Abstract
Bipolar disorder (BD) and metabolic disturbance represent a chronic state of low-grade inflammation and corticostriatal circuitry alterations. Herein, we aimed to investigate whether plasma leptin, an adipokine that plays a key role in the interplay of metabolism and inflammation, is associated with corticostriatal connectivity in patients with BD. Twenty-eight BD I patients, 36 BD II patients and 66 healthy controls were enrolled and completed the Hamilton Depression Rating Scale, the Young Mania Rating Scale, and the Recent Life Change Questionnaire. Fasting plasma leptin and C-reactive protein (CRP) levels were measured, and corticostriatal connectivity was examined using functional magnetic resonance imaging (fMRI). The relationships between leptin, CRP and body mass index (BMI) identified in the controls and BD II patients were absent in the BD I patients. We did not find a significant group difference in the leptin level; nevertheless, the negative correlation between leptin level and corticostriatal connectivity (ventrolateral prefrontal cortex and inferior temporal gyrus) observed in the healthy controls was absent in the BD patients. The disproportionate increase in leptin level with increasing BMI in BD indicated a potential inflammatory role of white adipose tissue in BD. Furthermore, higher CRP levels in BD I patients might induce leptin resistance. Collectively, our results implied vulnerability to inflammatory and metabolic diseases in patients with BD, especially BD I.
Similar content being viewed by others
Introduction
Bipolar disorder (BD), characterized by emotion dysregulation, increases the relative risk of mortality1, which is partially mediated by metabolic disturbance2,3,4. Furthermore, evidence has shown that aberrant immune signaling contributes to all stages of BD, and also to metabolic disturbance5,6. Recent structural and functional studies have directly implicated reward system dysregulation in BD7,8,9, and evidence has shown that core network functional abnormalities lead to aberrant reward-processing, and the neural circuitry in BD is highly associated with systemic inflammation10,11. Such systemic inflammation might drive changes in dopaminergic corticostriatal circuitry connectivity12,13, and a recent large-scale study indicated that both BD and body mass index (BMI) were associated with similar regional brain volumes, including that of the basal ganglia14.
Leptin is secreted by white adipose tissue and regulates energy homeostasis. There is direct brain-adipose crosstalk through leptin, which regulates the energy balance and motivation through action at distinct neural circuits15,16,17,18. In addition, there is compelling evidence implicating leptin as an important modulator of the inflammatory process. Leptin can increase the production of pro-inflammatory cytokines and modulates both innate and adaptive immune responses2,19,20. Leptin itself has been found to be able to stimulate C-reactive protein (CRP) synthesis from the liver and endothelial cells21. A positive and independent relationship between peripheral leptin and CRP has been found previously in healthy subjects22. Taken together, leptin could modulate the reward system and plays a key role in the interplay of metabolism and inflammation23.
Both leptin and CRP are linked directly and independently through a number of pathophysiological mechanisms to BD2,24,25,26. However, whether the pathophysiological processes induced by leptin or CRP are enhanced or redundant in BD remains unknown27. We previously reported altered corticostriatal circuitry in BD that was associated with the level of CRP28. In the current study, we aimed to investigate the relationship between peripheral leptin and CRP levels. Regarding the role of the caudate nucleus in both metabolic control and BD29, we also investigated the possible correlation between leptin and caudate-seeded corticostriatal connectivity in patients with BD30,31,32. We hypothesized that the alterations in caudate-seeded functional connectivity (FC) in the corticostriatal circuitry may be linked to leptin metabolic feedback dysregulation in BD patients.
Results
Demographic and clinical data
Twenty-eight BD I patients, 36 BD II patients and 66 age-, sex-, life events-, and BMI-matched healthy controls were enrolled in this study. Most of the BD patients received mood stabilizer treatment, including valproic acid (n = 14, 22.6%), valproic acid plus antipsychotics (n = 23, 35.9%), lithium (n = 2, 0.03%), and lithium plus antipsychotics (n = 8, 12.5%). Only one patient received valproic acid plus lithium, and one received valproic acid plus lithium plus antipsychotics; eight patients (12.5%) received only antipsychotics, 3 (4.7%) only antidepressants, and 4 (6.3%) did not take any medication. There were no group differences in the usage of valproic acid (p = 0.182), olanzapine (p = 0.100), quetiapine (p = 0.832), amisulpride (p = 0.466), aripiprazole (p = 0.404), or lurasidone (p = 0.824); however, increased usage of lithium (p = 0.013), risperidone (p = 0.008) and clozapine (p = 0.018) was observed in the BD I patients (Table 1).
There were no significant differences in the demographic data of all groups, with the exception of education level (Table 1); both the BD I and BD II patients had lower education levels according to the post-hoc two-sample t-test. The BD I patients in this study scored 3.71 ± 5.44 on the 17-item Hamilton Depression Rating Scale (HDRS) and 2.00 ± 3.80 on the 11-item Young Mania Rating Scale (YMRS) (Table 1), and 17 (60.7%) were euthymic (scores of fewer than 7 on the HDRS and YMRS). The BD II patients scored 5.00 ± 4.93 on the HDRS and 1.18 ± 1.78 on the YMRS (Table 1), and 23 (63.9%) were euthymic. Nevertheless, both the BD I and BD II patients, in comparison with the healthy controls, demonstrated higher HDRS and YMRS scores according to the post-hoc two-sample t-test.
Correlation analyses
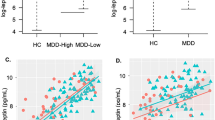
There were no significant differences in BMI, plasma leptin level or CRP level between groups (Table 1). The plasma leptin level was sub-significantly correlated with the CRP level among the healthy controls (r = 0.264, p = 0.054) and BD II patients (r = 0.527, p = 0.002), but this was not the case in the BD I patients (r = − 0.006, p = 0.978). Similarly, BMI was significantly correlated with the CRP level among the healthy controls (r = 0.466, p = 0.000) and BD II patients (r = 0.708, p = 0.000), but not in the BD I patients (r = 0.085, p = 0.700). In contrast, both the BD I and BD II patients exhibited significant correlations between the plasma leptin level and BMI (r = 0.432/0.472, p = 0.040/0.006, respectively); however, the healthy controls showed a sub-significant correlation (r = 0.263, p = 0.055).
Leptin and corticostriatal circuitry connectivity
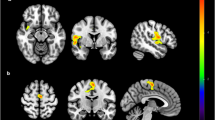
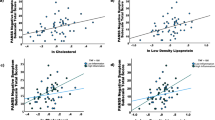
Among the healthy controls, the dorsal caudate (DC)-ventrolateral prefrontal cortex (vlPFC), DC-inferior temporal gyrus and DC-putamen FC were negatively correlated with the plasma leptin level (r = − 0.597, p < 0.001; r = − 0.532, p < 0.001; r = − 0.345, p = 0.011) (Table 2, Fig. 1). In contrast, the DC-putamen FC was positively correlated with the plasma leptin level in the BD I patients (r = 0.609, p = 0.002), while a significant positive correlation between plasma leptin level and the DC-inferior temporal gyrus FC was observed in the BD II patients (r = 0.362, p = 0.035).
Brain regions whose functional connectivity with the left dorsal caudate was correlated with the plasma leptin level, with between-group differences. The left dorsal caudate-seeded functional connectivity was significantly correlated with the plasma leptin level among the patients with (A) bipolar I disorder and (B) bipolar II disorder. The coordinates of the peak voxel are presented in Table 2. Significance was thresholded at the uncorrected voxel level p = 0.001, followed by the FWE-corrected cluster level p = 0.05. The color bar denotes the t-scores. Figures are displayed according to neurological convention (left = left).
The right DC-seeded FC analyses yielded similar results (Supplemental Table S1). The ventral rostral putamen and the dorsal caudal putamen-seeded FC analyses yielded similar results only in the BD II patients (Supplemental Table S2), and not in the BD I patients. These supplementary analyses supported the specificity of the DC circuitry among the BD patients. Furthermore, after regressing out the effects of BMI, age and CRP, the correlations between leptin and corticostriatal connectivity yielded similar results (Supplemental Tables S3, S4).
Between-group differences in corticostriatal circuitry connectivity
After identifying associations between plasma leptin, CRP and corticostriatal connectivity (Table 2, Fig. 1), we compared the between-group differences in corticostriatal circuitry connectivity (Table 3) to further investigate the potential effects of leptin in the BD patients.
The BD I patients exhibited significantly decreased FC between the DC and the putamen and parahippocampal gyrus as compared with the healthy controls, and between the DC and the orbitofrontal cortex (OFC) as compared with the BD II patients (Table 3). The BD II patients showed decreased FC between the DC and the OFC, vlPFC, dorsolateral prefrontal cortex, posterior parietal cortex, inferior temporal gyrus, parahippocampal gyrus, supplementary motor area, thalamus, pons, and cerebellum as compared with the healthy controls (Table 3).
Discussion
In line with a previous meta-analysis study, our results showed that the leptin level was not altered in the BD patients as compared with the healthy controls2. Interestingly, the relationship between leptin and CRP found in the controls and BD II patients was absent in the BD I patients, indicating that higher CRP levels in BD I patients might induce leptin resistance33. Moreover, such dysregulation may arise from obesogenic diets, medications and/or BD per se34,35. Although we still cannot conclude whether the pathophysiological processes induced by leptin or CRP are enhanced or redundant, their possible additive or even synergistic effects need to be taken into consideration, as both leptin and CRP might increase simultaneously in BD. Our results also implied vulnerability to inflammatory and metabolic diseases in BD I patients.
Network-based studies have identified core network functional abnormalities in different states of BD10. The results here were in line with a unified model, suggesting that emotional dysregulation in BD arises from dynamic alterations in circuits that involve the perception of interoception36. In the current study, we demonstrated that there were significant correlations between the level of leptin and the corticostriatal circuitry in healthy controls, indicating a leptin-modulated effect on the interoceptive reward circuitry37,38. The results showed that the negative correlation between leptin and DC-seeded FC was reversed in both the BD I and BD II patients, implying that the aforementioned leptin-modulated corticostriatal circuitry was dysregulated. Furthermore, the dysregulated regions involved the emotion-regulation circuitry, including the vlPFC (Table 2, Fig. 1), as has been well-documented in BD patients11,36. The interaction between affective and interoceptive reward circuitries might explain the high vulnerability to metabolic syndrome of patients with BD2,36,39. Evidence has also shown that changes in BMI and insulin sensitivity are associated with brain intrinsic functional reorganization40. In BD patients, whether central leptin resistance plays a key role in leading to dysregulated corticostriatal circuits and affects food choice requires further investigation41,42,43.
The results showed that the BD I patients exhibited altered DC-putamen FC. The putamen is involved in reward-seeking and motivation behaviors, including food-seeking, and its FC is associated with childhood BMI44. The local striatal circuitry plays a critical role in the computing of sensory, motor and limbic information into behavioral and cognitive outputs, and impaired function in terms of an amplifier or filter may induce dysfunction of lateral inhibition45. Such lateral inhibition is modulated by dopamine, and inhibitory imbalance is a predominant theory in psychosis, which is only manifested in BD I, and not BD II45,46.
In contrast, the BD II patients showed diffuse corticostriatal hypo-connectivity, including the dorsolateral prefrontal cortex and parahippocampal gyrus, which is important for reward and decision-making in terms of food choices47. Furthermore, the BD II patients exhibited DC-OFC hypo-connectivity that was significantly lower than that in the BD I patients (Table 3), and showed significant dysregulation of leptin in the DC-vlPFC FC (Table 2, Fig. 1). Obese humans exhibit vlPFC-OFC hyper-connectivity, which has a substantial trend in terms of a negative correlation with the level of leptin48, while leptin injections may down-regulate sensitivity to food via hypothalamus-OFC hypo-connectivity49. As the OFC is involved in emotional processes, while the vlPFC encodes certainty and predictions36, our results further supported that BD II is not a milder form of BD I, but presents with wide-ranging functional abnormalities in the reward circuitry11,50,51.
Our study had limitations in terms of the cross-sectional design and in not considering eating habits. This should be taken into account in subsequent study. Given that the BD patients may have been a number of variables that could confound the observed associations; nevertheless, after regressing out the effects of BMI, age and CRP, our main findings remained robust (Supplemental Tables S3, S4).
Conclusions
The results of this study connected circuits controlling mood and energy balance with corticostriatal circuitry52. Moreover, the dysregulated circuitry in BD implied vulnerability to inflammatory and metabolic diseases, especially in BD I. These results provided insights that could kindle hope for the development of novel circuitry-based treatments for BD53.
Methods
Ethics approval and consent to participate
All participants provided their written informed consent. The study was approved by the Institutional Review Board of National Cheng Kung University Hospital and was conducted in accordance with the Declaration of Helsinki.
Subjects
All patients were recruited from the psychiatric outpatient department at National Cheng Kung University Hospital, while the healthy controls were recruited from the community through advertisement. All participants, who were either Mandarin or Taiwanese speakers, were screened by a psychiatrist using the Chinese version of the Mini International Neuropsychiatry Interview (MINI)54, the HDRS55,56, and the YMRS. All patients were diagnosed by a psychiatrist to determine eligibility according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). A proportion of the subjects (BD I: n = 15, 54%; BD II: n = 25, 69%; healthy controls: n = 48, 73%) overlapped with those enrolled in our previous published studies28,57,58.
The exclusion criteria for all the participants were: (1) any psychiatric (other than BD and tobacco use disorder), neurological, autoimmune, serious surgical, or severe physical illnesses, such as acute coronary syndrome, kidney dialysis, hepatic failure, or transplant; (2) any head injury history with loss of consciousness; (3) any medications that could affect the immune system or use of anti-inflammatory medications; (4) any contraindications in relation to magnetic resonance imaging (MRI), such as having a metal implant, a pacemaker implant, or claustrophobia; (5) plans for pregnancy or a positive pregnancy test.
Experimental design
After enrolment in the study, all patients received treatment as usual, and the administration of medications was recorded. There was no significant relapse of BD that necessitated clinical care changes during the study. All participants in the three groups completed the Recent Life Change Questionnaire (RLCQ) to assess the type and magnitude of life events during the previous 12 months59. The RLCQ, which consists of 39 items querying representative life change events, is the Taiwanese version of the Schedule of Recent Experience60, which consisted originally of 43 life change events, later termed life change units61. Blood samples were taken for the measurement of fasting plasma leptin and CRP levels, and FC was examined during the resting state using functional magnetic resonance imaging (fMRI). For detailed information on image acquisition, image preprocessing, definition of the seeds in the left DC and other areas among the basal ganglia (for additional testing to examine specificity), and seed-based FC maps, please refer to our published papers57,58.
Levels of fasting plasma leptin and high-sensitivity C-reactive protein
The participants were instructed to fast for at least 9 h prior to each examination. Blood samples for the leptin and CRP assays were collected between 08:00 and 10:00 am in 5-mL EDTA tubes and stored at 4 °C in a fridge. Plasma was isolated by centrifugation at 1800×g for 15 min at 4 °C and immediately stored at − 80 °C. The levels of fasting plasma leptin (Linco Research, St Louis, MO, USA) and CRP (eBioscience, San Diego, CA, USA) were measured using ELISA methods. For detailed information on the catalog numbers, minimum detectable levels, and intra- and inter-assay coefficients of the ELISA kits used for the measurement of leptin and CRP, please see Supplemental Table S5. Dilute hs-CRP samples from 1:30 to 1:1000 with Assay Buffer.
Statistical analyses
SPSS Statistics 20.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Missing data were excluded from the calculations, and the numbers of participants with missing data are indicated in the footnote of each table. Results were considered significant at p < 0.05 (two-tailed). As most of the variables shown in Table 1 were not normally distributed in a Gaussian manner, a Kruskal–Wallis H test was conducted to examine between-group (BD I patients, BD II patients and healthy controls) differences in the demographic characteristics, HDRS score, YMRS score, life events, and plasma levels of leptin and CRP. The post-hoc Mann–Whitney U test was performed whenever the between-group difference was significant. Pearson correlation analyses were performed to test the relationships among the plasma leptin level, CRP level, and BMI.
Image analysis
One-way ANOVA was employed to analyze the FC maps using SPM12 (Wellcome Trust Centre for Neuroimaging, London, https://www.fil.ion.ucl.ac.uk/spm/). Statistical maps were computed to identify changes in the DC-seeded FC for between-group comparisons. Significance was thresholded at the uncorrected voxel-level p = 0.001, followed by the voxel-level family-wise error (FWE) rate-corrected cluster-level at p = 0.05 for whole-brain multiple comparisons.
Another one-way ANOVA was performed to determine the correlations between the DC-seeded FC and the leptin level. We entered the demeaned (in SPM12) values as regressors to identify brain regions exhibiting higher correlations with the DC-seeded FC among the three groups. Significance was thresholded at the uncorrected voxel-level p = 0.001, followed by the FWE-corrected cluster-level at p = 0.05.
As SPM12 reports peak coordinates as identified within a confluent cluster, there can be multiple peaks that sit on different brain regions/areas (e.g., Brodmann area). We reported one representative peak for each region/area. To display 3D imaging, we used MRIcroGL for 3D rendering (Department of Psychology, University of South Carolina http://www.mccauslandcenter.sc.edu/mricrogl). To show the regression results in scatterplots, we extracted the DC-seeded FC values in each brain region showing higher correlations with the DC-seeded FC among the three groups (peak MNI coordinates are presented in Tables 2, 3, radius = 3 mm). The corresponding correlation coefficients (r) and p values were analyzed using SPSS Statistics 20.0.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Staudt Hansen, P. et al. Increasing mortality gap for patients diagnosed with bipolar disorder—A nationwide study with 20 years of follow-up. Bipolar Disord. 21, 270–275 (2019).
Fernandes, B. S. et al. Leptin in bipolar disorder: A systematic review and meta-analysis. Eur. Psychiatry 35, 1–7 (2016).
Wei, J. et al. A plasma metabolomics study suggests alteration of multiple metabolic pathways in patients with bipolar disorder. Psychiatry Res. 299, 113880 (2021).
Sanchez-Autet, M. et al. Association between neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and c-reactive protein levels and metabolic status in patients with a bipolar disorder. World J. Biol. Psychiatry 23, 1–27 (2021).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150 (2014).
Jones, G. H., Vecera, C. M., Pinjari, O. F. & Machado-Vieira, R. Inflammatory signaling mechanisms in bipolar disorder. J. Biomed. Sci. 28, 45 (2021).
Nabulsi, L. et al. Bipolar disorder and gender are associated with frontolimbic and basal ganglia dysconnectivity: A study of topological variance using network analysis. Brain Connect. 9, 745–759 (2019).
Nabulsi, L. et al. Frontolimbic, frontoparietal, and default mode involvement in functional dysconnectivity in psychotic bipolar disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 140–151 (2020).
Nabulsi, L. et al. Aberrant subnetwork and hub dysconnectivity in adult bipolar disorder: A multicenter graph theory analysis. Cereb. Cortex 32, 2254–2264 (2022).
Martino, M. & Magioncalda, P. Tracing the psychopathology of bipolar disorder to the functional architecture of intrinsic brain activity and its neurotransmitter modulation: A three-dimensional model. Mol. Psychiatry 27, 793–802 (2022).
Phillips, M. L. & Swartz, H. A. A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 171, 829–843 (2014).
Felger, J. C. & Treadway, M. T. Inflammation effects on motivation and motor activity: Role of dopamine. Neuropsychopharmacology 42, 216–241 (2017).
Marsland, A. L. et al. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav. Immun. 62, 162–170 (2017).
McWhinney, S. R. et al. Association between body mass index and subcortical brain volumes in bipolar disorders-ENIGMA study in 2735 individuals. Mol. Psychiatry 26, 6806–6819 (2021).
Fernandes, M. F. et al. Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab. 22, 741–749 (2015).
Davis, J. F. et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol. Psychiatry 69, 668–674 (2011).
Hommel, J. D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Caron, A., Lee, S., Elmquist, J. K. & Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 19, 153–165 (2018).
Tsai, S. Y. et al. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J. Affect. Disord. 136, 110–116 (2012).
La Cava, A. Leptin in inflammation and autoimmunity. Cytokine 98, 51–58 (2017).
Hribal, M. L., Fiorentino, T. V. & Sesti, G. Role of C reactive protein (CRP) in leptin resistance. Curr. Pharm. Des. 20, 609–615 (2014).
Shamsuzzaman, A. S. et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation 109, 2181–2185 (2004).
Abella, V. et al. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 13, 100–109 (2017).
Fernandes, B. S. et al. C-reactive protein concentrations across the mood spectrum in bipolar disorder: A systematic review and meta-analysis. Lancet Psychiatry 3, 1147–1156 (2016).
Solmi, M. et al. Peripheral levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and interleukin-1beta across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 97, 193–203 (2021).
Rummel, C., Inoue, W., Poole, S. & Luheshi, G. N. Leptin regulates leukocyte recruitment into the brain following systemic LPS-induced inflammation. Mol. Psychiatry 15, 523–534 (2010).
Goldsmith, D. R. et al. Protein and gene markers of metabolic dysfunction and inflammation together associate with functional connectivity in reward and motor circuits in depression. Brain Behav. Immun. 88, 193–202 (2020).
Tseng, H. H. et al. Peripheral inflammation is associated with dysfunctional corticostriatal circuitry and executive dysfunction in bipolar disorder patients. Brain Behav. Immun. 91, 695–702 (2021).
Nakamura, Y., Ozawa, S. & Koike, S. Caudate functional connectivity associated with weight change in adolescents. Front. Hum. Neurosci. 14, 587763 (2020).
Morawetz, C., Steyrl, D., Berboth, S., Heekeren, H. R. & Bode, S. Emotion regulation modulates dietary decision-making via activity in the prefrontal-striatal valuation system. Cereb. Cortex 30, 5731–5749 (2020).
Loonen, A. J. M., Kupka, R. W. & Ivanova, S. A. Circuits regulating pleasure and happiness in bipolar disorder. Front. Neural Circuits 11, 35 (2017).
Robinson, J. L. et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 60, 117–129 (2012).
Chen, K. et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat. Med. 12, 425–432 (2006).
Pitharouli, M. C. et al. Elevated c-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: Results from the UK biobank. Am. J. Psychiatry 178, 522 (2021).
Godfrey, J. R. et al. Obesogenic diet-associated C-reactive protein predicts reduced central dopamine and corticostriatal functional connectivity in female rhesus monkeys. Brain Behav. Immun. 88, 166–173 (2020).
Perry, A., Roberts, G., Mitchell, P. B. & Breakspear, M. Connectomics of bipolar disorder: A critical review, and evidence for dynamic instabilities within interoceptive networks. Mol. Psychiatry 24, 1296–1318 (2019).
Contreras-Rodriguez, O., Martin-Perez, C., Vilar-Lopez, R. & Verdejo-Garcia, A. Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biol. Psychiatry 81, 789–796 (2017).
Jastreboff, A. M. et al. Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care 37, 3061–3068 (2014).
Williams, L. J. et al. Lifetime psychiatric disorders and body composition: A population-based study. J. Affect. Disord. 118, 173–179 (2009).
Kullmann, S. et al. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 33, 1052–1061 (2012).
Clarke, R. E., Verdejo-Garcia, A. & Andrews, Z. B. The role of corticostriatal-hypothalamic neural circuits in feeding behaviour: Implications for obesity. J. Neurochem. 147, 715–729 (2018).
Neseliler, S. et al. Neurocognitive and hormonal correlates of voluntary weight loss in humans. Cell Metab. 29, 39–49 (2019).
Schmidt, L. et al. Resting-state connectivity within the brain’s reward system predicts weight loss and correlates with leptin. Brain Commun. 3, 005 (2021).
Assari, S. & Boyce, S. Resting-state functional connectivity between putamen and salience network and childhood body mass index. Neurol. Int. 13, 85–101 (2021).
Burke, D. A., Rotstein, H. G. & Alvarez, V. A. Striatal local circuitry: A new framework for lateral inhibition. Neuron 96, 267–284 (2017).
Lanillos, P. et al. A review on neural network models of schizophrenia and autism spectrum disorder. Neural Netw. 122, 338–363 (2020).
Enax, L. & Weber, B. Neurobiology of food choices—Between energy homeostasis, reward system, and neuroeconomics. e-Neuroforum 7, 13–22 (2016).
Devoto, F. et al. Altered brain resting-state functional connectivity in obese patients is associated with plasma levels of leptin. Diabetes 67, 2077 (2018).
Hinkle, W., Cordell, M., Leibel, R., Rosenbaum, M. & Hirsch, J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS ONE 8, e59114 (2013).
Gong, J. et al. Large-scale network abnormality in bipolar disorder: A multimodal meta-analysis of resting-state functional and structural magnetic resonance imaging studies. J. Affect. Disord. 292, 9–20 (2021).
Caseras, X., Lawrence, N. S., Murphy, K., Wise, R. G. & Phillips, M. L. Ventral striatum activity in response to reward: Differences between bipolar I and II disorders. Am. J. Psychiatry 170, 533–541 (2013).
Waterson, M. J. & Horvath, T. L. Neuronal regulation of energy homeostasis: Beyond the hypothalamus and feeding. Cell Metab. 22, 962–970 (2015).
Gautron, L., Elmquist, J. K. & Williams, K. W. Neural control of energy balance: Translating circuits to therapies. Cell 161, 133–145 (2015).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl 20), 22–33 (1998).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960).
Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296 (1967).
Wei, S. Y. et al. Dysregulation of oxytocin and dopamine in the corticostriatal circuitry in bipolar II disorder. Transl. Psychiatry 10, 281 (2020).
Hsieh, Y. T. et al. Childhood neglect is associated with corticostriatal circuit dysfunction in bipolar disorder adults. Psychiatry Res. 295, 113550 (2021).
Pai, L., Wen, H.-C., Lu, R.-B. & Kuo, M.-L. The questionnaire of life stress cognition of adults-revised (full text in Chinese). Chin. J. Mental Health (Taiwan) 3, 195–205 (1985).
Rahe, R. H. Epidemiological studies of life change and illness. Int. J. Psychiatry Med. 6, 133–146 (1975).
Miller, M. A. & Rahe, R. H. Life changes scaling for the 1990s. J. Psychosom. Res. 43, 279–292 (1997).
Acknowledgements
The authors thank all the participants in this study and extend particular appreciation to Chien Ting Lin and Yi-Han Han from National Cheng Kung University for their technical and experimental assistance. They thank the Mind Research and Imaging Center (MRIC) at National Cheng Kung University for consultation and instrument availability. The MRIC is supported by the Ministry of Science and Technology.
Funding
This work was supported by the National Science and Technology Council, Taiwan (MOST 108-2320-B-006-004, MOST 108-2314-B-006-045, MOST 110-2320-B-006-022, MOST 107-2314-B-006-082, MOST 108-2628-B-006-004, MOST 107-2320-B-006-071) and National Cheng Kung University Hospital (NCKUH-11004018).
Author information
Authors and Affiliations
Contributions
Corresponding author P.S.C. designed the study and wrote the protocol. H.H.C. contributed to the statistical analyses. S.-Y.W. wrote the first draft of the manuscript. H.-H.T., W.H.C., Y.K.Y., and P.S.C. managed the data collection. All authors interpreted the analysis of the results and helped to revise the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, SY., Tseng, HH., Chang, H.H. et al. Associations of leptin and corticostriatal connectivity in bipolar disorder. Sci Rep 12, 21898 (2022). https://doi.org/10.1038/s41598-022-26233-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26233-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.