedical Engineering, Polish Academy of Science, Warsaw, Poland.
Author information
Authors and Affiliations
Contributions
B.S.: conceptualization, simulations and writing of the original manuscript. M.M.S.: conceptualization, collection of clinical data and writing of the original manuscript. K.J.P.: conceptualization, simulations and data processing. A.B.: conceptualization and collection of clinical data. M.D.: conceptualization and writing of the original draft. M.K.: conceptualization, writing of the initial draft and engineering support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stankiewicz, B., Mierzewska-Schmidt, M., Pałko, K.J. et al. A new method of ventilation inhomogeneity assessment based on a simulation study using clinical data on congenital diaphragmatic hernia cases. Sci Rep 12, 22635 (2022). https://doi.org/10.1038/s41598-022-27027-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27027-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.