Abstract
Suppressor of mek1 (Dictyostelium) homolog 2 (Smek2), was identified as one of the responsible genes for diet-induced hypercholesterolemia (DIHC) of exogenously hypercholesterolemic (ExHC) rats. A deletion mutation in Smek2 leads to DIHC via impaired glycolysis in the livers of ExHC rats. The intracellular role of Smek2 remains obscure. We used microarrays to investigate Smek2 functions with ExHC and ExHC.BN-Dihc2BN congenic rats that harbor a non-pathological Smek2 allele from Brown-Norway rats on an ExHC background. Microarray analysis revealed that Smek2 dysfunction leads to extremely low sarcosine dehydrogenase (Sardh) expression in the liver of ExHC rats. Sarcosine dehydrogenase demethylates sarcosine, a byproduct of homocysteine metabolism. The ExHC rats with dysfunctional Sardh developed hypersarcosinemia and homocysteinemia, a risk factor for atherosclerosis, with or without dietary cholesterol. The mRNA expression of Bhmt, a homocysteine metabolic enzyme and the hepatic content of betaine (trimethylglycine), a methyl donor for homocysteine methylation were low in ExHC rats. Results suggest that homocysteine metabolism rendered fragile by a shortage of betaine results in homocysteinemia, and that Smek2 dysfunction causes abnormalities in sarcosine and homocysteine metabolism.
Similar content being viewed by others
Introduction
Exogenous hypercholesterolemic (ExHC) rats serve as a model of diet-induced hypercholesterolemia (DIHC)1. Although their serum cholesterol levels are within the normal range without dietary cholesterol, they rapidly develop hypercholesterolemia when fed with a diet containing cholesterol. We previously conducted linkage analysis of ExHC with Brown Norway (BN) rats as a control to identify the female-specific responsible loci Dihc1 on chromosome.5 and a unisex-responsible locus Dihc2, 4.1 Mbp region at the 3′ end, on chromosome.142,3. Further investigation identified a responsible gene, suppressor of mek1 (Dictyostelium) homolog 2 (Smek2) from the Dihc2 region3. The ExHC rats have a 10-bp deletion mutation in the coding region of the Smek2 gene3. This deletion leads to disrupted SMEK2 protein function that is not fatal in rats. With respect to the pathological mechanism of DIHC in ExHC rats, we found that compared with the original Sprague–Dawley (SD) strain, ExHC rats have impaired liver glucose metabolism (low expression of liver-type phosphofructokinase, Pfkl, a rate-limiting enzyme of glycolysis)4, low de novo fatty acid synthesis in the liver due to a shortage of required precursors5, and that this shortage of fatty acids leads to hepatic secretion of β-VLDL that is slowly uptaken by the liver5. However, the mechanism of Pfkl downregulation by the loss-of-function of Smek2 is unclear. Furthermore, analyses of multiple changes in hepatic lipid metabolism might have overlooked some metabolic changes. The overall picture of Smek2 function remains incomplete. Yoon et al.6 reported that SMEKs act as the activity-regulating domain of the transcriptional factor, cAMP response element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2). The ubiquitous transcriptional factor CREB regulates cell growth, differentiation, neuro-signals and other processes7. The CREB co-activators, CRTCs, regulate hormone secretion and nutritional metabolism. Abnormal transcriptional regulation can exert powerful effects in vivo, and gain- and loss-of-function mutations in transcriptional factors cause inherited anomalies and diseases8,9,10,11. For instance, mutations in hepatocyte nuclear factor-1a (HNF-1a) cause maturity-onset diabetes of the young (MODY3)9. Mutations in the CREB-binding protein (CREBBP) gene cause Rubinstein-Taybi syndrome (RSTS)12. Activated CREBBP acts on RNA polymerase via TFIIB, which is a basic transcription complex and promotes transcription13. An accumulation of abnormalities in the cascade reaction is thought to result in RSTS. Abnormal transcription factors are major problems. We previously found that a Smek2 deficiency affects lipid metabolism5 and glucose metabolism4. Genes such as Smek2, which are not fatal if deficient, but can have widespread effects, are associated with the development of non-communicable diseases that manifest late in life. Here, we investigated the influence of dysfunctional Smek2 in the livers of ExHC rats using microarray analysis.
Microarrays have been used for decades to exhaustively analyze gene expression14,15, and functions16,17,18, especially of target genes, such as LATS1 in human cell lines and PAP1 in Arabidopsis plants17,18. Microarrays are optimal for analyzing the influences of Smek2 dysfunction. Here, we compared the effects of SMEK2 dysfunction between ExHC and congenic ExHC.BN-Dihc2BN rats (congenic rats) that were established by backcross inbreeding during Smek2 identification3, and harbor the low-responsive BN allele of Dihc2 on the high-responsive ExHC background genome. This comparison decreased noise derived from genetic differences between ExHC and congenic rats facilitated analyses of Smek2 function. We also estimated the influence of the screened genes on metabolism in ExHC rats.
Results
Growth parameters and serum lipid levels
Table 1 shows the growth parameters and serum lipid levels. Initial and final body weight, as well as daily food intake did not significantly differ. However, the ExHC rats tended to gain weight (P = 0.053), and food efficiency was significantly increased. Dietary cholesterol did not affect growth parameters. Dietary cholesterol increased serum cholesterol levels in both strains, but significantly more so in ExHC, than in congenic rats. Serum triacylglycerol levels were significantly higher after 2% cholesterol diet in both strains. Serum phospholipid levels were significantly increased by dietary cholesterol and significantly higher in ExHC rats than congenic rats.
Microarray findings
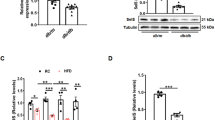
Microarray analysis revealed 251 and 377 genes that were upregulated and downregulated, respectively, in ExHC, compared with congenic rats (data not shown). Table 2 shows the top 10 genes with downregulated expressioned in ExHC rats, the most significant being the mRNA expression of Sardh, encoding sarcosine dehydrogenase. The binary logarithm of fold change of expression levels in ExHC rats to congenic rats was − 9.571 (~ 1/760 of congenic rats). Protein expressions of SARDH was not observed in the liver of ExHC rats regardless of dietary cholesterol contents (Fig. 1).
Protein expressions in the livers of congenic (ExHC.BN-Dihc2BN) (C) and ExHC (E) rats. (A) SARDH, (B) CBS and BHMT. n = 6/group, Dots and bar in each graph represent the values of each rats and the average of group, respectively. Differences among groups were analyzed with two-way ANOVA with Tukey–Kramer post-hoc test. n.d.; not detected, n.s.; not significant.
Determination of hepatic mRNA expression
Table 3 shows the hepatic mRNA expression of genes associated with homocysteine metabolism. The mRNA expression of Sardh and betaine-homocysteine methyltransferase (Bhmt) was significantly lower, and that of cystathionine beta-synthase (Cbs) and of phosphatidylethanolamine N-methyltransferase (Pemt) was significantly higher in ExHC than in congenic rats. In contrast, dietary cholesterol intake significantly decreased Bhmt and Pemt mRNA levels in both rat strains. Although an interaction was found between strain and dietary cholesterol in Smek2, there was no significant difference by dietary cholesterol with Tukey–Kramer post-hoc test.
Determination of hepatic protein expression
Figure 1 shows the hepatic protein expressions of SARDH, CBS, BHMT in the liver. In the liver of ExHC rats, SARDH protein was not detected (Fig. 1A). There was no significant difference on the expressions of BHMT (Fig. 1B). Although the interaction between strain and dietary cholesterol on CBS expression was significant, there was no significant difference after Tukey–Kramer post-hoc test.
Analysis of serum and liver parameters
Table 4 shows the serum and hepatic parameters. Serum sarcosine and homocysteine levels were significantly increased in ExHC rats regardless of the presence or absence of dietary cholesterol, and serum betaine levels were decreased compared with those of congenic rats. Levels of hepatic sarcosine were significantly higher, and those of PE were lower (increased PC/PE ratio) in ExHC compared with congenic rats. Although dietary cholesterol did not affect serum sarcosine levels, dietary cholesterol decreased hepatic sarcosine and increased hepatic SPH levels, regardless of strain. No interaction was found between strain and dietary cholesterol in any of the parameters.
Determination of Sardh mRNA levels in cells with Smek2 knockdown
Small-interfering RNA (siRNA) against Smek2 suppressed Smek2 mRNA expression by 80% and Sardh mRNA expression by 31% in rat hepatocytes (Fig. 2).
Discussion
We investigated the function of the Smek2 gene, which is responsible for DIHC3, by comparing the transcriptomes of ExHC (tentatively Smek2−/−) and congenic (tentatively Smek2+/+) rats. The pathogenic ExHC allele of Smek2 exacerbated the response to dietary cholesterol (approx. + 223% to congenic rats) and increased serum phospholipid levels (+ 8 ~ 20%). Dietary cholesterol decreased serum triacylglycerol levels by approx. 40% in both strains. These observed changes were consistent with the previously reported results3,4,5,45. The findings revealed that ExHC rats developed hypersarcosinemia due to Smek2 dysfunction via dysregulated Sardh mRNA expression. Sarcosine (monomethylglycine) is synthesized from glycine by S-adenosylmethionine demethylation (Fig. 3). Sarcosine accumulation is related to emotional instability, growth failure19, and prostate cancer20 in humans. However, the relationship between sarcosine metabolism and the onset of these diseases has remained obscure. A sar−/− mouse model of hypersarcosinemia with a mutation in Sardh has been established, and this mouse develops average plasma sarcosine concentrations of 785 μM21. However, the sarcosine content of individual tissues from sar−/− mice has not been investigated. Humans with SARDH mutations also develop hypersarcosinemia22 and have rather variable plasma sarcosine levels (760–53 μM)19. The normal range of plasma sarcosine is 3–0.5 μM in healthy persons19. However, sarcosine metabolism is regulated by the dietary intake of nutrients associated with one-carbon metabolism, such as folic acid and cobalamin23,24. Hypersarcosinemia is induced in humans who are folic acid-deficient, with sarcosine levels ranging from 40 to 0.6 μM25. The ExHC rats (average sarcosine level: 300 μM) were hypersarcosinemic based on these values. During homocysteine metabolism, sarcosine is formed by glycine methylation (Fig. 3). A folate deficiency typically induces homocysteinemia26, and the ExHC rats developed both hypersarcosinemia and homocysteinemia21. ExHC rats developed abnormal amino acid levels in blood in addition to abnormal lipid parameters observed previously5. This is the first study to show that abnormal sarcosine metabolism caused by decreased Sardh expression in the liver can lead to homocysteinemia. The blood homocysteine levels normal SD rats were approximately 5 μM27 and increased to approximately 20 μM due to deficiency of vitamin B12 or folic acid27. Mutations in Bhmt, betaine-homocysteine methyltransferase, induce serum homocysteine levels to ~ 50 μM in mice28. Homocysteinemia was relatively severe (average 30 μM) in ExHC, compared with congenic rats that developed mild homocysteinemia (average 15 μM). Taken together, these results indicated that the ExHC rats had developed hypersarcosinemia with homocysteinemia.
Diagram of methionine/homocysteine metabolism. Reactions are shown as arrows in lines. Gene symbols for related enzymes are described in circles. Bhmt; betaine-homocysteine methyltransferase, Cbs; cystathionine beta-synthase, Dmgdh; dimethylglycine dehydrogenase, Gamt; guanidinoacetate methyltransferase, Gnmt; glycine N-methyltransferase, Ms; methionine synthase, Mthfr; methylenetetrahydrofolate reductase, Pcyt1a; phosphate cytidylyltransferase 1, Pemt; phosphatidylethanolamine N-methyltransferase, Sardh; sarcosine dehydrogenase.
Serum homocysteine levels in ExHC rats were approximately 50% of those in congenic rats. The 4.1 Mbp region on chromosome.14 of the congenic rats was derived from Brown Norway (BN) rats, and the rest of their genome was derived from ExHC rats. These facts indicate that an important regulator of Sardh is located in the Dihc2 region, which contains 33 genes including Smek23. However, the change was insufficient to explain why a similar change to that of Sardh expression was not evident in any other gene except Smek2 between ExHC and congenic rats3. Since Smek2 knockdown reduced Sardh expression in rat hepatocytes (Fig. 2), Smek2 is probably involved in Sardh regulation. The Smek2 protein can regulate protein phosphatase 4 (PP4)6, which is required for expression of the stress response transcriptional regulator, DAF-1629, which in turn is required for high-homocysteine-stress resistance in the nematode C. elegans30. Another study has shown that Smek suppresses the expression of Wnt signaling genes by promoting histone deacetylase31. Activation of the Wnt/β-catenin signal suppresses sterol regulatory element binding protein-1c (SREBP-1c), the master transcriptional regulator of lipid synthesis32,33. The expression of SREBP-1c is not affected in the ExHC rat liver5. Therefore, Wnt signaling might contribute little to Smek2 function. Sarcosinemia and homocysteinemia in ExHC rats might be mediated by these or other pathways rather than being directly regulated by Smek2.
Homocysteinemia is induced experimental animals by a deficiency of dietary vitamin B12 or folic acid23,24. Both cause a depression in homocysteine catabolism (methionine cycle) due to the depression of the folic acid metabolism. The nutritional conditions between ExHC and congenic rats did not significantly differ in the present study, so these deficiencies did not cause homocysteinemia. Betaine is a methyl donor for the conversion of homocysteine to methionine. Lower serum betaine levels suggested that homocysteinemia in ExHC rats is caused by a delay in homocysteine catabolism due to a betaine deficiency. Betaine is normally generated by the conversion of choline or glycine via sarcosine. The excessive accumulation of sarcosine in hepatocytes implied that betaine synthesis from glycine was low in ExHC rats. Additionally, a decrease in PE indicated that betaine synthesis from choline was also low in ExHC rats. This was considered to be a result of preferentially synthesizing PC, which is necessary for the formation of cell and lipoprotein membranes, from choline as well as betaine. The betaine deficiency in the livers of ExHC rats was caused by a lack of hepatic choline. Phosphatidylcholine is needed to construct stable lipid membranes because it has less lipid bilayer flexion than PE, PA, and lysophospholipids34,35. The ExHC rat liver slowly re-uptakes β-VLDL secreted from it5. The hepatic PC requirement in ExHC rats might be higher than that of other rat strains. Additionally, a decrease in PC supply from the de novo synthesis pathway, which is another source of PC, might be suppressed in ExHC rats because of the impaired fatty acid synthesis. In fact, PE, which is also a precursor of PC, was reduced in the livers of ExHC rats. Taken together, betaine was reduced because choline was preferentially used for PC synthesis in the liver of ExHC rats. As another regulatory factor, genes involved in one-carbon metabolism such as Bhmt and Sardh are regulated by intracellular and extracellular osmotic pressure36. Osmotic pressure around hepatic cells might be high because large amounts of sarcosine solute accumulated in the livers of ExHC rats. Hepatic Bhmt mRNA levels were significantly corelated with hepatic sarcosine levels (R = − 0.59, P < 0.01) and serum homocysteine levels (R = − 0.55, P < 0.01). However, hepatic BHMT protein expression levels show no significant co-relationship. Similar results were observed about hepatic CBS expressions. There should be a proportional relationship between mRNA levels and protein levels, but CBS and BHMT in this study did not. Since reports on post-translational regulation of CBS and BHMT were not found, the detail of this unlink between mRNA and protein levels was unknown. As one possibility, hepatic protein levels of BHMT and CBS may be saturated both in congenic and ExHC rats since both strains also develop homocysteinemia as described above. In this study, because the amount of increases in Cbs mRNA levels was as small as 15%, and the protein levels were not linked, CBS was considered to be unrelated to development of homocysteinemia. On the other hand, Bhmt mRNA levels decreased by Smek2 dyfunction, and by cholesterol feeding, respectively. But, BHMT protein levels were not changed in this study. There is a report that BHMT protein was also proportionally decreased linked to mRNA levels in high-fat-diet fed mouse37. As another possibility that BHMT protein levels did not reflect mRNA levels, Smek2 dysfunction may affect BHMT protein regulation in the liver. In any case, the changes of enzymes related to homocysteine metabolism were not direct causes for severe homocysteinemia in ExHC rats. Taken together, hepatic choline metabolism might be involved in the regulation of homocysteine metabolism in ExHC rats.
The decreased PC/PE ratio of the liver is linked to the progression of fatty liver and NASH in rats38,39. The normal range of the hepatic PC/PE ratio in mice is 1.5–2.040. The PC:PE ratios of both congenic and ExHC rats in this study were within this range. The PC:PE ratio of 1.9 indicated that the liver remained normal in ExHC rats. The present study showed that anomalies in Smek2 resulted in disrupted sarcosine, homocysteine, lipid, and carbohydrate metabolism, but might be protective only in terms of maintaining a robust hepatic lipid composition.
In conclusion, microarray analysis revealed that hepatic mRNA levels of Sardh (sarcosine dehydrogenase) were significantly lower in ExHC (Smek2−/−), than in congenic (Smek2+/+) rats. The ExHC rats developed hypersarcosinemia and further homocysteinemia due to abnormal sarcosine metabolism caused by decreased Sardh expression. In addition to regulating glucose metabolism4,5, Smek2 is also important for regulating amino acid metabolism.
Materials and methods
Animals and diets
Ethical approval for the experiments was granted by the Animal Care and Use Committee, Kyushu University (Authorization no.: A22-160-2). This study proceeded according to the guidelines for animal experiments at the Faculty of Agriculture and Graduate Course at Kyushu University. The study was carried out in compliance with the ARRIVE guidelines. Male ExHC/Sea and congenic rat colonies were maintained through brother-sister mating (Nutrition Chemistry Laboratory, Faculty of Agriculture, Kyushu University). All rats had free access to an NMF, non-purified diet (Oriental Yeast Co., Tokyo, Japan) and deionized water in a temperature-controlled room at 22–25 °C with a 12-h light cycle (0800–2000). Control and cholesterol diets were formulated according to the AIN76™ formula41 as (weight %): 0 or 2 cholesterol, 10 olive oil (fatty acid composition %: 10.1 palmitic acid, 0.6 palmitoleic acid, 3.8 stearic acid, 80.7 oleic acid, 4.1 linoleic acid, 0.4 α-linolenic acid, 0.3 eicosenoic acid, fatty acid as described5, 20 casein, 15 corn starch, 5 cellulose, 3.5 mineral mixture (AIN76™), 1 vitamin mixture (AIN76™), 0.3 DL-methionine, 0.2 choline bitartrate and sucrose to 100. Rats of each strain were randomly assigned to control diet group or cholesterol diet group (n = 6/group) at 4 weeks old. In this study, we chose a small sample size to screen for genetic alterations that were not found in previous studies. Therefore, the original intention was to collect basic evidence of genetic variation in a simple design of experiments. After feeding with these diets for 2 weeks, the rats were beheaded and blood samples were collected. Livers were immediately excised and serum was separated from blood by centrifugation at 1750×g at 4 °C for 5 min.
Determination of serum lipid levels
Serum cholesterol, triacylglycerol and phospholipid levels were measured using enzyme kits (T-CHO KAINOS, Triglyceride E-test WAKO and Phospholipid C-test WAKO) (KAINOS Laboratories, Inc., Tokyo, Japan and Fujifilm Wako Pure Chemical Corporation, Osaka, Japan).
RNA extraction
Total cellular RNA was isolated from liver tissues and cultured cells using the acid-guanidium-phenol-chloroform (AGPC) method as described5 and incubated with DNase I (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37 °C for 30 min to prevent DNA contamination.
Microarray analysis
Synthesis of Cy3-labeled cRNA probe
Samples of RNA from rats fed with cholesterol (n = 4/group) were analyzed using microarrays. Since the number of individuals that can be analyzed by microarray at one time was limited, four rats per group were selected with serum cholesterol levels close to the averages of each group. Cyanine3 (Cy3)-labeled complementary RNA (cRNA) was synthesized with following kits as described by the manufacturers. Complementary DNA (cDNA) was synthesized from RNA samples selected using RNA Spike-In Kit, One-Color (Agilent Technologies Inc., Santa Clara, CA, USA). Cy3-labeled cRNA was synthesized from cDNA, amplified using Quick Amp Labeling Kit, One-Color (Agilent), then purified using RNeasy mini spin columns (Qiagen, Hilden, Germany).
Microarray hybridization and data analysis
Cy3-labeled cRNA (1.65 μg) was fragmented using Covaris M (Covaris Inc., Woburn, MA, USA), then hybridized to the Whole Rat Genome (4 × 44 K) Oligo Microarray (Agilent) using a Gene Expression Hybridization Kit (Agilent). Slides were washed three times with Gene Expression Wash Buffers 1 and 2 (Agilent). The slides were dried and visualized using an Agilent Microarray Scanner (Agilent). The density of spots was measured by Feature Extraction, the software provided with the microarray scanner. After background correction and normalization, we compared levels of gene expression corresponding to the density of each spot, between the ExHC and congenic rats.
Determination of hepatic protein levels
Hepatic protein levels of SARDH (100 kDa), CBS (61 kDa), and BHMT (45 kDa), were measured by western blotting. Liver tissues (50 mg) were homogenized in 500 μL of 10 mM Tris–HCl containing 1 mM EDTA (pH 7.4) using a biomasher. Protein concentrations in liver homogenates were measured with a commercial kit (DC™ Protein Assay) (Bio-Rad Laboratories, Inc., CA, USA). The lysate solutions containing 50 ug protein were transferred to an 0.6 ml-tubes, vacuum dried, and then redissolved in 50 µl of sample buffer (0.M Tris–HCl, 2% SDS, 10% glycerol, 1.55% dithiothreitol). This protein solutions (15 µl/well) were applied to SDS-PAGE (5% acrylamide gel for stacking, 10% acrylamide gel for separating). Protein in gel was transblotted on Amersham™ Hybond™ P0.45 PVDF membrane (GE Healthcare, IL, USA). The blocking of the PVDF membrane was done with blocking reagent (Blocking One, Nacalai tesque, Kyoto, Japan). Each protein on the membrane was stained with anti-SARDH rabbit IgG antibody (1:3000) (22762-1-AP) (Proteintech Group, Inc., IL, USA), anti-CBS rabbit IgG antibody (1:3000) (14787-1-AP) (Proteintech), and anti-BHMT rabbit IgG antibody (1:3000) (15965-1-AP) (Proteintech) for 1st antibody, and anti-rabbit IgG antibody (1:3000) (NA934) (Merck KGaA., Darmstadt, Germany) for 2nd antibody. Antibodies were with Tween20-containing phosphate buffered saline (PBS-T) containing 1% Skim Milk for immunoassay (Nacalai tesque). As an internal standard protein, hepatic levels of β-actin (43 kDa) or GAPDH (34 kDa) were detected with anti-β-actin rabbit IgG antibody (1:3000) (Code: PM053) (Medical & biological laboratories Co., LTD., Tokyo, Japan) anti-GAPDH mouse IgG antibody (1:25,000) (60004-1-AP) (Proteintech), anti-rabbit IgG antibody and anti-mouse IgG antibody (1:3000) (NA931) (Merck KGaA). BHMT expression was measured next to GAPDH. For re-probing of BHMT, anti-GAPDH IgG antibody on the membrane was stript with WB Stripping Solution (Code# 05680-21) (Nacalai tesque). These proteins were detected with ECL™ Prime Western Blotting Detection Reagent (GE Healthcare) and LAS4000 (GE Healthcare). Intensities of observed protein bands were measured with KyPlot 6.0 (KyensLab Inc.) (https://www.kyenslab.com/en-us/). Whole pictures of menbranes were provided as Supplemental figures (S1, S2).
Smek2 knockdown
Preparation of lipoprotein deficient serum (LPDS)
We prepared lipoprotein deficient serum (LPDS) from Gibco (LOT#366208) fetal bovine serum (FBS) (Thermo Fisher Scientific Inc.). A solution of potassium bromide (32.8 g) in 100 mL of FBS was ultracentrifuged at ~ 145,000×g using an RP-55T rotor (Hitachi Koki Co., Tokyo, Japan) for 40 h in an SCP70H2 ultracentrifuge (Hitachi Koki Co.), then LPDS was collected from the ultracentrifuge tubes and dialyzed against PBS twice for 1 h each, then overnight at 4 °C. Dialyzed LPDS sterilized using syringe filters with 0.45-μm pores (Whatman International Ltd., Maidstone, UK) was included in cell culture medium.
Cell culture
MCA-RH7777 rat hepatoma cells (DS Pharma Biomedical Co., Ltd., Osaka, Japan) were maintained in basal media, DMEM (pH 6.9) (Thermo Fisher Inc.) containing 10% v/v FBS, 1.0 × 105 IU/L penicillin, 0.1 g/L streptomycin, and 3.7 g/L NaHCO3 under a 5% CO2 atmosphere at 37 °C. Cells that reached 80%–90% confluence were subcultured for 48 h, then diluted to 3 × 105/mL in NEAA (non-essential amino acid) medium, DMEM containing 10% v/v LPDS, 0.28 mM alanine, 0.33 mM asparagine, 0.35 mM proline monohydrate, 0.23 mM asparagusic acid, 0.51 mM glutamic acid.
Smek2 siRNA treatment
Diluted cells (1 mL) were seeded in 12-well plates and incubated for 24 h. Thereafter, 200 μL of siRNA-lipid complex, DMEM containing 0.075 μM Smek2 siRNA or negative control siRNA (Cosmobio Co., Ltd., Tokyo, Japan) (Table 5), and Lipofectamine RNAi MAX (1.5 μL; Thermo Fisher Scientific Inc.) were added to the wells. The medium was replaced 24 h after transfection, then total RNA was extracted from the cells after 24 h thereafter.
Determination of hepatic mRNA levels
Complementary DNA for real-time reverse transcription polymerase chain reactions (RT-PCR) was synthesized from total RNA (1.0 μg) using Transcriptor First Strand cDNA Synthesis Kits (Roche Holdings AG., Basel, Switzerland). The expression of 10 genes was measured using real-time RT-PCR with SYBR Premix EX Taq II kits and a Thermal Cycler Dice Real Time System TP800 (Takara, Bio Inc., Kusatsu, Japan). The amplification program comprised 10 s of denaturation at 95 °C, annealing for 15 s, and elongation for 10 s at 72 °C. The mRNA levels were normalized to the internal standard β-glucuronidase gene (Gusb). Table 6 shows the primer sequences used for RT-PCR.
Determination of serum and hepatic sarcosine levels
Serum and hepatic sarcosine levels were measured using sarcosine assay kits (BioVision, San Francisco, CA, USA). Liver tissues (50 mg) were homogenized in 500 μL of 10 mM Tris–HCl containing 1 mM EDTA (pH 7.4) using a biomasher. Enzymes in the homogenates were deactivated by incubation at 60 °C for 10 min, then serum and hepatic sarcosine levels were measured in 20 and 10 μL of liver homogenates and serum samples, respectively.
Determination of serum betaine levels
Serum betaine (trimethylglycine) levels were measured using high-performance liquid chromatography (HPLC) as described42. Serum samples (50 μL) were mixed with an equal volume of 100 mM KH2PO4 in screw-top microcentrifuge tubes, then derivatization solution (900 μL; 12.5 mM 18-crown-6, 50 mM 4-bromophenacyl bromide in acetonitrile) was added. The tubes were vacuum-packed in plastic bags and placed in a water bath at 80 °C for 1 h. After cooling to room temperature, the samples were centrifuged at 1000×g. Supernatants (15 μL) were directly injected into the HPLC comprising a 600 Controller (Waters Corp., Milford, MA, USA), a 486 Tunable Absorbance Detector (Waters Corp.), Supelcosil™ LC-SCX HPLC column, particle size 5 μm, 25 cm × 4.6 mm) (Sigma-Aldrich, St. Louis, MO, US), mobile phase, acetonitrile:ultra-pure-water (9:1) containing 22 mM choline, at a flow rate of 1.5 mL/min.
Determination of serum homocysteine levels
Serum homocysteine levels were measured by using reverse phase HPLC as described43. Serum samples (200 μL) were incubated at 4 °C for 30 min in 10% tri-n-butylphosphine in dimethyl formamide (20 μL), then cold trichloroacetic acid (200 μL) was added. Protein was precipitated by centrifugation at 1000×g for 5 min. Tubes containing 200 μL of supernatant, 200 μL of 0.25 M boric acid (pH10.5), 100 μL of 1.0 mg/mL SBD-F (fluorescent substance) in 2.5 M boric acid (pH7), were vacuum-packed in plastic bags and shaken at 60 °C for 1 h. Samples were iced and filtered using a Millipore filter (DISMIC-3cp) with 0.45-μm pores (Advantec, Tokyo, Japan). Filtrates (10 μL) were injected into the HPLC (600 Controller; 470 scanning fluorescence detector (excitation and emission, 385 and 515 nm, respectively; Bondasphere 5 μ, C18 300 Å, particle size, 5 μm, 15 cm × 3.9 mm; [all from Waters Corp.], mobile phase, 0.1 M phosphoric acid [pH 3] containing 10% v/v methanol; flow rate, 1 mL/min).
Fractionation of hepatic phospholipid levels
Hepatic lipids were extracted as described by Folch et al.44. Hepatic phospholipids were fractionated by thin-layer chromatography (TLC) using the solvent described by Wada et al.45. Phospholipids separated from hepatic lipids in the following order from the bottom of the TLC plate: lysophosphatidylcholine (LPC), sphingomyelin (SPH), phosphatidylcholine (PC), phosphatidylserine and phosphatidylserine (PS + PI), phosphatidylethanolamine (PE), and neutral lipids (NL). Hepatic lipids (1 μL) were dried, fused to 50 μL of hexane, and spotted onto TLC plates (Merck). The hexane was evaporated from the plates, then the lipids were separated in a chamber containing gas from the solvent (CHCl3:MetOH:acetic acid: ultrapure water, 50:30:8:4) until the solvent reached the top of the TLC plates (~ 2 h). The TLC plates were removed from the chamber and dried. Lipids on the plates were visualized by exposing them to iodic gas in another chamber. Thereafter, colored phospholipid bands on the plates were edged with pencil in a fume hood and scraped into test tubes using a razor.
Determination of hepatic phospholipid levels
Phospholipids were mixed with 1 mL of 70% perchloric acid in tubes and heated at 180 °C for 30 min to 1 h until the solution became clarified. After cooling to room temperature, the following were added to the tubes in the order: ultrapure water (5 mL), 2.5% ammonium molybdate (1 mL), and 10% ascorbic acid (1 mL). The tubes were vortex-mixed, placed in boiling water for 5 min with a marble as a tap, then cooled to room temperature in a water bath, The absorbance of each solution was measured at 820 nm.
Statistical analysis
We extracted and compared genes with altered expression levels in the microarray analysis between ExHC and congenic rats. Values with P = 0.01 were considered significantly different. Differences in data of in vivo experiment were assessed using two-way analyses of variance (ANOVA). When interaction between two elements was identified, the data were analyzed using Tukey–Kramer post-hoc tests. Differences in data of in vitro experiment were assessed using Student’s t-test. Values with P < 0.05 were considered significantly different. Data are shown as means with standard error of the means (SEM). Data were statistically analyzed using Excel 2011 with the Statcel 3 add-in (Microsoft Corp., Redmond, WA, USA).
Data availability
The data underlying the results presented in the study are available from Zenodo (https://zenodo.org/) (reserved https://doi.org/10.5281/zenodo.7370257).
Abbreviations
- AGPC:
-
Acid-guanidium-phenol-chloroform
- ANOVA:
-
Analyses of variance
- BN rat:
-
Brown Norway rat
- CREB:
-
CAMP response element-binding protein
- CREBBP:
-
CAMP response element-binding protein binding protein
- CRTC2:
-
CAMP response element-binding protein-regulated transcription coactivator 2
- congenic rat:
-
Congenic ExHC.BN-Dihc2BN rat
- Cy3:
-
Cyanine3
- DIHC:
-
Diet-induced hypercholesterolemia
- ExHC rat:
-
Exogenously hypercholesterolemic rat
- HNF-1a:
-
Hepatocyte nuclear factor-1a
- HPLC:
-
High-performance liquid chromatography
- LATS1:
-
Large tumor suppressor kinase 1
- LPC:
-
Lysophosphatidylcholine
- LPDS:
-
Lipoprotein deficient serum
- MODY3:
-
Maturity-onset diabetes of the young
- NL:
-
Neutral lipids
- PAP1:
-
Production of anthocyanin pigment 1
- PBS:
-
Phosphate buffered saline
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- Pfkl:
-
Liver-type phosphofructokinase
- PI:
-
Phosphatidylinositol
- PS:
-
Phosphatidylserine
- RSTS:
-
Rubinstein-Taybi syndrome
- SPH:
-
Sphingomyelin
- Sardh:
-
Sarcosine dehydrogenase
- SD rat:
-
Sprague–Dawley rat
- SEM:
-
Standard error of the means
- SREBP-1c:
-
Sterol regulatory element binding protein-1c
- TLC:
-
Thin-layer chromatography
- VLDL:
-
Cholesterol ester-rich very low density lipoprotein
References
Imai, Y., Matsumura, H., Shino, A., Oka, K. & Suzuoki, Z. Induction of aortic lipid deposition in a high-response (ExHC) rat fed a diet containing cholesterol and cholic acid. Atherosclerosis https://doi.org/10.1016/0021-9150(77)90072-7 (1977).
Asahina, M., Sato, M. & Imaizumi, K. Genetic analysis of diet-induced hypercholesterolemia in exogenously hypercholesterolemic rats. J. Lipid Res. https://doi.org/10.1194/jlr.M500257-JLR200 (2005).
Asahina, M. et al. Identification of SMEK2 as a candidate gene for regulation of responsiveness to dietary cholesterol in rats. J. Lipid Res. https://doi.org/10.1194/jlr.M800135-JLR200 (2009).
Tanaka, Y. et al. Low utilization of glucose in the liver causes diet-induced hypercholesterolemia in exogenously hypercholesterolemic rats. PLoS ONE https://doi.org/10.1371/journal.pone.0229669 (2020).
Tanaka, Y. et al. Unavailability of liver triacylglycerol increases serum cholesterol concentration induced by dietary cholesterol in exogenously hypercholesterolemic (ExHC) rats. Lipids Health Dis. https://doi.org/10.1186/1476-511X-13-19 (2014).
Yoon, Y. S. et al. Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.1012665107 (2010).
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/nrm3072 (2011).
Chao, C. S. et al. Novel GATA6 mutations in patients with pancreatic agenesis and congenital heart malformations. PLoS ONE https://doi.org/10.1371/journal.pone.0118449 (2015).
Gragnoli, C. et al. Maturity-onset diabetes of the young due to a mutation in the hepatocyte nuclear factor-4 alpha binding site in the promoter of the hepatocyte nuclear factor-1 alpha gene. Diabetes https://doi.org/10.2337/diacare.46.10.1648 (1997).
Okada, T. et al. A complex polymorphic region in the brain-derived neurotrophic factor (BDNF) gene confers susceptibility to bipolar disorder and affects transcriptional activity. Mol. Psychiatry https://doi.org/10.1038/sj.mp.4001822 (2006).
Postma, A. V. et al. A gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.107.168294 (2008).
Kamenarova, K. et al. Identification of a novel de novo mutation of crebbp in a patient with Rubinstein-Taybi syndrome by targeted next-generation sequencing: A case report. Hum. Pathol. https://doi.org/10.1016/j.humpath.2015.09.004 (2016).
Parker, D. et al. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol. Cell Biol. https://doi.org/10.1128/mcb.16.2.694 (1996).
Schena, M. et al. Microarrays: Biotechnology’s discovery platform for functional genomics. Trends Biotechnol. https://doi.org/10.1016/s0167-7799(98)01219-0 (1998).
Brown, P. O. & Botstein, D. Exploring the new world of the genome with DNA microarrays. Nat. Genet. https://doi.org/10.1038/4462 (1999).
Fan, Q. et al. Microarray study of gene expression profile to identify new candidate genes involved in the molecular mechanism of leptin-induced knee joint osteoarthritis in rat. Hereditas https://doi.org/10.1186/s41065-017-0039-z (2017) (eCollection 2018).
Hao, Y., Chun, A., Cheung, K., Rashidi, B. & Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. https://doi.org/10.1074/jbc.M709037200 (2008).
Tohge, T. et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. https://doi.org/10.1111/j.1365-313X.2005.02371.x (2005).
Levy, H. L., Coulombe, J. T. & Benjamin, R. Massachusetts metabolic disorders screening program: III. Sarcosinemia. Pediatrics 74, 509–513 (1984).
Sreekumar, A. et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature https://doi.org/10.1038/nature07762 (2009).
Harding, C. O. et al. sar: a genetic mouse model for human sarcosinemia generated by ethylnitrosourea mutagenesis. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.89.7.2644 (1992).
Bar-joseph, I. et al. Mutations in the sarcosine dehydrogenase gene in patients with sarcosinemia. Hum. Genet. https://doi.org/10.1007/s00439-012-1207-x (2012).
Blom, W. & Fernandes, J. Folic acid dependent hypersarcosinaemia. Clin. Chim. Acta https://doi.org/10.1016/0009-8981(79)90445-5 (1979).
MacMillan, L. et al. Cobalamin deficiency results in increased production of formate secondary to decreased mitochondrial oxidation of one-carbon units in rats. J. Nutr. https://doi.org/10.1093/jn/nxx057 (2018).
Allen, R. H., Stabler, S. P. & Lindenbaum, J. Serum betaine, N, N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism https://doi.org/10.1016/0026-0495(93)90198-w (1993).
Lin, J. Y., Kang, S. S., Zhou, J. M. & Wong, P. W. Homocysteinemia in rats induced by folic acid deficiency. Life Sci. https://doi.org/10.1016/0024-3205(89)90225-7 (1989).
Blaise, S. et al. Mild neonatal hypoxia exacerbates the effects of vitamin-deficient diet on homocysteine metabolism in rats. Pediatr. Res. https://doi.org/10.1203/01.PDR.0000161406.19231.98 (2005).
Teng, Y. W., Cerdena, I. & Zeisel, S. H. Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J. Nutr. https://doi.org/10.3945/jn.112.166835 (2012).
Sen, I. et al. DAF-16/FOXO requires protein phosphatase 4 to initiate transcription of stress resistance and longevity promoting genes. Nat. Commun. https://doi.org/10.1038/s41467-019-13931-7 (2020).
Drobny, A. et al. Betaine-rich sugar beet molasses protects from homocysteine-induced reduction of survival in Caenorhabditis elegans. Eur. J. Nutr. https://doi.org/10.1007/s00394-019-01944-3 (2020).
Lyu, J., Jho, E. H. & Lu, W. Smek promotes histone deacetylation to suppress transcription of Wnt target gene brachyury in pluripotent embryonic stem cells. Cell Res. https://doi.org/10.1038/cr.2011.47 (2011).
Abiola, M. et al. Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS ONE 4, e8509 (2009).
Zhang, S., Yan, L. & Kim, S. M. Vanadium-protein complex inhibits human adipocyte differentiation through the activation of β-catenin and LKB1/AMPK signaling pathway. PLoS ONE 15, e0239547 (2020).
Gong, J. et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. https://doi.org/10.1083/jcb.201104142 (2011).
Holte, L. L., Peter, S. A., Sinnwell, T. M. & Gawrisch, K. 2H nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys. J. https://doi.org/10.1016/S0006-3495(95)80422-4 (1995).
Hoffmann, L. et al. Osmotic regulation of hepatic betaine metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. https://doi.org/10.1152/ajpgi.00332.2012 (2013).
Li, Y. et al. Betaine alleviates high-fat diet-induced disruptionof hepatic lipid and iron homeostasis in mice. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23116263 (2022).
Arendt, B. M. et al. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl. Physiol. Nutr. Metab. https://doi.org/10.1139/apnm-2012-0261 (2013).
Ling, J., Chaba, T., Zhu, L. F., Jacobs, R. L. & Vance, D. E. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology https://doi.org/10.1002/hep.24782 (2012).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Rev. Biomembr. https://doi.org/10.1016/j.bbamem.2017.04.006 (2017).
American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc Committee on standards for nutritional studies. J. Nutr. 107, 1340–1348 (1997).
Laryea, M. D., Steinhagen, F., Pawliczek, S. & Wendel, U. Simple method for the routine determination of betaine and N, N-dimethylglycine in blood and urine. Clin. Chem. 44, 1937–1941 (1998).
Araki, A. & Sako, Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. https://doi.org/10.1016/0378-4347(87)80438-3 (1987).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Wada, M. & Sugano, M. The practical methods for utilization of thin-layer chromatography to the analysis of glycerolipids from animal tissues. Sci. Bull. Fac. Agric. Kyushu Univ. 26, 505–516 (1972).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 19580141 (MSato) and 23・1071 (YT). The authors thank Editage (http://www.editage.jp) for the English language editing.
Author information
Authors and Affiliations
Contributions
M.Sato designed and supervised the implementation of this study; K.I. supervised the implementation of this study; Y.T., M.K., S.N., C.Y., M.A., M.Sakamoto, B.S., and K.T. performed experiments; Y.T., M.K., K.T., and M.Sato analyzed data and wrote the paper; All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanaka, Y., Kawano, M., Nakashima, S. et al. Mutation in Smek2 regulating hepatic glucose metabolism causes hypersarcosinemia and hyperhomocysteinemia in rats. Sci Rep 13, 3053 (2023). https://doi.org/10.1038/s41598-022-26115-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26115-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.