Abstract
Optical techniques are utilized for the non-invasive analysis of the zebrafish cardiovascular system at early developmental stages. Being based mainly on conventional optical microscopy components and image sensors, the wavelength range of the collected and analyzed light is not out of the scope of 400–900 nm. In this paper, we compared the non-invasive optical approaches utilizing visible and near infrared range (VISNIR) 400–1000 and the shortwave infrared range (SWIR) 900–1700 nm. The transmittance spectra of zebrafish tissues were measured in these wavelength ranges, then vessel maps, heart rates, and blood flow velocities were calculated from data in VISNIR and SWIR. An increased pigment pattern transparency was registered in SWIR, while the heart and vessel detection quality in this range is not inferior to VISNIR. Obtained results indicate an increased efficiency of SWIR imaging for monitoring heart function and hemodynamic analysis of zebrafish embryos and larvae and suggest a prolonged registration period in this range compared to other optical techniques that are limited by pigment pattern development.
Similar content being viewed by others
Introduction
Zebrafish (Danio rerio) is a small cyprinid fish. Powerful genetic tools, small body size, many offspring, and relative ease of laboratory manipulation make zebrafish one of the most common model organisms for studying various processes in vertebrates, including human diseases1,2,3. Last decades D. rerio have been actively used for modeling cardiovascular pathologies due to the presence of human disease genes in the genome4 and optical transparency of the embryos5. The zebrafish heart contains the most specialized structures and signaling pathways intrinsic to mammals and humans6,7,8. The basic vascular anatomy of the developing D. rerio also shows strong similarity to that of other vertebrates9. Mentioned advantages suggest that zebrafish models will keep their relevance in studying cardiovascular diseases in the future10.
Optical approaches are the most common for non-invasive studying of the zebrafish cardiovascular system at early developmental stages. These techniques are utilized in the zebrafish model as a tool for examining congenital heart defects11, cardiomyopathy12,13,14, as well as the effects of various influences on the cardiovascular system functioning15,16,17. Moreover, transparent zebrafish embryos can be used for studying cardiovascular phenotypes, which would be lethal in humans and other mammals, as they obtain oxygen by passive diffusion from water for the first several days of development8. Optical approaches are also applied to studying the impact of blood flow on the arrest and the extravasation of circulating tumor cells in zebrafish embryos18. It opens prospects for evaluating antitumor drug efficacy via studying the relationship between hemodynamics in the zebrafish model and cancer cell migration ability2.
Nowadays, optical techniques deliver outstanding imaging capabilities. Optical coherence tomography enables micrometer-resolution visualization of the internal zebrafish anatomy19. It becomes a reliable tool for identifying and studying specific tissues when coupled with photoacoustic20, angiographic21, or other imaging modalities. Confocal22 and light sheet23 microscopy provide a more detailed structure of zebrafish’s heart and vessels but require long-term scanning and blood cells labeling. Fluorescence of the dyes allows accurate high-contrast cardiovascular imaging24. Without them, extensive image segmentation and analysis algorithms are necessary25. Label-free and cost-effective optical approaches to heartbeat measurements and vessel mapping are mainly based on time-lapse analysis26,27 which means tracking the temporal variations related to blood pulsations in bright-field microscopic images.
Regardless of the physical principle, a significant restriction of optical techniques in visible light is that embryos lose their transparency due to pigment pattern formation. In wild-type zebrafish, this process commences at the prim-5 stage of embryonic development, approximately 24 h post fertilization 28. Then a few metamorphic melanophores scatter over the dorsal and ventral myotomes starting the transformation to the adult melanophore pattern in larvae5. It limits long-term experimental plans that utilize optical approaches by 1–2 weeks post-fertilization. Efforts have been made to develop techniques that keep the transparency of zebrafish embryos for a longer period29. Using 1-phenyl-2-thiourea that blocks the formation of melanophores due to the reduction of tyrosinase activity is proposed for this purpose30. Transparent zebrafish mutants as TraNac (tra b6/b6; nac w2/w2), casper (roy a9/a9; nac w2/w2), and crystal (alb b4/b4; nac w2/w2; roy a9/a9) are available31,32,33. However, genomic interventions34 or the application of chemical agents35 can affect experimentation results. Moreover, experimental plans may suggest utilizing wild-type zebrafish exclusively. Thus, the development of optical approaches allowing to ignore melanocytes is highly relevant for the zebrafish embryos model and fish embryos in general.
Optical techniques in SWIR (900–1700 nm) can be one of the ways to overcome this issue. Light in this range has advantageous properties such as reduced scattering and a higher penetration depth in biological tissues36. The availability of SWIR cameras enables multiple high sensitivity imaging modalities. In this paper, we demonstrate experimentally that the optical transparency of zebrafish tissues in SWIR is higher than in VISNIR that makes typical cardiovascular imaging observations much more effective.
Materials and methods
Specimen preparation
Wild-type D. rerio (AB strain) were obtained from the commercial distributor and maintained in the Laboratory of Physiology and Toxicology (Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences). We kept zebrafish larvae in glass aquaria at 26 °C in a 12-h light–dark cycle and fed them with Artemia flakes. We put a selected individual into a 35 mm dish filled with water for each experiment and moved it back after image acquisition. We applied tricaine methanesulfonate (TMS, MS-222) solution at a concentration of 0.168 mg/mL within 1 min at 26 °C for anesthesia. After the experiments, the development, survival rate, morphology, and vital activity in the processed larvae did not differ significantly from those of not subjected specimens.
All methods were carried out in accordance with relevant guidelines and regulations. The study was approved by the Institutional Animal Care and Use Committee at the Papanin Institute for Biology of Inland Waters (protocol 6, date of approval: 25 February 2022 https://ibiw.ru/index.php?p=downloads&id=46958). In addition, the study was carried out in accordance with ARRIVE guidelines.
Experimental setup
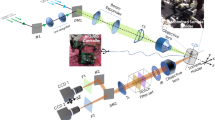
For image acquisition, we put the dish on a precise XYZ translation stage of transmitted light microscope BW Optics MF608. It has a 100 W halogen lamp equipped with continuous intensity adjustment and powered from the stabilized DC power supply. This light source has a wide irradiance spectrum that covers both VISNIR and SWIR. Adjustable lenses (collector and condenser) and diaphragms (field and aperture) of the microscope provide an even bright-field Kohler illumination of the sample. The imaging system consists of a microscopic objective (4 × NA 0.1), tube lens, adapter lens, and camera. Objective magnification may be adjusted depending on the task. It should be high enough to resolve single vessels and is a compromise of spatial resolution, depth of view, and data collection time. For a detailed analysis of a particular organ, one can install a 10 × or 20 × objective but make sure that the depth of focus is still acceptable (see Table 1). When a whole-body vessel image is required, it is necessary to scan the specimen by moving the translation stage with overlap between the fields of view at adjacent positions. The higher the resolution required, the more images and time we need to calculate vessel maps and stitch the whole-body panorama.
For VISNIR image acquisition, we had a monochrome CMOS camera IDS uEye UI-3360CP-NIR-GL Rev.2 (2048 × 1088 pixels, 5.5 × 5.5 μm2 pixel pitch). For SWIR imaging, we installed InGaAs camera Allied Vision Goldeye G-032 TEC2 (640 × 512 pixels, 15 × 15 μm2 pixel pitch). To compensate for the difference in the formats of these sensors and achieve closer values of optical magnification in VISNIR and SWIR, we applied 0.5 × and 1 × adapters, correspondingly.
Additionally, we inserted narrow band (FWHM 10 nm) filters with central wavelengths from 450 to 1600 nm with a 50 nm step into the microscope illumination system for spectral transmission measurements.
Before the experiments, we made sure that the optical transmission of microscope components was high enough in SWIR and carried out geometrical calibration of the setup. For this purpose, we acquired images of the grid distortion target and measured residual image distortion and illumination non-uniformity.
A series of N = 3000 images in VISNIR (12 bit, 1200 × 1200) and SWIR (14 bit, 640 × 512) at a 50 Hz frame rate within 60 s were collected in each experiment.
Data processing
We applied a well-proven image processing pipeline described earlier27 to detect and quantify cardiac activity. First, we eliminated non-uniformity in illumination by subtracting low frequency components from all images and normalizing them. Second, we implemented precise pixel-to-pixel image stabilization necessary to compensate for relative twists and turns between the specimen’s parts. Third, we analyzed temporal intensity variations in each pixel of the well-matched image stack and detected pixels in which these signals have periodic shapes and relate to cardiac activity. Fourth, we obtained vessel images, calculated heartbeat, and measured blood flow velocity.
Experimental protocol
The study consisted of two stages (Fig. 1). The first one aimed to measure the spectral properties of zebrafish tissues and prove the potential advantages of SWIR over VISNIR in terms of optical transmittance. In the second stage, we compared the quantitative characterization of the cardiovascular system in VISNIR and SWIR by calculating vessel maps, heart rates, and blood flow velocities.
Results
Optical transparency measurements
Figures 2a and 2b show VISNIR (400–900 nm) and SWIR (900–1700 nm) images of the eye, yolk, muscles, heart, and otoliths – organs that have different structures and properties37. We manually selected regions related to them (color-shaded areas in Fig. 2c) and marked the pigment pattern to see if its weak transparency in VISNIR differs in SWIR.
By installing the narrow-band filters into the microscope illumination system, we acquired a series of 15 spectral images of anesthetized zebrafish in the wavelength range 450–1600 nm, calculated wavelength dependences of the averaged intensity in selected organs I(λi) (i = 1,2,..,15) and specimen-free background B(λi), and evaluated transmission spectra as T(λi) = I(λi) / B(λi). Figure 2d demonstrates the box plots T(λi) averaged over 5 species. Due to the high water absorbance around 1450 nm shown by the grey vertical stripe in Fig. 2d, we consider the values of T(λi) in this wavelength band to be unreliable and, therefore, skip it. Data shown in Fig. 2d clearly indicates 1.5–2.5 times increased optical transmission T(λi) of all zebrafish organs in SWIR. Muscles, yolk, heart, and pigmented skin are consistently 70–90% transparent in the whole range of 850–1600 nm.
Cardiovascular study
Vessel imaging. Figure 3 illustrates typical VISNIR and SWIR images of zebrafish bodies. Once the images of the raw series are matched, videocapillaroscopy processing leads to calculation of blood flow maps, i.e. two-dimensional distributions of intensity variations and vessel images via highlighting the pixels in which intensity variations correspond to cardiac activity27. The detection of vessels from VISNIR images in the areas of strong pigmentation is barely possible. In the individual shown in Fig. 3, the pigment pattern marked with yellow covers a large part of vena cardinalis and partially arteria segmentalis. It immediately results in the absence of these vessels in the reconstructed vessel image. The pigment is transparent and, therefore, almost does not affect the blood flow detection capability of the image processing algorithm in SWIR. The vessel map reconstructed from the SWIR image series demonstrates a much more detailed structure of vessels including the ones hidden by pigmentation patterns.
We collected 8 image stacks with 20–30% overlap, calculated vessel maps, and stitched them into seamless panoramas to obtain high-resolution vessel images of the whole animal in VISNIR and SWIR (Fig. 4). In the SWIR panorama (Fig. 4b), one may see multiple vessels in the head, body, and tail which are absent in VISNIR (Fig. 4a). Again, due to transparency in SWIR, pigmentation all over the fish almost does not affect optical measurements.
Heart rate. Heart rate is a primary indicator of cardiac function and overall health. Its measurement is a mandatory and well-established procedure performed by multiple optical techniques38,39,40,41,42. One of the most straightforward is photoplethysmography (PPG), i.e. tracking volumetric variations of blood circulation. We implemented this approach for heartbeat measurement in VISNIR and SWIR. We selected the heart area (highlighted with a color frame in Fig. 5a) and calculated the temporal dependency of its average intensity for this purpose (Fig. 5b). After subtracting low-frequency components, we obtained normalized PPG signals (Fig. 5c) that may be analyzed and compared. Our experiments show that both VISNIR and SWIR imaging provide clear and stable periodic PPG signals. Though the heartbeat signal in VISNIR has 4–5 times higher amplitude and a more detailed shape, both signals suit well for heart rate measurements. Fourier analysis allows calculating the dominating frequencies, i.e. periods of these signals. In the example shown in Fig. 6, the heart rates measured in VISNIR and SWIR are 161 bpm and 164 bpm correspondingly. These values are close to the data obtained in previous studies22,43.
Raw images (a) and temporal dependences of blood flow velocity (b) in aorta dorsalis (red) and vena cardinalis (blue): VISNIR (left) and SWIR (right). Scale bar: 0.5 mm. Blood flow velocity in vena cardinalis was calculated only in SWIR due to the opaqueness of pigmentation that covers this vessel in VISNIR.
Blood flow velocity. Another parameter crucial for quantitative characterization of cardiac function and understanding of cardiovascular hemodynamics is blood flow velocity22,44. Figure 6a shows raw images of a zebrafish body acquired in VISNIR and SWIR. We manually selected the areas of aorta dorsalis (red) and vena cardinalis (blue) (see Fig. 6a and 6b), transformed their trajectories into straight lines by detecting central lines of these vessels and calculating normals to them in each point, and tracked relative shifts between the adjacent images27. Then, we may calculate blood flow velocity as a product of this shift and frame rate of 50 Hz. The pigmentation pattern almost completely covers vena cardinalis in VISNIR (see Fig. 6a). Thus, its detection and analysis are feasible only in SWIR. Figure 6b shows temporal dependences of blood flow velocity in aorta dorsalis and vena cardinalis. The phase shift between the signals in aorta dorsalis and vena cardinalis, their shapes, velocity values (VISNIR: 576 ± 450 μm/s, SWIR: 574 ± 510 μm/s and 564 ± 112 μm/s), and other features correspond well to the data obtained by alternative methods22,44. We may also extract heart rate from these signals by simple frequency analysis. It is 161 bpm in VISNIR and 164 bpm in SWIR in the example shown in Fig. 6. These values are consistent with that obtained from PPG signals in the heart area (Fig. 5c).
Table 2 summarizes the quantitative data related to the presented cardiovascular studies in VISNIR and SWIR for two zebrafish 4 dpf larvae. The signal-to-noise ratio (SNR) of PPG signal shows the reliability of cardiac activity measurements. The vessel density was defined as the proportion of the pixels related to vessels with detected blood flow over the total amount of pixels occupied by the specimen. Quantifications in Table 2 reveal the advantages of cardiovascular studies in SWIR over conventional VISNIR imaging. The processing of SWIR images is much more informative and reliable in terms of vessel mapping and blood flow velocity measurement due to the increased transparency of zebrafish organs and pigmentation.
Discussion
Our evaluation of the VISNIR and SWIR imaging showed a significantly increased optical transmission for zebrafish tissues and greater transparency of pigment pattern in SWIR. At the same time, the quality of heart and vessels detection in the images obtained in this range is not inferior to VISNIR. PPG signals derived from SWIR images are consistent with the heart rhythm. In this way, relatively simple changes in technical implementation make it possible to extend the period for the non-invasive studying of the cardiovascular system in zebrafish larvae and juveniles by the optical methods up to the formation of definitive scales5. This technique reveals new prospects for utilizing zebrafish as models for development and disease via long-term experimental plans for studying congenital heart defects, cardiomyopathy, and the effects of various influences on the functioning of the cardiovascular system11,12,13,14,15,16,17. In addition, long-term experiments are preferred for studying the dynamics of metastasis via circulating tumor cells in the zebrafish bloodstream2. Extended registration of cardiovascular performance and angiography in such studies can be applicable for predicting the efficiency of personalized cancer therapy and exploring mechanisms that drive metastasis and responses to therapy.
Transparent zebrafish larvae are also widely used for the assessment of drug-induced cardiotoxicity45. This kind of toxicity is one of the most frequent reasons for drug withdrawals46. Harmful reactions identified with the zebrafish model before clinical trials reduce the cost of testing new drugs45,47. Optical approaches are often applied to evaluating the functioning of the D. rerio cardiovascular system for drug screening47,48,49. Revealed advantages of SWIR imaging can provide an evaluation of the chronic cardiotoxicity in zebrafish with the non-invasive technique. The prolonged SWIR imaging registration period will allow more accurate preclinical studies, including delayed effects of low concentrations of drugs and possible bioaccumulation of drugs. Moreover, long-term experimental plans are necessary to assess the pharmacodynamics of drugs. Thus, the approach described and tested in the present study can significantly expand the possibilities of D. rerio utility as a drug screening tool.
Scales cover the zebrafish with age, and an adult iridophore-xanthophore pigment pattern appears in addition to melanophores5. It can reduce the transparency of the adult zebrafish in the SWIR range. SWIR imaging is suitable for monitoring heart function and hemodynamic analysis in juvenile zebrafish and fry. Imaging of 10 dpf zebrafish in VISNIR and SWIR can be found in the Supplementary Video. The main advantage of SWIR imaging is the almost complete transparency of the melanophores. It allows extending the imaging period in wild-type zebrafish by 2–3 weeks compared to optical techniques and abandoning genetic or biochemical manipulations to increase tissue transparency. This approach can be used to build complex long-term experimental plans in the zebrafish model (for example, when studying the pharmacodynamics of drugs, delayed effects and bioaccumulation of drugs and xenobiotics, the dynamics of metastasis via circulating tumor cells in the bloodstream, etc.).
The zebrafish is by far the most in-demand fish in biomedical experiments today. A smaller but significant part of studies is carried out on medaka Oryzias latipes50. Nowadays, some other fish species among teleosts are explored for modeling human disease51. The early development of fish species is quite similar37, so the non-invasive optical approaches with SWIR imaging most likely can be implemented for studying the cardiovascular performance and angiography in most teleosts species at early development.
Conclusion
Zebrafish tissues and their pigmentation pattern demonstrate increased transparency in SWIR in comparison to VISNIR. This finding expands the scope of optical techniques for cardiovascular research as monitoring cardiac activity and blood flow imaging. Even in the presence of a pigment, SWIR imaging allows non-invasive mapping and quantification of vessels located behind it. We believe that the results of this study may improve the efficiency of multiple optical techniques (photoplethysmography, optical coherence tomography, hyperspectral imaging, etc.) widely used for studying developmental dynamics and disorders in zebrafish.
Data availability
The main data generated and analyzed during this study are included in this published article. The additional datasets generated and analyzed during the current study are available from the corresponding author on reasonable requests.
References
Fontana, B. D., Mezzomo, N. J., Kalueff, A. V. & Rosemberg, D. B. The developing utility of zebrafish models of neurological and neuropsychiatric disorders: A critical review. Exp. Neurol. 299, 157–171. https://doi.org/10.1016/j.expneurol.2017.10.004 (2018).
Chen, X., Li, Y., Yao, T. & Jia, R. Benefits of zebrafish xenograft models in cancer research. Front Cell Dev Biol 9, 616551. https://doi.org/10.3389/fcell.2021.616551 (2021).
Lai, K. P., Gong, Z. & Tse, W. K. F. Zebrafish as the toxicant screening model: Transgenic and omics approaches. Aquat. Toxicol. 234, 105813. https://doi.org/10.1016/j.aquatox.2021.105813 (2021).
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503. https://doi.org/10.1038/nature12111 (2013).
Parichy, D. M., Elizondo, M. R., Mills, M. G., Gordon, T. N. & Engeszer, R. E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 238, 2975–3015. https://doi.org/10.1002/dvdy.22113 (2009).
Staudt, D. & Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 46, 397–418. https://doi.org/10.1146/annurev-genet-110711-155646 (2012).
Liu, C. C., Li, L., Lam, Y. W., Siu, C. W. & Cheng, S. H. Improvement of surface ECG recording in adult zebrafish reveals that the value of this model exceeds our expectation. Sci. Rep. 6, 25073. https://doi.org/10.1038/srep25073 (2016).
Bowley, G. et al. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 179, 900–917. https://doi.org/10.1111/bph.15473 (2022).
Isogai, S., Horiguchi, M. & Weinstein, B. M. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 230, 278–301. https://doi.org/10.1006/dbio.2000.9995 (2001).
Hogan, B. M. & Schulte-Merker, S. How to plumb a pisces: Understanding vascular development and disease using zebrafish embryos. Dev. Cell 42, 567–583. https://doi.org/10.1016/j.devcel.2017.08.015 (2017).
Liang, J. et al. Elevated glucose induces congenital heart defects by altering the expression of tbx5, tbx20, and has2 in developing zebrafish embryos. Birth Defects Res. A Clin. Mol. Teratol. 88, 480–486. https://doi.org/10.1002/bdra.20654 (2010).
Norton, N. et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am. J. Hum. Genet. 88, 273–282. https://doi.org/10.1016/j.ajhg.2011.01.016 (2011).
Zou, J. et al. An internal promoter underlies the difference in disease severity between N- and C-terminal truncation mutations of Titin in zebrafish. eLife https://doi.org/10.7554/elife.09406 (2015).
Brodehl, A. et al. Mutations in ILK, encoding integrin-linked kinase, are associated with arrhythmogenic cardiomyopathy. Transl. Res. 208, 15–29. https://doi.org/10.1016/j.trsl.2019.02.004 (2019).
Denvir, M. A., Tucker, C. S. & Mullins, J. J. Systolic and diastolic ventricular function in zebrafish embryos: Influence of norepenephrine, MS-222 and temperature. BMC Biotechnol. 8, 21. https://doi.org/10.1186/1472-6750-8-21 (2008).
Santoso, F. et al. Development of a simple ImageJ-based method for dynamic blood flow tracking in zebrafish embryos and its application in drug toxicity evaluation. Inventions 4, 65. https://doi.org/10.3390/inventions4040065 (2019).
Meng, H., Liang, J., Zheng, X., Zhang, K. & Zhao, Y. Using a high-throughput zebrafish embryo screening approach to support environmental hazard ranking for cardiovascular agents. Sci. Total Environ. 702, 134703. https://doi.org/10.1016/j.scitotenv.2019.134703 (2020).
Follain, G. et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell 45, 33-52.e12. https://doi.org/10.1016/j.devcel.2018.02.015 (2018).
Kagemann, L. et al. Repeated, noninvasive, high resolution spectral domain optical coherence tomography imaging of zebrafish embryos. Mol. Vis. 14, 2157–2170 (2008).
Haindl, R. et al. Functional optical coherence tomography and photoacoustic microscopy imaging for zebrafish larvae. Biomed. Opt. Express 11, 2137–2151. https://doi.org/10.1364/boe.390410 (2020).
Yang, D., Yuan, Z., Yang, Z., Hu, M. & Liang, Y. High-resolution polarization-sensitive optical coherence tomography and optical coherence tomography angiography for zebrafish skin imaging. J. Innov. Opt. Health Sci. 14, 2150022. https://doi.org/10.1142/s179354582150022x (2021).
Watkins, S. C. et al. High resolution imaging of vascular function in zebrafish. PLoS ONE 7, e44018. https://doi.org/10.1371/journal.pone.0044018 (2012).
Schlaeppi, A., Graves, A., Weber, M. & Huisken, J. Light sheet microscopy of fast cardiac dynamics in zebrafish embryos. J. Vis. Exp. 174, 62741. https://doi.org/10.3791/62741 (2021).
Simms, V. A., Bicknell, R. & Heath, V. L. Development of an ImageJ-based method for analysing the developing zebrafish vasculature. Vasc. Cell 9, 2. https://doi.org/10.24238/13221-9-1-172 (2017).
Chen, Y., Fingler, J., Trinh, L. & Fraser, S. Label-free imaging of developing vasculature in zebrafish with phase variance optical coherence microscopy. Proc. SPIE 9716, 97160A. https://doi.org/10.1117/12.2209653 (2016).
Kang, C. P. et al. An automatic method to calculate heart rate from zebrafish larval cardiac videos. BMC Bioinform. 19, 169. https://doi.org/10.1186/s12859-018-2166-6 (2018).
Machikhin, A. S., Burlakov, A. B., Volkov, M. V. & Khokhlov, D. D. Imaging photoplethysmography and videocapillaroscopy enable noninvasive study of zebrafish cardiovascular system functioning. J. Biophoton. 13, e202000061. https://doi.org/10.1002/jbio.202000061 (2020).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. https://doi.org/10.1002/aja.1002030302 (1995).
Inyushin, M., Meshalkina, D., Zueva, L. & Zayas-Santiago, A. Tissue transparency in vivo. Molecules 24, 2388. https://doi.org/10.3390/molecules24132388 (2019).
Karlsson, J., Von Hofsten, J. & Olsson, P. E. Generating transparent zebrafish: A refined method to improve detection of gene expression during embryonic development. Mar. Biotechnol. 3, 522–527. https://doi.org/10.1007/s1012601-0053-4 (2001).
White, R. M. et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189. https://doi.org/10.1016/j.stem.2007.11.002 (2008).
Antinucci, P. & Hindges, R. A crystal-clear zebrafish for in vivo imaging. Sci. Rep. 6, 29490. https://doi.org/10.1038/srep29490 (2016).
Wenz, R., Conibear, E., Bugeon, L. & Dallman, M. Fast, easy and early (larval) identification of transparent mutant zebrafish using standard fluorescence microscopy. F1000Research 9, 963. https://doi.org/10.12688/f1000research.22464.1 (2020).
D’Agati, G. et al. A defect in the mitochondrial protein Mpv17 underlies the transparent casper zebrafish. Dev. Biol. 430, 11–17. https://doi.org/10.1016/j.ydbio.2017.07.017 (2017).
Bohnsack, B. L., Gallina, D. & Kahana, A. Phenothiourea sensitizes zebrafish cranial neural crest and extraocular muscle development to changes in retinoic acid and IGF signaling. PLoS ONE 6, e22991. https://doi.org/10.1371/journal.pone.0022991 (2011).
Marcos-Vidal, A., Vaquero, J. J. & Ripoll, J. Optical properties of tissues in the near infrared: Their relevance for optical bioimaging. In Near Infrared-Emitting Nanoparticles for Biomedical Applications (eds Benayas, A. et al.) 1–20 (Springer, 2020). https://doi.org/10.1007/978-3-030-32036-2_1.
Kunz, Y. W. Developmental Biology of Teleost Fishes (Springer, 2004).
Scardina, G. A., Ruggieri, A. & Messina, P. Oral microcirculation observed in vivo by videocapillaroscopy: A review. J. Oral Sci. 51, 1–10. https://doi.org/10.2334/josnusd.51.1 (2009).
Chojnowski, M. M., Felis-Giemza, A. & Olesińska, M. Capillaroscopy-a role in modern rheumatology. Reumatologia 54, 67–72. https://doi.org/10.5114/reum.2016.60215 (2016).
Nilsson, G. & Wardell, K. Laser Doppler perfusion monitoring and imaging of blood microcirculation. Proc. SPIE https://doi.org/10.1117/12.180808 (1994).
Mennes, O. A., Van Netten, J. J., Slart, R. H. J. A. & Steenbergen, W. Novel optical techniques for imaging microcirculation in the diabetic foot. Curr. Pharm. Des. 24, 1304–1316. https://doi.org/10.2174/1381612824666180302141902 (2018).
Heeman, W., Steenbergen, W., van Dam, G. & Boerma, E. C. Clinical applications of laser speckle contrast imaging: A review. J. Biomed. Opt. 24, 1–11. https://doi.org/10.1117/1.Jbo.24.8.080901 (2019).
Gierten, J. et al. Automated high-throughput heartbeat quantification in medaka and zebrafish embryos under physiological conditions. Sci. Rep. 10, 2046. https://doi.org/10.1038/s41598-020-58563-w (2020).
Benslimane, F. M. et al. Cardiac function and blood flow hemodynamics assessment of zebrafish (Danio rerio) using high-speed video microscopy. Micron 136, 102876. https://doi.org/10.1016/j.micron.2020.102876 (2020).
Lane, S., More, L. A. & Asnani, A. Zebrafish models of cancer therapy-induced cardiovascular toxicity. J. Cardiovasc. Dev. Dis. 8, 8. https://doi.org/10.3390/jcdd8020008 (2021).
Stevens, J. L. & Baker, T. K. The future of drug safety testing: Expanding the view and narrowing the focus. Drug Discov. Today 14, 162–167. https://doi.org/10.1016/j.drudis.2008.11.009 (2009).
Maciag, M., Wnorowski, A., Mierzejewska, M. & Plazinska, A. Pharmacological assessment of zebrafish-based cardiotoxicity models. Biomed. Pharmacother. 148, 112695. https://doi.org/10.1016/j.biopha.2022.112695 (2022).
Han, Y., Zhang, J. P., Qian, J. Q. & Hu, C. Q. Cardiotoxicity evaluation of anthracyclines in zebrafish (Danio rerio). J. Appl. Toxicol. 35, 241–252. https://doi.org/10.1002/jat.3007 (2015).
Huang, Y. et al. Famoxadone-cymoxanil induced cardiotoxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 205, 111339. https://doi.org/10.1016/j.ecoenv.2020.111339 (2020).
Lin, C. Y., Chiang, C. Y. & Tsai, H. J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 23, 19. https://doi.org/10.1186/s12929-016-0236-5 (2016).
Schartl, M. Beyond the zebrafish: Diverse fish species for modeling human disease. Dis. Model. Mech. 7, 181–192. https://doi.org/10.1242/dmm.012245 (2013).
Acknowledgements
The experiments were carried out on the base of the Center for Collective Usage of the Scientific and Technological Center of Unique Instrumentation of the Russian Academy of Sciences. This study was supported by Russian Science Foundation (project 22-49-08012 https://rscf.ru/en/project/22-49-08012/).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.M., A.B. and V.K.; methodology, M.V.; software, M.V.; validation, A.M. and M.V.; formal analysis, A.B. and V.K.; investigation, V.B.; resources, D.K.; data curation, A.M. and V.B.; writing – original draft preparation, A.M., V.K. and A.B.; writing – review and editing, V.B., A.M. and D.K.; visualization, M.V. and V.B.; supervision, A.M., A.B. and V.K.; project administration, A.M.; funding acquisition, A.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Volkov, M., Machikhin, A., Bukova, V. et al. Optical transparency and label-free vessel imaging of zebrafish larvae in shortwave infrared range as a tool for prolonged studying of cardiovascular system development. Sci Rep 12, 20884 (2022). https://doi.org/10.1038/s41598-022-25386-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25386-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.