Abstract
Whole Exome Sequencing (WES) studies provide important insights into the genetic architecture of serious mental illness (SMI). Genes that are central to the shared biology of SMIs may be identified by WES in families with multiple affected individuals with diverse SMI (F-SMI). We performed WES in 220 individuals from 75 F-SMI families and 60 unrelated controls. Within pedigree prioritization employed criteria of rarity, functional consequence, and sharing by ≥ 3 affected members. Across the sample, gene and gene-set-wide case–control association analysis was performed with Sequence Kernel Association Test (SKAT). In 14/16 families with ≥ 3 sequenced affected individuals, we identified a total of 78 rare predicted deleterious variants in 78 unique genes shared by ≥ 3 members with SMI. Twenty (25%) genes were implicated in monogenic CNS syndromes in OMIM (OMIM-CNS), a fraction that is a significant overrepresentation (Fisher’s Exact test OR = 2.47, p = 0.001). In gene-set SKAT, statistically significant association was noted for OMIM-CNS gene-set (SKAT-p = 0.005) but not the synaptic gene-set (SKAT-p = 0.17). In this WES study in F-SMI, we identify private, rare, protein altering variants in genes previously implicated in Mendelian neuropsychiatric syndromes; suggesting pleiotropic influences in neurodevelopment between complex and Mendelian syndromes.
Similar content being viewed by others
Introduction
Neuropsychiatric syndromes, such as schizophrenia (SCZ), bipolar disorder (BD), obsessive compulsive disorder (OCD) and substance use disorders (SUDs) (referred hereafter as serious mental illness [SMI]) often cluster in families1,2. Next generation sequencing (NGS) can be used to explore the genetics of complex, common disorders, using both case–control and family-based designs3. These include common variant genome wide association studies (GWAS) and exome wide studies that scan for rare coding variants4,5. Such methods have provided useful details about the contribution of rare variants to the genetic architecture of SMI, often complementing the common variant contributions identified in GWAS.
Case–control studies typically exclude relatives to minimize sampling bias and potential false positive associations. About 10% of persons affected with SMI have an affected first degree relative. The increased occurrence of potential disease relevant variants, in densely affected pedigrees, may offer clues towards genes and pathways involved in the neurobiology of SMI. Many studies employing whole exome sequencing (WES) in psychiatry have analyzed families with multiple affected members (reviewed in6). These studies have generally focused on pedigrees with a cluster of individuals affected with a specific SMI such as BD or SCZ. Family-based studies in SCZ7,8,9, BD10,11,12,13 and OCD14 have identified multiple rare de novo and loss of function variations relevant to the biology of each syndrome. Similarly, case–control association studies in SCZ4,5 and BD15,16 have also identified the contribution of rare variants of large effect, advancing the current understanding of genetic architecture of these syndromes. An overview of recent findings from family based and case–control sequencing studies in SMI is presented in the Supplement S1.
In summary, we note a trend towards a higher burden of rare, protein altering variations, across cases with different SMI syndromes, when compared to controls17,18. These variants tend to be overrepresented in genes that are integral to neurodevelopment and synaptic function, and these ontologies are often identified across SMI syndromes19,20,21. Some genes with a higher burden of rare variants, identified in the family studies, have also been implicated in other severe neuropsychiatric syndromes, with features of anomalies in neurodevelopment and neurodegeneration22,23. There seems to be considerable overlap of rare variants that contribute to the risks of SMI, across syndromes. This convergence has also been observed for common variants24, as well as across genes, gene networks25,26, molecular pathways27, and brain imaging endophenotypes28. A recent analyses that integrated GWAS findings across 11 major psychiatric syndromes, suggests a shared genetic architecture across syndromes at bio-behavioural, functional genomic and molecular genetic levels29. A co-aggregation of SMI syndromes, and overlapping symptom dimensions, are often seen within a family1,2. Rare variants, identified in families with multiple ill members, may thus explain a proportion of the risk in the population. They are obviously of great heuristic value, to explore the pathobiology of the disease, and correlates of clinical features.
In this study, we examined the occurrence of rare, deleterious variants in individuals from families that had multiple affected members (as identified in Accelerator Program for Discovery in Brain Disorders using Stem Cells (ADBS))30. Within pedigree segregation, as well as cross-sample case–control association tests, were used to prioritize risk variants. We examined the functional and clinical significance of the prioritized genes and variants, including evolutionary conservation, mutation intolerance, brain expression, protein function and disease relevance. Specifically, we examined if the genes carrying the prioritized variants are overrepresented in Mendelian neuropsychiatric syndromes, and synaptic genes (as attempted for SCZ in the Xhosa population4). In families segregating a variant in a gene linked to a syndrome, we also reviewed the clinical profile of affected individuals for symptoms and signs of the particular Mendelian syndrome.
Results
Sample
We sequenced 280 (131 females) individuals, including 220 from 75 families (F-SMI), and 60 unrelated-controls. Of the F-SMI samples, 160 were cases diagnosed with a SMI: SCZ (n = 63), BD (n = 80), OCD (n = 7), SUD (n = 7), complex SMI (n = 3) along with 60 family-controls without a lifetime diagnosis of mental illness. The demographic profile of the sample with age and sex distribution and illness profile of the affected sample is provided (Table 1). Fifty-one (68%) of the 75 families had cases with diagnosis across ≥ 2 of the 4 illness categories noted above.
Within the F-SMI familial sample, the median number of samples from cases per family were 2 (range: 1–6) and family-controls was 1 (range:1–7). A subset of 16 multi-sample F-SMI (≥ 3 exome samples) was selected for analysis of within pedigree segregation of putatively deleterious variants.
Variant profile
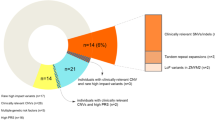
Variant calling the exomes of 280 samples resulted in identification of 793,818 unique variants. Among these, the median (interquartile range) number of synonymous and non-synonymous SNVs per sample were 9279 (2146) and 8721 (2433) respectively. The median (interquartile range) count of rare variants (minor allele frequency [MAF] < 0.1%) per sample was 717 (515). A breakdown of the variant profile in the sample is provided in Fig. 1. A total of 3556 unique single nucleotide polymorphisms and small insertion deletions were defined as rare putatively deleterious (RPD) variants based on the criteria of rarity (-MAF < 0.1%) and deleteriousness (loss of function or missense predicted deleterious) as further enumerated in the methods.
Conceptual overview—exome sequencing was done for individuals from families with multiple members with serious mental illness (SMI). The table shows the meaningful (significant) variants detected. Details of one representative gene (GEMIN5) are shown. The seven variants marked above have been implicated in Neurodevelopmental Disorder with Cerebellar Atrophy and Motor dysfunction: NEDCAM earlier. The variant marked below rs544452250 (5-154268943-C-G; E1433Q) was predicted to be deleterious, and was shared across three individuals with schizophrenia, BPAD and Substance use disorder, in one family.
Within pedigree segregation of private variants
Among 14 of the 16 multi-sample high-density families, we identified a total of 78 RPD variants in 78 different genes that were shared by ≥ 3 affected members within a family and were absent in the 60 unrelated-controls, defined as shared-broad RPD (sb-RPD) (Supplementary Table 1). 77 variants were private to one among the 14 pedigrees. Only the variant at the position chr5:154268943 in GEMIN5 gene was shared by 3/6 and 3/3 cases from families D002 and D012 respectively (Fig. 1, Table 2).
Of the 78 prioritized sb-RPD variants, 46 variants had MAF < 0.01% and 36 of these were absent or noted in no more than one unaffected family control and were defined as shared-stringent-RPD (ss-RPD) (Supplementary Table 1a).
In the set of 59 pedigrees with ≤ 2 case samples, 15 of the same 78 genes harboring sb-RPD were noted to carry RPD variants that were absent in the 60 unrelated-controls (Supplementary Table 2). Additionally, at the variant level, 4 of the 78 RPD variants prioritized from the set of 14 families, were also noted in this latter set of 59 families (Supplementary Table 3).
Overrepresentation analysis
Twenty of these 78 genes (25%) in the sb-RPD set are implicated in monogenic neurodevelopmental syndromes, with both dominant and recessive modes of inheritance in the Online Mendelian Inheritance in Man (OMIM) database. We performed an overrepresentation analysis of our gene-list on a list of 2450 genes having a Central Nervous System (CNS) phenotype annotation in OMIM clinical synopsis (OMIM-CNS) among 20,203 protein coding genes in the human genome (Supplementary Table 4). The prioritized gene list from multi-sample F-SMI was significantly enriched for genes implicated in monogenic CNS syndromes (Fisher’s Exact test OR = 2.51, 95%CI = 1.43–4.25, p = 0.001). These gene-phenotype relationships along with the genomic coordinates and pathogenicity prediction of the identified variants in this sample are described in Table 2.
The effect size of the overrepresentation was much larger in the gene harboring ss-RPD subset, defined with stringent prioritization criteria as 13 out of 36 prioritized genes (37%) were noted in OMIM-CNS gene-set (Fisher’s Exact test OR = 4.11, 95%CI = 1.91–8.48, p = 0.0002). These genes are marked within Table 2.
To check if the observed overrepresentation was specific to the CNS, we derived 19 additional gene-lists that encompassed genes implicated in OMIM syndromes affecting other organ systems (e.g. ‘cs_head_and_neck_head’, ‘cs_cardiovascular’ ‘cs_respiratory’ etc.). Among the 20 OMIM derived gene-lists (CNS+ above 19), for the 78 sb-RPD gene set, statistically significant overrepresentation at p < 0.0025 (0.05/20) was noted for gene lists annotated with clinical synopsis terms ‘central nervous system’ (pcor = 0.026), ‘head and neck’ (pcor = 0.037), and a nominally significant overrepresentation for ‘peripheral nervous system (0.057) (Supplement S2 and S3 and Supplementary Table 4). Among the 36 genes in ss-RPD gene-set, across 20 OMIM clinical synopsis term gene lists, only OMIM-CNS list derived from clinical synopsis term ‘central nervous system’ (pcor = 0.011) showed statistically significant overrepresentation. These results suggest that the prioritized gene lists harboring sb-RPD and ss-RPD variants were specifically overrepresented in clinical conditions involving these systems.
We performed an overrepresentation analysis in a set of genes (n = 516) carrying RPDs in the 60 unrelated controls that MAF < 0.01% in gnomAD South Asian and global samples similar to the threshold used in defining ss-RPD subset. While we noted a statistically significant overrepresentation (OR = 1.77, 95%CI = 1.41 to 2.22), the magnitude of effect was significantly lower when compared to the effect size noted for the ss-RPD gene-set (OR = 4.11, 95%CI = 1.91 to 8.48) consisting of 36 genes prioritized in within-pedigree analysis (z = 2.03, one tailed p-value = 0.01). Furthermore, the observed overrepresentation for control gene-set harboring RPDs was nonspecific as significant effects were noted for 13 out of 20 OMIM clinical synopsis genes-lists.
We also compared the effect size of the overrepresentation analysis statistic between genes harboring ‘segregating’ and ‘non-segregating’ RPDs among the cases within affected pedigrees. The magnitude of effect for overrepresentation was lower in the ‘non-segregating’ RPD gene set compared to segregating gene set (Supplementary S3, Supplementary Fig. 1).
Unlike the OMIM-CNS gene-list, we did not note an overrepresentation of the synaptic gene-list (1233 genes) from SynGO database31 for the set of 78 genes with sb-RPD variants segregating within pedigrees (Fisher’s Exact test OR = 1.5, 95%CI = 0.58–3.26, p = 0.34) or the 36 genes with ss-RPD variants (Fisher’s Exact test OR = 1.4, 95%CI = 0.28–4.62, p = 0.47).
Functional significance of prioritized genes
These 78 prioritized genes with sb-RPD variants in the within-pedigree analysis were significantly more conserved, in comparison with the background list of 20,125 remaining protein coding genes (mean (SD) conservation score (Zoonomia32) 0.63 (0.13) vs 0.59 (0.18), p = 0.014). Among these genes, 20 OMIM-CNS genes were significantly more mutation intolerant as compared to the other 59 genes, as suggested by lower LOEUF score33 ((mean (SD) − 0.6 (0.32) vs 0.99 (0.48), p = 0.001)) and a higher pLI score34 ((mean (SD) − 0.41 (0.49) vs 0.16 (0.36), p = 0.05)). The mean expression of these 20 genes was also significantly higher compared to the other 59 genes in the brain cortex (mean (SD) − 15.9 (15.3) vs 5.2 (7.5), p = 0.006); and rest of the brain (mean (SD) − 16.9 (15.2) vs 6.02 (8.4), p = 0.005).
Cross-pedigree association analysis using Sequence Kernel Association Test (SKAT)
We performed a geneset-level association analysis to examine genes with higher burden of RPD variants compared to controls. The analysis adjusted for kinship structure and population structure inferred by Principal Component Analysis. This analysis included two candidate gene-sets, viz. synaptic gene-set (1233 genes) from SynGO database31 and the OMIM-CNS gene-set (2450 genes) to examine the difference in the association of RPD variant burden in each of these gene-sets among cases and controls using SKAT (by treating these gene sets as a pooled SNP-set). After correcting for kinship and population structure we noted a statistically significant association for OMIM-CNS gene-set (pbonferroni = 0.005) but did not note a statistically significant association for the synaptic gene-set (pbonferroni = 0.17) (Table 3). These results remained robust in a sensitivity analysis that involved association analysis in ss-RPD variants with MAF < 0.01% with a significant association for OMIM-CNS gene-set (pbonferroni < 1e−7) but not for the synaptic gene-set (pbonferroni = 0.24). In a preliminary gene-level SKAT analysis, we noted genome wide significance (p < 2.4e−6) for a single gene POTEE and nominally significant associations (p < 0.05) for 9 additional genes with a higher variant burden among cases (Supplementary Table 5).
Discussion
Employing WES in families with multiple members detected to have SMIs, we identify segregation of rare deleterious variants in several genes. A significant proportion of these genes are implicated in neuropsychiatric syndromes with Mendelian inheritance, such as (GEMIN5, COASY, CACNA1B etc., see Table 2). Majority (78/79) of the prioritized variants were noted to be private to fourteen multi-sample pedigrees. In four of these families, review of health records revealed evidence of clinical features that overlapped with the primary OMIM syndrome. The 79 genes prioritized by pedigree analysis tended to be more conserved, and more intolerant of variation, when compared to the remaining set of 20,124 genes. In addition, in the cross-pedigree association analysis, cases harbored an increased burden of rare deleterious variants in genes involved in the structural and functional integrity of synapses, as compared to unrelated controls.
The overrepresentation of genes in within-pedigree prioritization was largely specific to disorders of ‘central nervous system’ (CNS), as genes with a clinical synopsis annotation to this category showed the strongest overrepresentation. Among 19 additional clinical synopsis categories, significant overrepresentation was noted for ‘head and neck’ term. This overlap with ‘head and neck’ may be explained by tightly interlinked developmental and molecular processes that regulate cranio-facial and brain development35. While there was evidence for increased burden of RPDs in genes relevant to CNS, we did not find a system-specific enrichment for genes harboring RPDs among 60 unrelated controls or the genes harboring non-segregating RPDs.
Among the 20 variants in genes implicated in CNS syndromes, three variants had been previously reported in the ClinVar database in the context of the primary Mendelian syndrome (Table 2). Of these, the variants in ADAMTS2 and JAG1 were noted to be of ‘uncertain significance’ and AFG3L2 variant was recorded as ‘likely benign’36. The remaining 17 variants have not been previously reported in the ClinVar database, in the context of a primary Mendelian syndrome. Interestingly, a duplication at the same site where we find a G allele insertion (Chr21: 47860904) in the PCNT gene (implicated in Microcephalic osteodysplastic primordial dwarfism type II), has been identified as a pathogenic variant in ClinVar, suggesting the significance of this site in protein function.
Pleiotropic influence for de novo variants and genes between SCZ, BD, autism and neurodevelopmental syndromes has been reported in large trio-based studies7,18,23. Our analysis also suggests genetic pleiotropy between Mendelian monogenic syndromes, and complex SMI syndromes. Ten families with variants in genes implicated in Mendelian neuropsychiatric syndrome did not have the ‘classical’ features of the primary syndrome. However, in four of these families we noted partial overlaps in clinical features, in some of the individuals with SMI (Supplementary Table 6). This may suggest incomplete penetrance and expression of the primary syndrome, and possible pleiotropic expression of a psychiatric syndrome for identified gene variants.
To examine if the observed pleiotropy between monogenic and complex SMI was specific to the current sample structure, population, or variant/gene prioritization approach we curated a list of 142 genes identified in 10 recent NGS studies in SCZ, BD, OCD and AD in the literature (Supplementary Table 7). Here too with 37 of 142 (26.1%) genes implicated in a Mendelian syndrome, we noted a statistically significant overrepresentation (Fisher’s Exact test OR = 2.29, p < 0.0001). Despite heterogeneity across these 10 studies with respect to the psychiatric syndrome, sample selection and variant prioritization methods, consistent evidence of overlap of risk between Mendelian and complex disease, at the level of aggregate list of genes harboring rare variants is noted.
Thus, while certain mutations in Mendelian disease genes result in early onset severe neurodevelopmental phenotype, other variants in the same genes may result in more subtle anatomical and functional consequences, that perhaps predispose to late-onset neuropsychiatric disorders37. These phenotype level effects of a given mutation, in a given gene, may further be moderated by background genetic effects38,39 and the role of the gene in neurodevelopment, and plasticity, over time40. Similar findings have been recently reported in a whole exome sequencing study of severe, extremely treatment resistant schizophrenia where authors note enrichment of genes with missense and loss of function variants, increased burden of Mendelian disease genes and specifically the loss of function genes to those syndromes with an annotation of behavioral phenotype41. Lastly, behavioral symptoms are frequently encountered with many Mendelian neurodevelopmental syndromes, and familial SMI could represent a cumulative consequence of multiple rare variants in more than one gene42.
Consistent with the enrichment of variants prioritized by within pedigree prioritization to OMIM-CNS gene-set, we noted a higher burden of RPD variants in the OMIM-CNS genes in across pedigree SKAT association analysis. However, a similar increased burden was not noted in the set of genes related to synaptic function. Both family-based and population-based genetic studies probing common and rare variants across a spectrum of SMI syndromes have implicated multiple synaptic genes with consequences on the structure and function of synapse18,43,44,45. As in the case–control WES study in South African Xhosa4, and using the same target list of genes, we observed rare predicted-deleterious variants in 104 genes related to synaptic structure and function. Of these, variants in genes represented by the GO component ‘integral component of presynaptic membrane’ (17/104) and ‘integral component of postsynaptic membrane’ (16/104), contributed the greatest proportion. Most (95%) of the rare-predicted deleterious variants in synaptic genes were private to a pedigree, or an individual, with only 6 genes having variants across two or more pedigrees (Supplementary Table 8).
In the gene-wise association test using SKAT in the cross-pedigree analysis (Supplementary Table 5), the signal noted at POTEE gene passed the threshold for genome-wide significance (p < 2.4E−6). Nominally significant associations were noted for nine additional genes (Supplementary Table 5). Variation in POTEE has not been previously reported in the context of neuropsychiatric syndromes, but differential expression of the gene product has been reported in substantia nigra in the neuroimmune syndrome such as Multiple Sclerosis46. CTBP2 gene which codes for C-terminal binding protein 2, an isoform of which is a major component of synaptic ribbons, also plays a critical role in regulating cell migration during neocortical development47. Genome wide significant loci for multiple brain cortical morphometric measurements such as sulcal depth, cortical thickness and cortical surface area have been mapped to ADAMTS20 gene in previous studies48,49. The SLC9B1 gene is within the cis regulatory region of a genome wide significant association locus in a recently published GWAS of schizophrenia50,51. The ABCD1 gene on chromosome X encodes peroxisomal membrane protein called adrenoleukodystrophy protein involved in the transport of very long chain fatty acids. More than 650 mutations in this gene have been implicated in adrenoleukodystrophy syndrome, with highly variable clinical manifestations that often include cognitive and neuropsychiatric symptoms52. SYNJ2 gene has highest relative expression in human brain and encodes inositol polyphosphate phosphatase and is involved in neurotransmitter recycling. Variations in this gene have not thus far been implicated in neuropsychiatric syndromes but chromatin modification in this gene has been identified in the context of SCZ53. In summary, among the nominally significant gene wise associations with a higher variant burden among cases identified in this study, some had evidence in the literature with relevance to SMI and neurodevelopment syndromes but need further examination in larger samples. Given the small number of case pedigrees and control samples examined in the current study, these results warrant confirmation in future studies with larger samples from the relevant population.
Some limitations are to be considered while interpreting the results of this study. While each F-SMI pedigree had multiple members affected with SMI, we could sample 3 or more affected individuals in only 16 such pedigrees. Hence the within-pedigree segregation analysis could only be performed in this subsample, reducing the power of this analysis to detect additional disease relevant variants. Augmenting sampling in remaining pedigrees may yield larger number of SMI relevant signals. A non-uniform structure of relatedness among sequenced samples within the pedigrees and absence of a sequenced unaffected family-control in 4 of the 16 pedigrees precluded filtering variants that segregated among unaffected members in every pedigree. We were also unable to perform family-based association testing in the present analysis. However, measurement of quantitative disease relevant endophenotypes within the same pedigrees will allow us to determine the impact of RPD variants on traits within unaffected members.
We noted an overrepresentation of OMIM-CNS genes but not the synaptic genes both in the within pedigree prioritization and cross-pedigree association analysis. These results highlight the consistency in the direction of results within the study but stand in contrast to previous studies that have reported an increased burden of rare functional variants in synaptic genes. These results lead us to speculate that in the context of genetic risk for complex neuropsychiatric syndromes in familial context, two-fold burden of effects from neurodevelopmental and synaptic function genes may contribute to final disease expression. Future family studies that sequence a larger number of affected and unaffected individuals within pedigrees and in addition characterize a spectrum of phenotype effects across lifespan may yield more robust signals of overlaps between monogenic neurodevelopmental syndromes and complex SMI.
Alignment and variant calling were performed with hg19, an earlier version of reference sequence for the human genome. A recent study has demonstrated that 0.9% of exome targets and up to 206 genes may fall in regions that are susceptible to discrepancies in the reference assemblies between hg19 and hg3854. We verified the prioritized genes in within-family segregation and cross-pedigree association analysis and observed that none of these overlapped with the discrepant genes or regions54. While our sample was adequately powered to confirm cross-pedigree association in pre-identified gene sets, the novel gene-wise associations identified in this analysis are would need confirmation in larger, diverse samples.
Conclusion
In this F-SMI WES study, we identify several private, rare, protein altering variants that segregate among the cases within a pedigree. We find suggestive evidence for overrepresentation of genes harboring these variants among genes implicated in monogenic forms of Mendelian neuropsychiatric syndromes, highlighting pleotropic influences in neurodevelopment and functioning. We also note a greater frequency of variants in genes involved in structural and functional integrity of synapses in cases compared to controls. The study demonstrates the usefulness of NGS approaches in F-SMI to identify disease relevant variants. Future studies, involving a larger number of families, with multiple affected members, and across diverse populations, may help us explore the contribution of rare coding variants to F-SMI. Validation of the functional impact of identified variants using cell models, combined with the application of in silico approaches to model protein structure alterations, and interactions, may help understand the convergent and divergent developmental mechanisms that underlie rare variants and risk of complex SMI.
Methods
Ethics statement
The study protocol was approved by the Institutional Ethics Committee of National Institute of Mental Health and Neurosciences, Bengaluru, India, and the study procedures conformed to the provisions of the Declaration of Helsinki. All participants provided written informed consent.
Sample
The families with multiple members with SMI (F-SMI) were identified as part of ADBS. This program is aimed at characterization of clinical and neurobiological phenotypes for neuropsychiatric syndromes and identification of genetic and molecular correlates of disease using cell models2,30,55. The diagnosis of SMI was established by independent clinical evaluation by two psychiatrists based on ICD-10 criteria56. Diagnosis and current and lifetime comorbidity were further evaluated and confirmed with Mini International Neuropsychiatric Inventory 5.0.057. The clinical status of the members belonging to the first two generations of all recruited participants was confirmed using Family Interview for Genetic Studies and pedigree charting58.
Cases were individuals with a diagnosis of SMI and family controls were unaffected individuals from the same families without a lifetime diagnosis of SMI or related syndromes. In addition, we identified unaffected unrelated individuals without family history of SMI, as ‘unrelated-controls’ from the population. Families with WES data from ≥ 3 cases were identified as ‘multi-sample F-SMI’ and selected for analysis of within-family segregation of variants as described below.
Sequencing, alignment, variant calling and quality assessment
The protocols for sequencing, alignment and variant calling including the quality controls (QC) have been previously published22 (Supplement S5). In brief, Illumina Nextera exome enrichment kits targeting 62.08 Mb of human genome were used for library preparation and the Illumina Hiseq platform was used for 100 base paired-end sequencing. Raw-read QC was performed with FastQC.0.10.1 and low-quality reads (< Q20) were excluded. Alignment to human genome build hg19/GRCH37 was performed using BWA (v-0.5.9). Realignment was performed with 1000G Phase1 INDELs using GATK (v-3.6) for removal of PCR duplicates and alignment artifacts. Single Nucleotide Polymorphisms (SNPs) and short insertion deletions (INDEL) variants were called with standard parameters (min coverage = 8, MAF ≥ 0.25 and P ≤ 0.001) using Varscan2 to generate sample-wise VCFs.
Annotation
VCFs were annotated with ANNOVAR59 tool for gene, region and filter based annotation options. Variant frequencies were obtained from the gnomAD—South Asian subset (N = 15,308). For in silico prediction of deleteriousness of coding variants, five functional annotations (SIFT60, LRT61, MutationTaster62, MutationAssessor63 and MetaSVM64) were used.
Variant prioritization
Variants were prioritized based on rarity (minor allele frequency ≤ 0.001 in gnomAD SAS and gnomAD global samples) and a ‘predicted-deleterious’ functional consequence. Predicted-deleteriousness of exonic Single nucleotide variants (SNVs) were defined as protein truncating variants (stop-gain, stop-loss or start loss, canonical splice sites) or missense variants predicted to have deleterious consequence in ≥ 4 of the 5 functional prediction annotation tools. Protein truncating or out-of-frame small insertion-deletions (indels) were similarly prioritized. All subsequent analysis involved the prioritized rare predicted-deleterious (RPD) variants. Minor allele frequencies of the prioritized variants were examined in remaining populations in gnomAD database to exclude rare variants specific to South Asian sample.
Analysis approach
To identify the RPDs that are putatively relevant to SMI syndromes we adopted two independent analytical approaches; a within-family prioritization of variants segregating with SMI and a cross-pedigree case–control association analysis.
Within-family variant selection
To identify high penetrance rare variants and to reduce potential false discoveries we defined an a priori criteria of sharing among ≥ 3 related cases with SMI within a pedigree, similar to an approach also used in a previous publication (12). To account for non-uniform relatedness structure between sampled cases across different pedigrees and the absence of an unaffected family control in 4 of the 16 multi-sample F-SMI (Supplement 6), we defined a broader set of shared RPD based on the criteria that were shared by ≥ 3 cases and were absent in unrelated technical controls from population and defined these as shared-broad RPD (sb-RPD). We further prioritized a second sub-set of shared RPDs with more stringent selection criteria and defined as shared-stringent RPD (ss-RPD). In addition to being shared by ≥ 3 cases within a pedigree, for these variants we chose a stringent MAF cut-off ≤ 0.0001 in gnomAD SAS and gnomAD global samples. Furthermore, these were either absent or were noted in not more than one in unaffected family control to allow for incomplete penetrance.
The resulting lists of shared-broad RPD (sb-RPD) and shared-stringent RPD (ss-RPD) variants and the genes harboring these variants were queried with RPDs noted in the cases in previously excluded families (< 3 samples from cases/family) to examine potential variant and gene level overlaps in SMI associated genes.
Case control association analysis
We performed gene-level and gene-set case–control association analysis between the cases and unrelated-controls, after accounting for the kinship and population sub-structure within the sample using Sequence Kernel Association Test (SKAT)65 as implemented in the R package SKAT66. A principal component analysis (PCA) was performed using common SNPs within the sample excluding uninformative variants to infer population sub-structure. The SKAT_null_emmax() function was used to approximately adjust for the overall genomic correlation among the individuals by fitting a null model for the binary case/control status incorporating the kinship matrix, first three principal components and three broad clusters observed in the PCA plot (Supplement S4). After covariate adjustment, the residuals obtained were permuted to obtain resampled residuals using the SKAT_Null_Model() function. Finally, association of RPD variants with case/control status was examined using SKAT() function. Up to 106 permutations were used per variant to obtain accurate p values in the critical range (p > e−5). For smaller p-values, the asymptotic SKAT p-values (by “davies” method” were retained). A p value threshold of 2.4e−6 in the permuted p value was set to infer genome wide significance for gene-level analysis after Bonferroni correction for 20,203 protein coding genes. Gene-set wide analysis p values were adjusted for Bonferroni correction for the two gene-sets tested.
Clinical and functional significance of the prioritized genes
The set of genes from within- family prioritization was examined for enrichment in genes implicated in monogenic neurodevelopmental syndromes in Online Mendelian Inheritance in Man (OMIM) database (OMIM-CNS genes) (Supplement S2) using the two tailed Fisher’s Exact test. The specificity of this enrichment to the CNS was verified by examining enrichment for 19 non-CNS gene lists among additional OMIM clinical synopsis categories (Supplement S2) and by repeating this analysis in genes harboring RPD variants in unrelated controls. In families where RPD variants in OMIM genes for particular monogenic CNS syndromes were noted, we examined the medical records for any overlapping OMIM-like clinical features. We further examined if the prioritized genes were overrepresented for synaptic genes derived from SynGO database31.
We examined the mutational constraint, tissue expression and evolutionary conservation using publicly available datasets. Mutational constraint was assessed by gnomAD LOEUF33 and ExAC pLI scores34. Brain and cortical expression were examined using the GTEx v8 with mean expression values per gene. Conservation scores derived from 240 species alignment were adopted from the Zoonomia consortium (Zoonomia fraction of CDS phyloP ≥ 2.270 (fdr 0.05))32. Comparisons between prioritized and the background list were performed using the Welch’s t-test.
Data availability
The datasets generated and/or analysed during the current study are available from the ADBS bio repository https://www.ncbs.res.in/adbs/bio-repository on registration and reasonable request.
References
Huang, M.-H. et al. Familial coaggregation of major psychiatric disorders among first-degree relatives of patients with obsessive-compulsive disorder: A nationwide study. Psychol. Med. 51, 680–687. https://doi.org/10.1017/S0033291719003696 (2020).
Sreeraj, V. S. et al. Psychiatric symptoms and syndromes transcending diagnostic boundaries in Indian multiplex families: The cohort of ADBS study. Psychiatry Res. 296, 113647. https://doi.org/10.1016/j.psychres.2020.113647 (2021).
Glahn, D. C. et al. Rediscovering the value of families for psychiatric genetics research. Mol. Psychiatry 24, 523–535. https://doi.org/10.1038/s41380-018-0073-x (2019).
Gulsuner, S. et al. Genetics of schizophrenia in the South African Xhosa. Science 367, 569–573. https://doi.org/10.1126/science.aay8833 (2020).
Singh, T. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516. https://doi.org/10.1038/s41586-022-04556-w (2022).
Kato, T. Whole genome/exome sequencing in mood and psychotic disorders. Psychiatry Clin. Neurosci. 69, 65–76. https://doi.org/10.1111/pcn.12247 (2015).
Howrigan, D. P. et al. Exome sequencing in schizophrenia-affected parent-offspring trios reveals risk conferred by protein-coding de novo mutations. Nat. Neurosci. 23, 185–193. https://doi.org/10.1038/s41593-019-0564-3 (2020).
Legge, S. E. et al. Genetic architecture of schizophrenia: A review of major advancements. Psychol. Med. https://doi.org/10.1017/S0033291720005334 (2021).
Li, M. et al. Novel genetic susceptibility loci identified by family based whole exome sequencing in Han Chinese schizophrenia patients. Transl. Psychiatry 10, 5. https://doi.org/10.1038/s41398-020-0708-y (2020).
Forstner, A. J. et al. Whole-exome sequencing of 81 individuals from 27 multiply affected bipolar disorder families. Transl. Psychiatry 10, 57. https://doi.org/10.1038/s41398-020-0732-y (2020).
Goes, F. S. et al. Exome sequencing of familial bipolar disorder. JAMA Psychiat. 73, 590–597. https://doi.org/10.1001/jamapsychiatry.2016.0251 (2016).
Sul, J. H. et al. Contribution of common and rare variants to bipolar disorder susceptibility in extended pedigrees from population isolates. Transl. Psychiatry 10, 74. https://doi.org/10.1038/s41398-020-0758-1 (2020).
Toma, C. et al. An examination of multiple classes of rare variants in extended families with bipolar disorder. Transl. Psychiatry 8, 65. https://doi.org/10.1038/s41398-018-0113-y (2018).
Halvorsen, M. et al. Exome sequencing in obsessive-compulsive disorder reveals a burden of rare damaging coding variants. Nat. Neurosci. 24, 1071–1076. https://doi.org/10.1038/s41593-021-00876-8 (2021).
Jia, X. et al. Investigating rare pathogenic/likely pathogenic exonic variation in bipolar disorder. Mol. Psychiatry https://doi.org/10.1038/s41380-020-01006-9 (2021).
Palmer, D. S. et al. Exome sequencing in bipolar disorder reveals shared risk gene AKAP11 with schizophrenia. medRxiv https://doi.org/10.1101/2021.03.09.21252930 (2021).
Ganna, A. et al. Quantifying the impact of rare and ultra-rare coding variation across the phenotypic spectrum. Am. J. Hum. Genet. 102, 1204–1211. https://doi.org/10.1016/j.ajhg.2018.05.002 (2018).
Nishioka, M. et al. Systematic analysis of exonic germline and postzygotic de novo mutations in bipolar disorder. Nat. Commun. 12, 3750. https://doi.org/10.1038/s41467-021-23453-w (2021).
Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184. https://doi.org/10.1038/nature12929 (2014).
Genovese, G. et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat. Neurosci. 19, 1433–1441. https://doi.org/10.1038/nn.4402 (2016).
Kenny, E. M. et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol. Psychiatry 19, 872–879. https://doi.org/10.1038/mp.2013.127 (2014).
Ganesh, S. et al. Exome sequencing in families with severe mental illness identifies novel and rare variants in genes implicated in Mendelian neuropsychiatric syndromes. Psychiatry Clin. Neurosci. 73, 11–19. https://doi.org/10.1111/pcn.12788 (2019).
Rees, E. et al. Schizophrenia, autism spectrum disorders and developmental disorders share specific disruptive coding mutations. Nat. Commun. 12, 5353. https://doi.org/10.1038/s41467-021-25532-4 (2021).
Brainstorm-Consortium et al. Analysis of shared heritability in common disorders of the brain. Science https://doi.org/10.1126/science.aap8757 (2018).
Cristino, A. S. et al. Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Mol. Psychiatry 19, 294–301. https://doi.org/10.1038/mp.2013.16 (2014).
Grotzinger, A. D. Shared genetic architecture across psychiatric disorders. Psychol. Med. https://doi.org/10.1017/S0033291721000829 (2021).
Gandal, M. J. et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697. https://doi.org/10.1126/science.aad6469 (2018).
Radonjic, N. V. et al. Structural brain imaging studies offer clues about the effects of the shared genetic etiology among neuropsychiatric disorders. Mol. Psychiatry 26, 2101–2110. https://doi.org/10.1038/s41380-020-01002-z (2021).
Grotzinger, A. D. et al. Genetic architecture of 11 major psychiatric disorders at biobehavioral, functional genomic and molecular genetic levels of analysis. Nat. Genet. 54, 548–559. https://doi.org/10.1038/s41588-022-01057-4 (2022).
Viswanath, B. et al. Discovery biology of neuropsychiatric syndromes (DBNS): A center for integrating clinical medicine and basic science. BMC Psychiatry 18, 106. https://doi.org/10.1186/s12888-018-1674-2 (2018).
Koopmans, F. et al. SynGO: An evidence-based, expert-curated knowledge base for the synapse. Neuron 103, 217–234.e214. https://doi.org/10.1016/j.neuron.2019.05.002 (2019).
Genereux, D. P. et al. A comparative genomics multitool for scientific discovery and conservation. Nature 587, 240–245. https://doi.org/10.1038/s41586-020-2876-6 (2020).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443. https://doi.org/10.1038/s41586-020-2308-7 (2020).
Fuller, Z. L., Berg, J. J., Mostafavi, H., Sella, G. & Przeworski, M. Measuring intolerance to mutation in human genetics. Nat. Genet. 51, 772–776. https://doi.org/10.1038/s41588-019-0383-1 (2019).
Naqvi, S. et al. Shared heritability of human face and brain shape. Nat. Genet. 53, 830–839. https://doi.org/10.1038/s41588-021-00827-w (2021).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. https://doi.org/10.1038/gim.2015.30 (2015).
Zhu, X., Need, A. C., Petrovski, S. & Goldstein, D. B. One gene, many neuropsychiatric disorders: Lessons from Mendelian diseases. Nat. Neurosci. 17, 773–781. https://doi.org/10.1038/nn.3713 (2014).
Chen, R. et al. Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nat. Biotechnol. 34, 531–538. https://doi.org/10.1038/nbt.3514 (2016).
Riordan, J. D. & Nadeau, J. H. From peas to disease: Modifier genes, network resilience, and the genetics of health. Am. J. Hum. Genet. 101, 177–191. https://doi.org/10.1016/j.ajhg.2017.06.004 (2017).
Ball, G. et al. Cortical morphology at birth reflects spatiotemporal patterns of gene expression in the fetal human brain. PLoS Biol. 18, e3000976. https://doi.org/10.1371/journal.pbio.3000976 (2020).
Zoghbi, A. W. et al. High-impact rare genetic variants in severe schizophrenia. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.2112560118 (2021).
Wang, Y. C. et al. Identification of ultra-rare missense mutations associated with familial schizophrenia by whole-exome sequencing. Schizophr. Res. 235, 60–62. https://doi.org/10.1016/j.schres.2021.07.027 (2021).
Forrest, M. P., Parnell, E. & Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 19, 215–234. https://doi.org/10.1038/nrn.2018.16 (2018).
Hall, J., Trent, S., Thomas, K. L., O’Donovan, M. C. & Owen, M. J. Genetic risk for schizophrenia: Convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 77, 52–58. https://doi.org/10.1016/j.biopsych.2014.07.011 (2015).
Taoufik, E., Kouroupi, G., Zygogianni, O. & Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. https://doi.org/10.1098/rsob.180138 (2018).
Chen, S., Lu, F. F., Seeman, P. & Liu, F. Quantitative proteomic analysis of human substantia nigra in Alzheimer’s disease, Huntington’s disease and Multiple sclerosis. Neurochem. Res. 37, 2805–2813. https://doi.org/10.1007/s11064-012-0874-2 (2012).
Wang, H. et al. ZEB1 represses neural differentiation and cooperates with CTBP2 to dynamically regulate cell migration during neocortex development. Cell Rep. 27, 2335–2353.e2336. https://doi.org/10.1016/j.celrep.2019.04.081 (2019).
Shadrin, A. A. et al. Vertex-wise multivariate genome-wide association study identifies 780 unique genetic loci associated with cortical morphology. Neuroimage 244, 118603. https://doi.org/10.1016/j.neuroimage.2021.118603 (2021).
van der Meer, D. et al. The genetic architecture of human cortical folding. Sci. Adv. 7, eabj9446. https://doi.org/10.1126/sciadv.abj9446 (2021).
Lam, M. et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 51, 1670–1678. https://doi.org/10.1038/s41588-019-0512-x (2019).
Li, Z. et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 49, 1576–1583. https://doi.org/10.1038/ng.3973 (2017).
Engelen, M. et al. X-linked adrenoleukodystrophy (X-ALD): Clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet. J. Rare Dis. 7, 51. https://doi.org/10.1186/1750-1172-7-51 (2012).
Gusev, F. E. et al. Chromatin profiling of cortical neurons identifies individual epigenetic signatures in schizophrenia. Transl. Psychiatry 9, 256. https://doi.org/10.1038/s41398-019-0596-1 (2019).
Li, H. et al. Exome variant discrepancies due to reference-genome differences. Am. J. Hum. Genet. 108, 1239–1250. https://doi.org/10.1016/j.ajhg.2021.05.011 (2021).
Sreeraj, V. S. et al. Cross-diagnostic evaluation of minor physical anomalies in psychiatric disorders. J. Psychiatr. Res. 142, 54–62. https://doi.org/10.1016/j.jpsychires.2021.07.028 (2021).
WHO. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (World Health Organization, 1992).
Sheehan, D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl 20), 22–33 (1998) (quiz 34-57).
Maxwell, M. E. (1992).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164. https://doi.org/10.1093/nar/gkq603 (2010).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081. https://doi.org/10.1038/nprot.2009.86 (2009).
Chun, S. & Fay, J. C. Identification of deleterious mutations within three human genomes. Genome Res. 19, 1553–1561. https://doi.org/10.1101/gr.092619.109 (2009).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 11, 361–362. https://doi.org/10.1038/nmeth.2890 (2014).
Reva, B., Antipin, Y. & Sander, C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic Acids Res. 39, e118–e118. https://doi.org/10.1093/nar/gkr407 (2011).
Dong, C. et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 24, 2125–2137. https://doi.org/10.1093/hmg/ddu733 (2015).
Lee, S. et al. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am. J. Hum. Genet. 91, 224–237. https://doi.org/10.1016/j.ajhg.2012.06.007 (2012).
SNP-Set (Sequence) Kernel Association Test v. 2.0.1 (2020).
Acknowledgements
The authors are grateful to all the patients, their family members and healthy volunteers who participated in the study. Financial support for the study was provided by Department of Biotechnology funded grants—BT/01/CEIB/11/VI/11/2012, entitled, “Targeted generation and interrogation of cellular models and networks in neuro-psychiatric disorders using candidate genes” and BT/PR17316/MED/31/326/2015 entitled, “Accelerator program for discovery in brain disorders using stem cells” (ADBS), Pratiksha Trust and The Institute of Stem Cells and Regenerative Medicine (InStem), Bengaluru, India.
The authors would like to thank the sequencing core facility at the Institute of Genomics and Integrative Biology (IGIB), Delhi (Dr. Faruq Mohammed) and the National Centre for Biological Sciences (NCBS), Bengaluru (Dr. Awadhesh Pandit) for sample processing and WES data generation.
The authors would like to thank all investigators of ADBS consortia for providing valuable inputs to the manuscript and having final approval of the manuscript. Suhas Ganesh is affiliated with Schizophrenia Neuropharmacology Research Group at Yale university and is supported by a NARSAD young investigator grant from the Brain and Behavior Research Foundation. Biju Viswanath is funded by the Intermediate (Clinical and Public Health) Fellowship (IA/CPHI/20/1/505266) of the DBT/Wellcome Trust India Alliance.
Author information
Authors and Affiliations
Consortia
Contributions
S.J., M.P., B.V. and ADBS consortium conceived the study. S.G., A.V., K.M. curated the WES data and performed analysis. D.I., K.N., R.K.N. curated clinical data and assisted with the WES analysis. S.B. provided inputs on statistical analysis and interpretation. P.F.S. provided inputs on bioinformatic analysis and interpretation. All authors contributed to writing, revising, and finalizing the manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ganesh, S., Vemula, A., Bhattacharjee, S. et al. Whole exome sequencing in dense families suggests genetic pleiotropy amongst Mendelian and complex neuropsychiatric syndromes. Sci Rep 12, 21128 (2022). https://doi.org/10.1038/s41598-022-25664-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25664-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.