Abstract
This nationwide population-based cohort study searched for demographic, comorbid, behavioral, and reproductive risk factors for idiopathic macular hole (MH) development using data provided by the Korean National Health Insurance Service. A total of 4,496,867 individuals aged 50–79 years who participated in the Korean National Health Screening Program in 2013 or 2014 were included. Participants were followed up until December 2018, and incident cases of idiopathic MH were identified. Prospective associations between incident idiopathic MH and various covariates were investigated using multivariable-adjusted Cox proportional hazard models. During an average follow-up period of 4.91 years, 3054 patients were newly diagnosed with idiopathic MHs. Women showed greater risk (hazard ratio of 1.71) and earlier presentation of idiopathic MH than men. Compared to the normal body mass index group, the obese group (≥ 25 kg/m2) showed a significantly lower risk of idiopathic MH. Among postmenopausal women, those with two or more children showed a greater risk of idiopathic MH than those who had not been pregnant, with a hazard ratio of 1.80. In conclusion, idiopathic MH occurred earlier and greater in women. Childbirth were associated with an increased risk of MH development, and obesity was associated with a lower risk of MH.
Similar content being viewed by others
Introduction
The macular hole (MH) is a full-thickness defect of the retinal tissue occurring at the center of the macula. MH can occur secondary to diabetic retinopathy, pathologic myopia, and other ocular condition, but most MH cases develop without obvious secondary cause and this condition is referred to as idiopathic MH. Idiopathic MH commonly affects people over 60 years of age and is caused by anteroposterior vitreomacular traction resulting from anomalous posterior vitreous detachment1. The prevalence of idiopathic MH ranges from 0.02 to 0.33%2,3, and the incidence rate of idiopathic MH in the general population was reported to be 7.80 per 100,000 person-years in the Olmsted County4 and 3.14 per 100,000 person-years in South Korea5. Approximately 10–16% of patients have MH in both eyes6,7, which implies that certain individuals have a greater tendency to develop MH. Nevertheless, the reason why some individuals have stronger anteroposterior vitreomacular traction and eventually progress to idiopathic MH is poorly understood.
Previous epidemiological studies attempted to identify the risk factors of MH and revealed that older age and female sex are significant risk factors for MH formation, with a reported female-to-male ratio from 2.0 to 3.3 from population-based studies4,5. However, risk factors for developing idiopathic MH other than age and sex are not yet known. Specifically, much remains unknown for the reasons why the disease develops frequently in postmenopausal women. A limited number of epidemiological studies have investigated other risk factors for MH development. Among those studies, the results were often inconsistent and limited by case–control design or the small number of study subjects, less than or about a hundred cases of MH8,9. Recently, a large-scale cohort study in the United States investigated the risk factors for developing idiopathic MH; however, did not sufficiently evaluate covariates such as behavioral or female reproductive factors10.
Herein, we performed a comprehensive risk factor analysis including demographic, comorbid, behavioral, and female reproductive factors from a nationwide population-based cohort to provide epidemiological basis for MH development and advance our understanding of the pathophysiology.
Methods
Setting
This was a nationwide, population-based, retrospective cohort study that used authorized data from the Korean National Health Insurance Service (NHIS) database. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Republic of Korea (IRB File Number 2020-11-074). The board waived the requirement for informed consent based on the use of de-identified public data and the retrospective design of the study.
The NHIS is the insurer in South Korea that provides mandatory universal medical care and holds all medical information and health claims data in the country. The NHIS runs the National Health Screening Program (NHSP)11, a free biennial general health examination provided to all Koreans aged above 40 years. The NHIS also extends the National Cancer Screening Program (NCSP) for those receiving general health examinations through the NHSP12. The NCSP provides examinations for the stomach, liver, colorectal, breast, and cervical cancer for all registered individuals at the age indicated for cancer screening (breast cancer examination beginning at 40 years of age).
The NHIS database stores data on demographic information (age, sex, income level, date of death); health claims (date of clinical visit, diagnosis code defined by the Korean Classification of Diseases seventh revision [KCD-7], prescription records); and those from the NHSP and NCSP (participants’ responses to questionnaires, anthropometric measurements, laboratory test results) of all registered Koreans. This database has been widely used in previous studies that identified risk factors for various diseases13,14. Detailed information regarding the database profiles has been provided elsewhere11,12.
Study design and participants
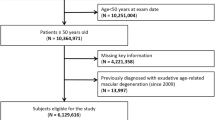
This study included two separate analyses (Fig. 1). In Analysis 1, we included both male and female participants who underwent NHSP, and investigated demographic, comorbid, and behavioral risk factors for MH development. In Analysis 2, only postmenopausal women who underwent NCSP were included to identify the association between female reproductive factors and the risk of MH development.
Among the entire South Korean population, participants who underwent national health examinations in 2013 and 2014 were identified. We included participants aged 50–79 years at baseline as most cases of posterior vitreous detachment would have already occurred in those over 80 years, and MH occurring before 50 years would have a secondary cause for MH development. Therefore, a total of 5,965,039 participants aged 50–79 years who participated in NHSP in 2013 and 2014 were identified. Individuals who had a history of vitrectomy (surgical code: S5121, S5122) from 2002 to the date of the examination were excluded from the study (n = 43,089) as they would not have vitreous that caused vitreofoveal traction and MH development. Individuals who had been diagnosed with retinal detachment (diagnostic code: H33), retinal vascular occlusion (diagnostic code: H34), uveitis (diagnostic code: H20, H30), and ocular trauma (diagnostic code: S05) from 2002 to the date of the examination were also excluded (n = 430,008) as they could cause secondary MH development. Individuals with diabetes mellitus at baseline and previous history of degenerative myopia (diagnostic code: H44.2) were then excluded as they are related to development of diabetic and myopic MH. Finally, 4,496,867 participants were included in Analysis 1.
Among those included in Analysis 1, 2,166,570 women underwent NCSP with NHSP and 1,918,134 postmenopausal women were identified. Individuals who had a history of hysterectomy (n = 182,945) were excluded from the study as they could have undergone simultaneous oophorectomy, influencing the participant's exposure to female hormones. Finally, 1,735,189 postmenopausal women were included in Analysis 2.
Clinical data and reproductive factors
Comorbid hypertension, diabetes, and dyslipidemia were identified based on the followings; (1) self-reported questionnaire responses; (2) health screening measurement results of blood pressure (hypertension, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg), fasting glucose (diabetes, fasting blood glucose levels ≥ 126 mg/dL), and total cholesterol (dyslipidemia, ≥ 240 mg/dL); (3) the presence of diagnostic codes (KCD-7 code: I15 for hypertension, E11–E14 for diabetes, E78 for dyslipidemia) combined with medication prescription codes within a year before the health screening examination. The history of stroke and heart disease was identified using self-reporting based on the NHSP questionnaire. The presence of chronic kidney disease was defined as a glomerular filtration rate of < 60 mL/min/1.73 m2 measured using blood sampling. Income level was categorized into quartiles according to the insurance premium level, which was determined by household income.
Data on health-related behaviors were collected using the NHSP questionnaire. Smoking status was divided into never-smokers and past/current smokers. Drinking habits were categorized into three levels: none, mild (< 6 times a week), and heavy (≥ 6 times a week). Those who had a moderate level of physical activity for more than 30 min a day for more than 5 days per week were categorized into the regular exercise group. The body mass index (BMI) was calculated as the weight (kg) divided by height squared (m2), and categorized as underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 23 kg/m2), overweight (23 ≤ BMI < 25 kg/m2), obese I (25 ≤ BMI < 30 kg/m2), and obese II (≥ 30 kg/m2), according to the Korean Society for the Study of Obesity15.
Reproductive factor variables were retrieved from the NCSP questionnaire responses. The questionnaire items included age at menarche and menopause, parity, history of hormone replacement therapy (HRT), and oral contraceptive pill use. Data on female reproductive factors were categorized as follows: age at menarche (< 14 years, 14–15 years, 16–17 years, and ≥ 18 years), age at menopause (< 40 years, 40–44 years, 45–49 years, 50–54 years, and ≥ 55 years), parity (0, 1, ≥ 2 children), duration of HRT (never, < 2 years, 2 to < 5 years, ≥ 5 years, and unknown), and duration of oral contraceptive pill use (never, < 1 year, ≥ 1 year, unknown).
Identification of incident MH development and follow-up
Incident MH cases were defined as those with both the diagnostic code for MH (H35.33) and the surgical codes for vitrectomy (S5121 or S5122) in the same claim after the date of the baseline examination5. Individuals with prior history of MH would not have been included in the study population, as we excluded those who had vitrectomy for any vitreoretinal disorder before the baseline examination. The incident date was defined as the date of the earliest claim that corresponded to the criteria for each patient. Incident MH cases that occurred after the diagnosis of retinal detachment, retinal vascular occlusion, uveitis, and ocular trauma were not counted as they could be secondary MH cases. Patients were followed from the date of health check-up to the date of incident MH, death, or the end of the study period (December 31, 2018), whichever came first.
Statistical analyses
The incidence rates of MH were calculated by dividing the number of incident cases by the total number of person-years. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazard models. The variables adjusted for each Cox model in Analysis 1 were as follows: model 1, age and sex; model 2, demographics (age, sex, income level) and comorbidities (hypertension, dyslipidemia, stroke, heart disease, and chronic kidney disease); model 3, demographics; comorbidities; and behavioral factors (smoking status, drinking habit, regular exercise, and BMI). In Analysis 2, the variables adjusted for each Cox model were as follows: model 1, age group; model 2, demographics (age, income level); comorbidities; and behavioral factors; model 3, demographics; comorbidities; behavioral factors; and reproductive factors (age at menarche, age at menopause, parity, duration of HRT, and duration of oral contraceptive pill). Additional sensitivity analyses were conducted in a broader population in which previous diabetes mellitus or degenerative myopia were not excluded. The purpose of sensitivity analyses was to examine the robustness of study results and to additionally evaluate the influence of diabetes mellitus and degenerative myopia on the development of overall MH (including diabetic MH and myopic MH). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Analysis 1: baseline characteristics
Table 1 presents the detailed baseline characteristics of the individuals studied in Analysis 1. The mean age of the participants was 59.4 years, and 54.1% were women. The average follow-up period was 4.92 years, and 2353 patients were newly diagnosed with idiopathic MH and underwent vitrectomy during the follow-up period.
Analysis 1: demographics, comorbidities, behavioral factors, and the risk of MH
Table 2 shows the incidence rates and HRs with 95% CIs for MH development according to various covariates. The incidence rate of idiopathic MH was 10.63 per 100,000 person-years. The age group from 65 to 69 years showed the highest risk of MH development during the follow-up period. In multivariable-adjusted analysis (model 3), female patients had a greater risk of idiopathic MH development with HR (95% CI) of 1.70 (1.50–1.93). Obese individuals (BMI ≥ 25 kg/m2) presented a lower risk of MH development than those with normal BMI (HR, 0.83; BMI, 25–30 kg/m2 and 0.80; BMI ≥ 30 kg/m2). Individuals in the highest income quartile had a greater risk of MH than those in the lowest income quartile with HR (95% CI) of 1.17 (1.05–1.30).
Table 3 presents the HRs and 95% CIs of idiopathic MH according to sex and its interaction with covariates in the multivariable-adjusted model (model 3). Men showed the greatest risk of MH development in the age group of 65–69 years, while women had the highest risk in the age group of 60–64 years (p-value for interaction < 0.001). No other variables showed any significant interactions between men and women.
Analysis 2: baseline characteristics
Table 4 shows the detailed baseline characteristics of the postmenopausal women included in Analysis 2. The mean age of the participants was 60.9 years. The average follow-up period was 4.91 years, and 1567 patients were newly diagnosed with MH and underwent vitrectomy during the follow-up period. The average age at menarche and menopause were 16.1 and 50.7 years, respectively. Approximately 90% of the participants had two or more children, and 20% had previously received HRT.
Analysis 2: female reproductive factors and the risk of MH
Table 5 shows HRs and 95% CIs of MH development according to female reproductive factors. In the fully adjusted model (model 3), participants who had given birth once or more had a greater risk of MH development compared to those who were nulliparous, with HR (95% CIs) of 1.58 (0.98–2.56) in those with having one child and 1.91 (1.15–3.18) in those with two or more children. Age at menopause and the history of HRT were not associated with the future risk of MH.
Sensitivity analyses
A total of 5,491,942 individuals were included in the additional sensitivity analyses. An average follow-up duration was 4.91 years and 3054 cases of MH developed. The age group from 65 to 69 years showed the highest risk of MH and obesity was associated with reduced risk of MH development (Supplemental Table 1). The history of giving birth was associated with greater risk of incident MH in postmenopausal women (Supplemental Table 2). The overall results of sensitivity analyses were similar to that of the main analyses. Diabetes mellitus and degenerative myopia were associated with increased risk of MH development.
Discussion
This nationwide population-based cohort study revealed that age, sex, obesity, and parity influence the risk of idiopathic MH formation. And the risks of idiopathic MH according to age were significantly different between men and women. There has been a paucity of epidemiologic studies investigating the risk factors of MH development. Some previous studies investigated various parameters including behavioral factors, reproductive factors, and biochemical data; however, were limited by a small number of MH cases and a case–control design8,9. A recent cohort study from the United States included a fairly large number of participants; however, only investigated demographic parameters and comorbidities as risk factors for MH10. The present study addressed the lapses of these previous studies by including the largest number of participants and assessing various covariates comprehensively and would provide solid epidemiological evidence on the risk of MH in the available literature.
The incidence of idiopathic MH according to individuals’ age showed an inverted U pattern, with peak MH incidence in the age group of 65 to 69 years. MH occurs as a complication of posterior vitreous detachment16, and the peak MH incidence may reflect peak posterior vitreous detachment in that age group. Older individuals over 70 years of age are more likely to have complete posterior vitreous detachment, leading to a lower risk of MH development after the age at peak incidence. In the subgroup analysis (Table 3), women showed an earlier presentation of MH with the highest MH incidence in 60–64 years, while men had the most significant MH incidence in 65–69 years. The earlier presentation of MH in women has also been reported in previous studies5,17,18, and is attributed to the earlier progression of posterior vitreous detachment in women than in men19.
Female sex was a significant risk factor for idiopathic MH development, with an HR of 1.70 compared to males. Although the female preponderance in MH has been widely reported in various epidemiological studies5,10, the exact underlying mechanism for this predisposition is unknown. The most widely accepted biological explanation for more frequent MH development in women is the sudden drop in estrogen levels at menopause20. Estrogen plays a role in collagen and hyaluronic acid synthesis21. The sudden drop of estrogen level at menopause may cause loss of vitreous collagen and glycosaminoglycan, which could lead to premature and anomalous posterior vitreous detachment and result in more significant and earlier development of MH in postmenopausal women10,20. Although this estrogen theory prevails in the related literature, not much epidemiological or biological research supporting the theory exists and some studies even contradict it. Only one case–control study reported that the MH group had a lower proportion of current estrogen users compared with control participants with marginal statistical significance (p = 0.04)8. In contrast, another case–control study did not show any association between MH and HRT9. The estrogen levels in the vitreous gel of eyes with MH were not different from or higher than that of eyes with other retinal diseases in previous studies22,23, which implies null or even detrimental effects of estrogen on MH.
In the present study, the history of postmenopausal HRT was not associated with the risk of idiopathic MH development, which does not support the estrogen theory. Other parameters related to lifetime estrogen exposure (age at menarche and age at menopause) were not associated with the risk of idiopathic MH. Instead, parity was significantly associated with the risk of developing idiopathic MH. The Eye Disease Case–Control Study Group has previously reported the same association8. According to their study, the odds ratio (95% CI) of having MH was 1.80 (1.02–3.10) for those who had parity once or more compared to those who had never been pregnant. The biological reasons for this association are unclear. However, there has been a hypothesis expecting the linkage between parity and MH24. Mehdizadeh et al. suggested that a higher incidence of MH development might be attributable to relaxin, a hormone produced during pregnancy24. Relaxin facilitates the birth process by widening the pubic bone and softening the cervix by modulating collagen metabolism, inhibiting collagen synthesis, and enhancing its breakdown by increasing matrix metalloproteinases25,26. They suspected that pregnancy-induced relaxin secretion may affect collagen and hyaluronic acid metabolism in the vitreous as well, and may induce premature vitreous syneresis and early anomalous posterior vitreous detachment24. Accordingly, MH would be more prevalent and present at a younger age in women than in men. Moreover, the effect size (HR) of the female sex was similar to the effect size of parity (1.70 for women compared with men, and 1.58 for those who had one child and 1.91 for those who had two or more children compared with those without children, model 3), indicating that nulliparous females have a risk of MH development similar to that of males. Collectively, our study results suggest that parity, rather than estrogen exposure, such as the history of HRT or age at menarche and menopause, is closely associated with the risk of idiopathic MH. The precise pathophysiology underlying the linkage between parity and the increased risk of MH development should be further elucidated.
Additionally, those who were obese (BMI ≥ 25 kg/m2) had a significantly lower risk of developing idiopathic MH. The association between obesity and MH has not been previously reported. One possible explanation for this association is the association between obesity and myopia. Researchers have previously reported that people with higher BMI tend to be less myopic27,28,29. It has been suggested that obese individuals tend to have a greater amount of retrobulbar fat, which then increases retrobulbar pressure and hinders axial elongation of the globe28,29. Hence, obese individuals in the present study might be less myopic, which would lead to a lower risk of MH. We were unable to confirm the association between BMI and myopia and MH in the present study owing to the lack of data regarding refraction and axial length. Future epidemiological studies to confirm the association between BMI, myopia, and MH may provide insights into the underlying mechanisms of the association between obesity and MH.
The highest income quartile level was associated with an increased risk of MH undergoing vitrectomy. This might be because people with greater income may receive proper treatment for visual deterioration, while those with less income may be more likely to ignore symptoms, not visit ophthalmology clinics, and not receive proper management such as vitrectomy for MH. We adjusted income levels in multivariable analysis, which would have decreased bias that can be attributed to those with a lower income level not going to the hospital.
Sensitivity analyses including patients with diabetes and degenerative myopia draw results similar to the main analyses, which might be attributable to the fact that most of MH cases are idiopathic. Degenerative myopia was associated with an increased risk of MH development with an HR of 5.60. MH cases in degenerative myopia would mostly be myopic MH, which is related to a more complex pathogenetic mechanism than idiopathic MH. Posterior scleral ectasia related to degenerative myopia increases the degree of anteroposterior and tangential traction force which can lead to MH formation30. In addition, progressive retinal layer thinning caused by axial elongation makes the foveal structure vulnerable to traction force and further promotes the MH formation31,32. Diabetes was also associated with an increased risk of MH development. MH development in diabetic retinopathy has been widely reported, particularly in association with fibrovascular proliferation and macular edema33,34. The vitreous in diabetic eyes undergo abnormal collagen cross-linking35, and the retinal hemorrhage facilitates posterior hyaloid thickening34, which may lead to stronger vitreoretinal adhesion. Additionally, diabetic macular edema with intraretinal exudation/cyst may render the retina more vulnerable to vitreofoveal traction and lead to MH34. These may also have resulted in the greater incidence of MH in patients with diabetes in the present study.
This study conducted multiple analyses, and could have an increased risk of type 1 statistical error. However, the p-values achieved from Cox regression analyses were less than 0.001 for age, sex, and their interaction; and there was a clear trend that the degree of BMI and childbirth correlate with the risk of idiopathic MH. These implies that our results were not derived from a mere random error.
The present study has some limitations that need to be addressed. First, the incident MH was identified using claims data. Therefore, we could not perform medical chart level or imaging-based verification of the disease. The study also might have missed those who were unable to access the healthcare system or who declined to get vitrectomy for MH. Therefore, the incidence of MH might have been underestimated. These are the inherent limitations of the claims data-based study. Second, the KCD-7 code for MH can also be used for lamellar hole, so we might have included some cases in which vitrectomy was implemented in lamellar holes. However, vitrectomy is not generally performed for lamellar holes in South Korea, so the majority of our cases would be full-thickness MH, and very few would be lamellar hole cases. Third, the female reproductive parameters were collected using a self-reported questionnaire. Therefore, there is a possibility of bias attributable to inaccurate recall. Forth, as aforementioned, our study did not have data regarding ocular measures and other ocular comorbidities. In addition, it is not possible to perform per-eye-based analyses with NHIS database. Therefore, we were unable to investigate and confirm the ocular risk factors (e.g. refraction, cataract surgery, intravitreal injection) for MH development. Further studies addressing these limitations are required.
In conclusion, the present nationwide population-based cohort study revealed that age, sex, BMI, and parity in women significantly influenced the risk of future idiopathic MH development. This study would promote a better understanding of the disease entity and could serve as evidence for future research regarding the underlying biological mechanism of idiopathic MH.
Data availability
The datasets analyzed in the current study were provided by the Korean NHIS. The data are available at https://nhiss.nhis.or.kr with the permission of the NHIS.
References
Meuer, S. M. et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: The beaver dam eye study. Ophthalmology 122, 787–795 (2015).
Mitchell, P., Smith, W., Chey, T., Wang, J. J. & Chang, A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology 104, 1033–1040 (1997).
Rahmani, B. et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology 103, 1721–1726 (1996).
McCannel, C. A., Ensminger, J. L., Diehl, N. N. & Hodge, D. N. Population-based incidence of macular holes. Ophthalmology 116, 1366–1369 (2009).
Cho, S. C., Park, S. J., Byun, S. J., Woo, S. J. & Park, K. H. Five-year nationwide incidence of macular hole requiring surgery in Korea. Br. J. Ophthalmol. 103, 1619–1623 (2019).
Choi, J. H. et al. Development of idiopathic macular hole in fellow eyes: Spectral domain optical coherence tomography features. Retina 40, 765–772 (2020).
Ezra, E. et al. Incidence of idiopathic full-thickness macular holes in fellow eyes. A 5-year prospective natural history study. Ophthalmology 105, 353–359 (1998).
Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am. J. Ophthalmol. 118, 754–761 (1994).
Evans, J. R. et al. Systemic risk factors for idiopathic macular holes: A case-control study. Eye (Lond) 12(Pt 2), 256–259 (1998).
Ali, F. S., Stein, J. D., Blachley, T. S., Ackley, S. & Stewart, J. M. Incidence of and risk factors for developing idiopathic macular hole among a diverse group of patients throughout the United States. JAMA Ophthalmol. 135, 299–305 (2017).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640 (2017).
Kim, Y., Jun, J. K., Choi, K. S., Lee, H. Y. & Park, E. C. Overview of the National Cancer screening programme and the cancer screening status in Korea. Asian Pac. J. Cancer Prev. 12, 725–730 (2011).
Yoo, J. E. et al. Female reproductive factors and the risk of Parkinson’s disease: A nationwide cohort study. Eur. J. Epidemiol. 35, 871–878 (2020).
Yoo, J. E. et al. Association of female reproductive factors with incidence of fracture among postmenopausal women in Korea. JAMA Netw. Open 4, e2030405 (2021).
World Health Organization. Regional Office for the Western, P. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. 1–55. (Health Communications Australia, 2000).
Johnson, M. W. Posterior vitreous detachment: Evolution and complications of its early stages. Am. J. Ophthalmol. 149, 371-382e371 (2010).
Steel, D. H. et al. Factors affecting anatomical and visual outcome after macular hole surgery: Findings from a large prospective UK cohort. Eye (Lond) 35, 316–325 (2021).
Wang, J. et al. Pre- and post-operative differences between genders in idiopathic macular holes. BMC Ophthalmol. 20, 365 (2020).
Hayashi, K., Sato, T., Manabe, S. I. & Hirata, A. Sex-related differences in the progression of posterior vitreous detachment with age. Ophthalmol. Retina 3, 237–243 (2019).
Nuzzi, R., Scalabrin, S., Becco, A. & Panzica, G. Gonadal hormones and retinal disorders: A review. Front. Endocrinol. (Lausanne) 9, 66 (2018).
Shah, M. G. & Maibach, H. I. Estrogen and skin. An overview. Am. J. Clin. Dermatol. 2, 143–150 (2001).
Nishikawa, Y. et al. A comparison of sex steroid concentration levels in the vitreous and serum of patients with vitreoretinal diseases. PLoS ONE 12, e0180933 (2017).
Inokuchi, N. et al. Vitreous estrogen levels in patients with an idiopathic macular hole. Clin. Ophthalmol. 9, 549–552 (2015).
Mehdizadeh, M., Jamshidian, M. & Nowroozzadeh, M. H. Macular hole epidemiology. Ophthalmology 117, 2442–2443 (2010).
Samuel, C. S., Butkus, A., Coghlan, J. P. & Bateman, J. F. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: The influence of estrogen and progesterone in regulating relaxin activity. Endocrinology 137, 3884–3890 (1996).
Naqvi, T. et al. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res. Ther. 7, R1 (2004).
Wong, T. Y., Foster, P. J., Johnson, G. J., Klein, B. E. & Seah, S. K. The relationship between ocular dimensions and refraction with adult stature: The Tanjong Pagar Survey. Invest. Ophthalmol. Vis. Sci. 42, 1237–1242 (2001).
Stojanov, O., Stokić, E., Sveljo, O. & Naumović, N. The influence of retrobulbar adipose tissue volume upon intraocular pressure in obesity. Vojnosanit Pregl. 70, 469–476 (2013).
Gunes, A., Uzun, F., Karaca, E. E. & Kalaycı, M. Evaluation of anterior segment parameters in obesity. Korean J. Ophthalmol. 29, 220–225 (2015).
De Giacinto, C., Pastore, M. R., Cirigliano, G. & Tognetto, D. Macular hole in myopic eyes: A narrative review of the current surgical techniques. J. Ophthalmol. 2019, 3230695 (2019).
Lin, C. W., Ho, T. C. & Yang, C. M. The development and evolution of full thickness macular hole in highly myopic eyes. Eye (Lond) 29, 388–396 (2015).
Cho, H. Y., Kim, Y. T. & Kang, S. W. Laser photocoagulation as adjuvant therapy to surgery for large macular holes. Korean J. Ophthalmol. 20, 93–98 (2006).
Yeh, P. T., Cheng, C. K., Chen, M. S., Yang, C. H. & Yang, C. M. Macular hole in proliferative diabetic retinopathy with fibrovascular proliferation. Retina 29, 355–361 (2009).
Brazitikos, P. D. & Stangos, N. T. Macular hole formation in diabetic retinopathy: The role of coexisting macular edema. Doc. Ophthalmol. 97, 273–278 (1999).
Sebag, J., Buckingham, B., Charles, M. A. & Reiser, K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch. Ophthalmol. 110, 1472–1476 (1992).
Acknowledgements
We would like to thank Dr. Kyunga Kim from the Statistics and Data Center, Research Institute for Future Medicine, Samsung Medical Center, for assisting with the statistical analyses.
Author information
Authors and Affiliations
Contributions
S.H., S.W.K., S.J.K., Y.C., S.R., and J.C. designed the study. S.H., J.C., K.Y.S., D.H.L., and D.W.S. analyzed and interpreted the data. S.H. wrote the final paper. S.W.K., S.J.K., D.H.L., D.W.S., D.C., Y.C., S.R., and J.C. reviewed the design, results, and final paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hwang, S., Kang, S.W., Kim, S.J. et al. Risk factors for the development of idiopathic macular hole: a nationwide population-based cohort study. Sci Rep 12, 21778 (2022). https://doi.org/10.1038/s41598-022-25791-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25791-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.