Abstract
In recent years, the cases of tramadol intoxication have become more frequent in many countries. However, most of the previous studies have been based on cases of tramadol intoxication, and the detailed information on the differences between postmortem distribution and diffusion of tramadol remains unclear. To investigate this issue systematically, we established a postmortem distribution model and two postmortem diffusion models. Then, gas chromatography-mass spectrometry (GC/MS) was used to measure the concentrations of tramadol in various biological specimens of fluids and tissues. In postmortem distribution, the results showed an uneven distribution of tramadol in various biological specimens, and the concentrations of tramadol in urine were significantly higher than those in other fluids. In postmortem diffusion, the results showed a dosage-dependent increase of tramadol concentration in most specimens; at all time points from 0.25 to 6 h after postmortem administration, the concentrations of tramadol in fluids were not significantly different from those in tissues, and the concentrations of tramadol in urine were lower than those in both tissues and other fluids in most time points. We recommend a quantitative examination of the specimens of both fluids and tissues to provide more evidence for the forensic identification, and the realization that there is a correlation between the concentrations of fluids and tissues is important for determining antemortem and postmortem administration of tramadol. This information can serve as ancillary data in inferring the contribution of a drug to death in cases of suspected tramadol poisoning.
Similar content being viewed by others
Introduction
As a synthetic opioid, tramadol has been widely used for its pain-relieving effects1,2,3,4. It was originally manufactured in Germany in 19775,6 and then in other countries some 20 years later. Tramadol, a pharmaceutical opioid, has been used as a painkiller for decades, but its non-medical use has caused a crisis of synthetic opioid overdoses in many subregions, in particular West, Central and North Africa, which requires urgent international attention7. Ojanperä et al. used the fatal toxicity index (FTI)—the absolute number of fatal poisonings caused by a particular drug—to evaluate the toxicity of 70 drugs and their relevance to fatal poisonings, and found that FTI of tramadol ranked 9th out of 70 drugs8. In a recent study in Denmark, the researchers analyzed opioid poisonings cases from 2006 to 2017 and found that 48 of the 408 deaths were caused by tramadol poisoning9.
Tramadol is used non-medically for the purposes of achieving pleasurable effects, i.e., to improve mood, prolonging the duration of sexual intercourse10,11, delaying the sensation of fatigue, and relieving pain, depression, anxiety, or other psychiatric disorders as a self-medication7. Therefore, there is a large market of tramadol for non-medical use, in which tramadol may be diverted from the licit production to the illicit markets, or be supplied by illicit manufactures at source. Due to its illicit and improper use, tramadol overdose-related intoxication and deaths are becoming frequent cases of drug poisoning in many countries6,12,13,14,15,16.
In cases of fatal intoxication, the interpretation of the probable cause of death relies mostly on the measurement of the concentrations of drugs. However, postmortem drug concentrations may vary according to the sampling site and the interval between death and postmortem specimen collection. This concept, known as postmortem redistribution (PMR), may complicate the interpretation of toxicological analyses. In fact, PMR may occur in either of the two cases: (1) antemortem (before death) administration of drug; (2) postmortem (after death) administration of drug17,18,19. Although PMR occurs in two cases likewise, the drug contributes to death differently, and the realization is important in forensic practice. Most previous research of PMR focuses on the cases of human unintentional death or suicide and falls into the category of antemortem administration of drug, so does our previous study of postmortem (antemortem administration of drug) redistribution of tramadol in poisoned rats20. Unlike conventional PMR, drug diffusion caused by postmortem administration has received little attention, i.e., drug was administered to dead individuals, which may occur when deaths from other reasons were disguised as acute tramadol intoxication. Thus, forensic scientists need to differentiate antemortem administration from postmortem administration for accurate identification.
To investigate this issue, we first explored the postmortem distribution of tramadol administered to live rabbits, simulating the state of individuals who died from acute intoxication. In order to determine whether postmortem diffusion occurs, and if so, the extent of passive diffusion, we designed two experiments to study the effects of dosage and length of time on postmortem diffusion by simulating the state of dead individuals to whom tramadol was administered. Finally, postmortem distribution and postmortem diffusion of tramadol were compared in rabbits, and the resulting correlation can be used to determine whether tramadol was administered before or after death.
Materials and methods
Drugs and chemicals
Standard tramadol (purity > 99.9%) was purchased from Institute of Forensic Science, Ministry of Public Security, China. Tramadol hydrochloride (50 mg/tablet) was purchased from Guangzhou Pui’s Pharmaceutical Co. Ltd, China. SKF525A, purchased from Standard Technology Development Co. Ltd, China, was prepared in methanol at a concentration of 1 g/L and used as the internal standard (IS). Both tramadol and IS were stored at 4 °C and protected from light. All other reagents were purchased from Sigma.
Animal experiments
The animals used in this study were 30 male rabbits weighing 2.0 ± 0.3 kg, provided by the Laboratory Animal Center of Shanxi Medical University, China. All rabbits fasted for 24 h before the experiment. All animal experiments complied with the ARRIVE guidelines, were approved by Institutional Animal Care and Use Committee of Shanxi Medical University, and performed in accordance with the current relevant legislation in China.
In the experiment of postmortem distribution, we administered tenfold LD50 (LD50 = 228 mg/kg) of tramadol intragastrically (ig) to six rabbits to make sure that the rabbits die from an overdose of tramadol. The autopsy was carried out immediately after the rabbits’ death and the specimens were collected separately for the study of tramadol’s postmortem distribution.
In two experiments of postmortem diffusion, all 24 rabbits (drug-free) were euthanized with aeroembolism before administration of tramadol. 2 h after the animals’ death, we used 9 rabbits and 15 rabbits to study the effects of dosage and length of time on tramadol’s postmortem diffusion, respectively, simulating the death of individuals shortly before tramadol administration. In the dosage effect group, three dosages of 1/8 LD50, 1/4 LD50, and 1/2 LD50 of tramadol were ig administered to three subgroups (n = 3) respectively, and all rabbits were then placed at room temperature (20 °C). 0.25 h after postmortem administration, the autopsy was carried out and the specimens were collected separately for detection. In the time effect group, all 15 rabbits were given ig a 1/4 LD50 dosage of tramadol and placed at room temperature (20 °C). The autopsy was carried out at 5 different time points (0.25 h, 0.5 h, 1 h, 3 h, and 6 h, n = 3) after postmortem administration and the specimens were collected separately for detection.
Extraction procedure
First, add 2 mL deionized water to each sample (1 mL or 1 g), homogenize the samples with a high-speed tissue homogenizer, and then add 20 μL (1 g/L) IS SKF525A to the homogenate. Next, add 10% HCl to the homogenate samples (pH value of 1–2) to precipitate protein, and adjust pH value of the supernatant solutions to 10 by adding 10% NaOH. The analytes were extracted twice from the samples with diethyl ether. The resulting organic extract was concentrated to dryness under water bath of 40 °C and the residue was dissolved in 20 μL ethanol for GC/MS analysis. Some samples, such as stomach, were highly concentrated and had to be properly diluted before analysis.
GC/MS
GC/MS analyses were performed on a Thermo Fisher Scientific TRACE DSQ system. Aliquots (1 μL) of the extract were injected into a DB-5 MS capillary column (30 m × 0.25 mm × 0.25 μm, J&W, Folsom, CA, USA). The adopted column temperature program was as follows: start with an initial temperature of 140 °C for 1 min, then rise to 250 °C at a rate of 30 °C/min, lastly, hold at 250 °C for 10 min. The carrier gas was helium with a constant flow at 1 mL/min. The temperature of ion source and transfer line was at 250 °C. Injection port temperature was also at 250 °C and the split injection mode was adopted (the split ratio was 10:1). The MS was operated in the selected ion monitoring mode, and the following ions were monitored: quantifier ion m/z 58 and qualifier ions m/z 77 and m/z 263 for tramadol; quantifier ion m/z 86 and qualifier ions m/z 99 and m/z 353 for the IS.
Method validation
Method validation was performed according to the guidelines of bioanalytical method validation introduced by U.S. FDA21. In the present study, to validate the results of bioanalytical method and facilitate the subsequent comparative analysis, we added standard tramadol and IS to each type of blank matrix samples of rabbits.
The linearity of the calibrator responses was estimated based on linear regression analysis, ranging from 0.05 to 96 μg/mL or μg/g, with a linearity expected to be > 0.99. Limit of detection (LOD) and lower limit of quantitation (LLOQ) were determined to estimate the sensitivity of the analytical method and calculated as the concentration of the inject sample to yield a signal-to-noise ratio of 3 and 10, respectively. For both LOD and LLOQ, the retention time was required to be within ± 2% and the ion ratio within ± 20% of the determined value of higher concentration standard samples, for valid results to be obtained.
Accuracy and precision were evaluated at three quality control (QC) concentrations (1, 16, and 64 μg/mL or μg/g) in six replicates (n = 6, respectively). Before each assay, we prepared QC samples were prepared before each assay by spiking blank samples with the tramadol reference standard solution at the determined concentrations. Accuracy was represented by the relative error of the mean (REM, in %), which was expected to lie within ± 15%. Intra- and inter-day precisions were represented by relative standard deviations (RSD, in %), which were supposed to be lower than 15%.
We compared the analytes with six concentrations of 0.5, 1, 4, 20, 40, and 80 μg/mL or μg/g to determine the recovery rates of tramadol from each matrix during the extraction process. And the analytes at each concentration were prepared in six replicates (n = 6, respectively).
Statistical analysis
Statistical analyses were performed with the statistical program of SPSS 11.5 and the data was represented by mean ± standard deviation (SD). As with all statistical measures, a P value of < 0.05 was considered significant in this study.
Ethics approval
All experiments were approved by Institutional Animal Care and Use Committee of Shanxi Medical University, and performed in accordance with the current relevant legislation in China. The authors complied with the ARRIVE guidelines.
Results
Method validation
A calibration curve for each biological matrix was generated. The calibration curves of tramadol were linear with a linearity > 0.99. The corresponding LOD and LLOQ of each biological matrix were gained, reaching at least 0.05 μg/mL (or μg/g) and 0.1 μg/mL (or μg/g), respectively. Accuracy and precision were determined based on the QC data. In our study, both % REM and the intra- and inter-day % RSD were found to be lower than 15%. At concentrations of 0.5, 1, 4, 20, 40, and 80 μg/mL (or μg/g), the ranges of average extraction recoveries of tramadol in all samples were: (92.2 ± 5.2)%–(109.4 ± 8.6)%, (96.7 ± 3.1)%–(107.6 ± 5.1)%, (98.0 ± 2.4)%–(104.6 ± 6.0)%, (94.8 ± 7.0)%–(105.8 ± 9.1)%, (95.5 ± 5.7)%–(102.7 ± 7.8)%, and (93.4 ± 5.9)%–(105.3 ± 6.3)% respectively. In conclusion, all validation results of GC/MS have met the requirements of bioanalytical method.
Postmortem distribution of tramadol in rabbits dying of poisoning
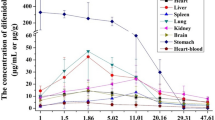
The results of postmortem distribution showed that tramadol was unevenly distributed in various biological specimens (Fig. 1). The concentrations of tramadol detected in the specimens of rabbits dying of poisoning were as follows: 163.0 ± 15.3 μg/g in kidney, 135.3 ± 14.5 μg/g in stomach, 130.2 ± 53.7 μg/g in liver, 112.5 ± 14.2 μg/g in spleen, 83.0 ± 19.4 μg/g in lung, 73.9 ± 32.2 μg/g in brain, 71.9 ± 12.2 μg/g in heart, 57.6 ± 19.9 μg/g in right forelimb muscles, 54.8 ± 17.4 μg/g in right posterior limb muscles, 1.4 ± 0.1 μg/mL in urine, 0.7 ± 0.1 μg/mL in bile, 0.7 ± 0.2 μg/mL in heart blood, and 0.4 ± 0.1 μg/mL in vitreous humor (Fig. 1).
It is noteworthy that the concentrations of tramadol in tissues (54.8–163.0 μg/g) were significantly higher than those in fluids (0.4–1.4 μg/mL). Additionally, the concentrations of tramadol in urine (1.4 μg/mL) were significantly higher than those in other fluids (0.4–0.7 μg/mL). These results suggested that, apart from conventional markers of blood, liver, kidney, and brain, urine also has the potential for identifying the individuals who died of poisoning, i.e., antemortem administration.
Tramadol’s postmortem diffusion in rabbits
24 rabbits (drug-free) were euthanized with aeroembolism. 2 h later, 9 rabbits and 15 rabbits were used to study the effects of dose and length of time on tramadol’s postmortem diffusion, respectively. Postmortem diffusion of tramadol was confirmed in both experiments.
The effect of dosage on tramadol’s postmortem diffusion in rabbits
3 subgroups were given a 1/8 LD50, 1/4 LD50, and 1/2 LD50 dosage of tramadol (n = 3) by ig respectively, and placed at room temperature (20 °C). 0.25 h after the postmortem administration, the autopsy was carried out and the specimens were collected separately for detection.
Tramadol was detected in all biological specimens at most dosages except that the concentrations of tramadol in vitreous humor, bile, urine, right forelimb muscles, right posterior limb muscles, brain, and lung were lower than LLOQ at the dosage of 1/8 LD50 (Fig. 2). A dosage-dependent effect was confirmed, because in the same kind of specimens, the higher the dosage of postmortem administration was, the higher the concentrations of tramadol were (*P < 0.05, compared with the concentrations of tramadol at the dose of 1/8 LD50, Fig. 2). Although there was no statistically significant difference in tramadol’s concentrations in stomach, heart, and liver at three different dosages, an increasing trend was observed. These results suggested that increasing the dosage in postmortem administration can promote the postmortem diffusion of the drug.
The effect of dosage on postmortem diffusion of tramadol in rabbits. VH vitreous humor, HB heart blood, RFLM right forelimb muscles, RPLM right posterior limb muscles. Data was represented by mean ± standard deviation. *P < 0.05, compared with the concentrations of tramadol in the dosage of 1/8 LD50.
The effect of length of time on tramadol’s postmortem diffusion in rabbits
15 rabbits were given a 1/4 LD50 dosage of tramadol by ig respectively, and placed at room temperature (20 °C). The autopsy was carried out at five different time points (0.25 h, 0.5 h, 1 h, 3 h, and 6 h, n = 3) after postmortem administration and the specimens were collected separately for detection.
Tramadol was detected in all biological specimens during 0.25–6 h after the postmortem administration (Fig. 3). It was noteworthy that the concentrations of tramadol in fluids (including vitreous humor, bile, urine, and heart blood) obviously increased at 6 h after the postmortem administration (*P < 0.05, compared with the concentrations of tramadol at 0.25 h, Fig. 3). In contrast, the concentrations of tramadol in stomach decreased (*P < 0.05, compared with the concentrations of tramadol at 0.25 h, Fig. 3). As to other tissues (kidney, liver, spleen, lung, brain, heart, right forelimb muscles, right posterior limb muscles), there was no statistically significant difference in concentrations at different time points from 0.25 to 6 h (Fig. 3). These results indicated that fluids may be more sensitive than tissues in assessing postmortem diffusion of drug.
Additionally, the concentrations of tramadol in fluids (0.1–8.3 μg/mL) were not significantly different from those in tissues (0.1–8.8 μg/g, except stomach). Despite the general upward trend, the concentrations of tramadol in urine were lower than those in both tissues and other fluids at most time points during 0.25 h to 6 h after postmortem administration.
Discussion
IN medicolegal investigations, the reference values (i.e., therapeutic, toxic, or lethal concentrations) are often used by forensic pathologists and toxicologists to assess the contribution of a drug to the death in fatalities. Therefore, the measurement of tramadol’s concentration is important for identifying the cause of death in the postmortem investigation. It was reported that the therapeutic concentration of tramadol in blood ranged from 0.1 to 0.3 μg/mL approximately1,13,22. If the concentration exceeds 1.0 μg/mL, the chances of toxic effects will increase23,24,25. In the present study, tramadol was widely distributed in all organs after ig administration (Fig. 1). With a mean of 0.7 μg/mL, the heart-blood concentration of tramadol seems not high. A probable explanation was that the relatively high dosage (10 LD50) led to rapid death in individuals; as a result, the drug had not been fully absorbed and sufficiently distributed in blood at the time of individuals’ death. This was confirmed by our previous study of postmortem redistribution20, in which the concentrations of tramadol were higher in blood (7.0 μg/mL) instead, though a lower dose (1/2 LD50) was used, possibly because of a longer survival time. Previous studies also showed that the observed concentration of a neuro-psychotropic drug in plasma may not accurately reflect the pharmacologic effects26,27,28. A recent study of postmortem drug distribution indicated that the detected concentrations of fifteen synthetic opioids, with the exception of norfentanyl, were higher in brain tissue than in blood29. In the present study, the concentration of tramadol in brain reached a high of 73.9 μg/mL, although it was 0.7 μg/mL in blood (Fig. 1). Additionally, the concentration of tramadol (1.4 μg/mL) in the specimen of urine was significantly higher than those in other fluids (0.4–0.7 μg/mL), which was consistent with previous cases of fatal intoxication due to tramadol alone25,30, indicating that the specimen of urine, like the conventional markers of blood, liver, kidney, and brain, can also be a sensitive marker to assess the individuals who died of poisoning, i.e., antemortem administration. These results suggested that quantitative analysis of different specimens is necessary for toxicological identification, because various specimens can provide more proof of drug intoxication in lethal cases. Moreover, it is important for medical toxicologists handling drug-related cases to note that postmortem administration of drugs (such as postmortem intravenous infusion or ig administration) may also lead to a lethal blood concentration of drugs, while the concentrations in tissues remain low. In such cases, an overdose of drugs was not the cause of death, although it seemed so, because postmortem diffusion may also lead to a lethal blood concentration. Thus, it is unreliable to assess the contribution of a drug to individuals’ death through its concentration in blood only, because it is also necessary to identify the correlation between the concentration of fluids and that of tissues before the cause of death can be determined, otherwise erroneous conclusions may be drawn.
PMR generally refers to the changes of drug concentrations occurring after death19, and involves the redistribution of drugs caused by concentration gradients, typically from high to low concentrations31,32. PMR may be influenced by two factors: (1) diffusion through blood vessels towards the surroundings and/or transparietal diffusion from organs to blood vessels; (2) spontaneous degradation and/or microorganism decomposition32,33. The extent of PMR will be influenced by factors of pharmacological characteristics (such as absorption route, state of ionization, volume of distribution, protein binding affinity, and lipophilicity), postmortem interval (PMI), site of drug sampling, and storage temperature of the corpse19,32,34. As our previous study demonstrated, both PMI and storage temperature will affect the PMR of tramadol20. In fact, PMR may occur in either of the two cases17,18,19: (1) antemortem administration of drug; (2) postmortem administration of drug. Although PMR occurs in two cases likewise, the drug contributes to death differently, and the realization is important in forensic practice. Human unintentional death or suicidal cases fall into the category of antemortem administration of drug, in which a rather large amount of drug is given perorally shortly before death. Drug will be rapidly absorbed and then widely distributed into well-perfused tissues via the circulatory system, such as liver, kidney, spleen, and lung. Once individuals die, a drug reservoir will be formed in the stomach and/or gut as a result of incomplete absorption of drug at the time of death.
Unlike most previous studies, this study focused on postmortem administration of drug, which was administered to dead individuals, and we investigated whether postmortem diffusion occurred as well as the extent of postmortem passive diffusion. Since tramadol was administered by ig to dead rabbits, the only drug absorption and distribution took place postmortem in the stomach. Therefore, a considerable “reservoir” of the substance was formed in the stomach, providing a concentration gradient for passive diffusion. Our results revealed that: (1) tramadol concentration showed a dosage-dependent increase in most specimens (Fig. 2); (2) tramadol concentration detected in stomach decreased over time, and increased in fluids (including vitreous humor, bile, urine, and heart blood); no significant difference was detected in other tissues (including right forelimb muscles, right posterior limb muscles, brain, lung, stomach, heart, liver, spleen, and kidney) during 0.25 h to 6 h after postmortem administration (Fig. 3). Tramadol was more readily redistributed in fluids possibly because the drug was dissolved19,35, which is consistent with previous studies in which blood, as a matrix on PMR, was sensitive to the change of drug concentration16,36. Additionally, it is noteworthy that the concentrations of tramadol in urine were lower than those in both tissues and other fluids at most time points during 0.25 h to 6 h after postmortem administration. The above-mentioned results indicated that postmortem ig administration of tramadol can likewise lead to considerable drug PMR (postmortem diffusion) in the body, which should be taken into account in forensic practice to avoid false conclusions.
Hence, it is necessary that the specimens of both fluids and tissues be quantitatively examined to provide more evidence for forensic identification, and the realization that there is a correlation between the concentrations of fluids and tissues is important for differentiating antemortem administration from postmortem administration. When administered by ig to live animals, drug was rapidly absorbed and then distributed into well-perfused tissues via the circulatory system due to the high Vd and liposolubility37,38; therefore, concentrations were high in fluids (especially urine and blood) and tissues (especially brain, liver, and kidney), and the differences in the concentrations of tramadol between fluids and tissues were significant. When administered by ig to dead animals, drug accumulated in the stomach alone and diffused through gastric wall towards the surrounding organs and blood vessels, so the differences in the concentrations of tramadol between fluids and tissues were not significant (though fluids are supposed to be more sensitive than tissues in assessing postmortem diffusion of drug), and the concentrations of drug in urine were lower than those in other fluids and tissues at most time points. This information can serve as ancillary data in inferring the contribution of a drug to death in medicolegal investigations.
Conclusion
The present study systematically explored the postmortem distribution and postmortem diffusion of tramadol in rabbits, aiming to differentiate antemortem administration from postmortem administration of tramadol through comparing tramadol concentrations in fluids and tissues. Our study provides valuable information for forensic toxicologists in assessing cases of suspected tramadol poisoning.
Data availability
All data generated during the study appear in the submitted article.
References
Faria, J. et al. Comparative pharmacology and toxicology of tramadol and tapentadol. Eur. J. Pain. 22(5), 827–844 (2018).
Miotto, K. et al. Trends in tramadol: Pharmacology, metabolism, and misuse. Anesth. Analg. 124(1), 44–51 (2017).
Grond, S. & Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 43(13), 879–923 (2004).
Shipton, E. A. Tramadol—Present and future. Anaesth. Intensive Care 28(4), 363–374 (2000).
Esmaeili, Z. et al. A disposition kinetic study of Tramadol in bile duct ligated rats in perfused rat liver model. Biomed. Pharmacother. 91, 251–256 (2017).
Tjäderborn, M., Jönsson, A. K., Hägg, S. & Ahlner, J. Fatal unintentional intoxications with tramadol during 1995–2005. Forensic Sci. Int. 173(2–3), 107–111 (2007).
United Nations Office on Drugs and Crime. World Drug Report 2019. https://wdr.unodc.org/wdr2019 (The United Nations, 2019).
Ojanperä, I., Kriikku, P. & Vuori, E. Fatal toxicity index of medicinal drugs based on a comprehensive toxicology database. Int. J. Legal Med. 130(5), 1209–1216 (2016).
Sørensen, A. M. S. et al. Short-term mortality following tramadol poisonings in Denmark. Basic Clin. Pharmacol. Toxicol. 131(1), 83–92 (2022).
Martyn-St James, M. et al. Tramadol for premature ejaculation: A systematic review and meta-analysis. BMC Urol. 15, 6 (2015).
Khan, A. H. & Rasaily, D. Tramadol use in premature ejaculation: Daily versus sporadic treatment. Indian J. Psychol. Med. 35(3), 256–259 (2013).
Chen, T. C., Chen, L. C. & Knaggs, R. D. A 15-year overview of increasing tramadol utilisation and associated mortality and the impact of tramadol classification in the United Kingdom. Pharmacoepidemiol. Drug Saf. 27(5), 487–494 (2018).
Randall, C. & Crane, J. Tramadol deaths in Northern Ireland: A review of cases from 1996 to 2012. J. Forensic Leg. Med. 23, 32–36 (2014).
Shadnia, S., Soltaninejad, K., Heydari, K., Sasanian, G. & Abdollahi, M. Tramadol intoxication: A review of 114 cases. Hum. Exp. Toxicol. 27(3), 201–205 (2008).
Handley, S., Patel, M. X. & Flanagan, R. J. Antipsychotic-related fatal poisoning, England and Wales, 1993–2013: Impact of the withdrawal of thioridazine. Clin. Toxicol. (Phila). 54(6), 471–480 (2016).
Nedahl, M., Johansen, S. S. & Linnet, K. Postmortem brain-blood ratios of codeine, fentanyl, oxycodone and tramadol. J. Anal. Toxicol. 45, 53–59 (2021).
De Letter, E. A. et al. Post-mortem redistribution of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) in the rabbit. Part I: Experimental approach after in vivo intravenous infusion. Int. J. Legal Med. 116(4), 216–224 (2002).
De Letter, E. A. et al. Post-mortem redistribution of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) in the rabbit. Part II: Post-mortem infusion in trachea or stomach. Int. J. Legal Med. 116, 225–232 (2002).
Yarema, M. C. & Becker, C. E. Key concepts in postmortem drug redistribution. Clin. Toxicol. (Phila). 43(4), 235–241 (2005).
Su, H. et al. Dynamic distribution and postmortem redistribution of tramadol in poisoned rats. J. Anal. Toxicol. 45, 203–210 (2021).
The Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (U.S Food & Drug Administration, 2018).
Clarot, F., Goullé, J. P., Vaz, E. & Proust, B. Fatal overdoses of tramadol: is benzodiazepine a risk factor of lethality?. Forensic Sci. Int. 134(1), 57–61 (2003).
Barbera, N. et al. A suicidal poisoning due to tramadol. A metabolic approach to death investigation. J. Forensic Leg. Med. 20, 555–558 (2013).
Jamali, B., Sheikholeslami, B., HosseinzadehArdakani, Y., Lavasani, H. & Rouini, M. R. Evaluation of the Ecstasy influence on tramadol and its main metabolite plasma concentration in rats. Drug Metab. Pers. Ther. 32(3), 137–145 (2017).
Musshoff, F. & Madea, B. Fatality due to ingestion of tramadol alone. Forensic Sci. Int. 116, 197–199 (2001).
Sheikholeslami, B., Gholami, M., Lavasani, H. & Rouini, M. Evaluation of the route dependency of the pharmacokinetics and neuro-pharmacokinetics of tramadol and its main metabolites in rats. Eur. J. Pharm. Sci. 92, 55–63 (2016).
Liu, M., Wang, P., Yu, X., Dong, G. & Yue, J. Intracerebral microdialysis coupled to LC–MS/MS for the determination tramadol and its major pharmacologically active metabolite O-desmethyltramadol in rat brain microdialysates. Drug Test Anal. 9, 1243–1250 (2017).
Altar, C. A. et al. Clinical validity of cytochrome P450 metabolism and serotonin gene variants in psychiatric pharmacotherapy. Int. Rev. Psychiatry. 25, 509–533 (2013).
Chessera, R., Pardi, J., Concheiro, M. & Cooper, G. Distribution of synthetic opioids in postmortem blood, vitreous humor and brain. Forensic Sci. Int. 305, 109999 (2019).
Moore, K. A. et al. Tissue distribution of tramadol and metabolites in an overdose fatality. Am. J. Forensic Med. Pathol. 20, 98–100 (1999).
Sastre, C., Bartoli, C., Baillif-Couniou, V., Leonetti, G. & Pelissier-Alicot, A. L. Post mortem redistribution of drugs: Current state of knowledge. Curr. Pharm. Des. 23(36), 5530–5541 (2017).
Pélissier-Alicot, A. L., Gaulier, J. M., Champsaur, P. & Marquet, P. Mechanisms underlying postmortem redistribution of drugs: A review. J. Anal. Toxicol. 27(8), 533–544 (2003).
Butzbach, D. M. The influence of putrefaction and sample storage on post-mortem toxicology results. Forensic Sci. Med. Pathol. 6, 35–45 (2010).
Brockbals, L. et al. Time-dependent postmortem redistribution of opioids in blood and alternative matrices. J. Anal. Toxicol. 42, 365–374 (2018).
Yonemitsu, K. & Pounder, D. J. Postmortem toxicokinetics of co-proxamol. Int. J. Legal Med. 104, 347–353 (1992).
Gerostamoulos, D. et al. The effect of the postmortem interval on the redistribution of drugs: A comparison of mortuary admission and autopsy blood specimens. Forensic Sci. Med. Pathol. 8, 373–379 (2012).
Barbosa, J. et al. Comparative metabolism of tramadol and tapentadol: A toxicological perspective. Drug Metab. Rev. 48, 577–592 (2016).
Oertel, R. et al. Distribution of metoprolol, tramadol, and midazolam in human autopsy material. J. Chromatogr A. 1218, 4988–4994 (2011).
Acknowledgements
This study was supported by two grants from National Natural Science Foundation of China (Grant No. 82130056 and 82072116).
Author information
Authors and Affiliations
Contributions
Y.W., Z.W. and K.Y. designed the research; L.W., C.Z., M.W. and B.L. performed most of the experiments and conducted the studies; H.S., L.W., A.W. and W.N. prepared the manuscript and edited the paper. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, H., Wang, L., Wurita, A. et al. A comparative study of postmortem distribution and postmortem diffusion of tramadol in rabbits. Sci Rep 13, 1702 (2023). https://doi.org/10.1038/s41598-022-25459-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25459-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.