Abstract
Nowadays Nano metals have received an eminent compromise of attention. Even though different nanostructure of same metal maybe gives different results in wide range applications. Copper oxide (CuO-NPs) and Copper Nano wires (CuO-NWs) were prepared in controlled size via the alternating current Arc discharge process. Deionized water and argon gas were the chosen dielectric medium during the process to obtain 2 different forms of copper oxides. By changing the dielectric material from deionized water to argon gas the shape of CuO nanoparticles changed from spherical (CuO-NPs) to wires (CuO-NWS). The yield prepared depicted the purity of the prepared CuO, and their diameters were about 10 ± 5 nm and 30 ± 3 nm for CuO-NWs and CuO-NPs respectively. In vitro cytotoxic effect of the prepared CuO-NWs & CuO-NPs using human normal lung fibroblast cell line (WI-38 cells) revealed that CuO-NWs & CuO-NPs CC50 values were 458.8 and 155.6 µg/mL respectively. Both yields showed potent antibacterial activity against different multi-drug resistant Acinetobacter baumannii strains. A complete eradication of the bacterial growth was noticed after 4 Hrs incubation with CuO-NWs. Moreover, CuO-NWs showed superior antibacterial activity (with minimum inhibitory concentration reached 1.8 µg/mL) over CuO-NPs. The detailed antibacterial activity mechanism of CuO-NWs was further investigated; data proved the precipitation and adsorption of the nanoparticles on the bacterial cell surface leading to cell deformation with reactive oxygen species increment. The results explicated that the nanoparticles shape plays an essential role in the antibacterial activity. Rotational Arc discharge machine might be a promising tool to obtain various metal nanostructures with low cost and environmentally friendly with potent activity.
Similar content being viewed by others
Introduction
Bacterial resistance to antibiotics has increased dramatically over the past two decades, and it presents a serious worry for medical experts worldwide. An efficient way to inhibit bacteria is to use metal nanoparticles due to their tiny size that immobilizes the activity of bacteria1. Saravanan et al.2 reported the emerged drug resistance mechanism through extended spectrum β-lactamases enzyme which can hydrolyze a variety of β-lactam antibiotics. In this contest, Multidrug resistant bacteria (MDR) have emerged as a serious problem for public health which resulted from antibiotics abuse and misuse3. One of the most prevalent MDR bacteria is Acinetobacter baumannii. A. baumannii belongs to Gram-negative bacilli (GNB) causing extensive drug resistance (XDR) and carbapenem resistant (CRAb) infections which limit its treatment options4. Many hospitals acquire infections caused by A. baumannii (pneumonia, septicemia and blood stream infections (BSI)) resulting in a significantly high mortality rate among hospitalized patients5. Colistin (COL) was the last resort for XDR and CRAb infections, though the resistance to polymyxins (including colistin) had been widely and rapidly reported6. This raised a struggle to discover new active compounds against colistin resistant strains7. Nanotechnology has been a promising field in the combat against the ever-growing number of antimicrobial-resistant microorganisms, especially through metal nanoparticles application8,9. Nanoparticles have different characteristics compared to bulk materials of identical chemical composition. Since the mechanistic action of nanoparticles is to attack many sites and targets within the bacterial cell, no microbial resistance was reported against metal nanoparticles10.
Nano-metals have piqued the scientific imagination due to their excellent electrical, optical, and structural properties11,12,13. Metals oxides have a variety of attractive characteristics, making it suitable for use in a wide range of applications12. Semiconductor transition metal oxide Nano-architecture as construction blocks has received important attention due to their novel structures and characteristic impact on multi-discipline applications recently. Cu nanoparticles showed great potential applications in medicine14, agriculture15, devices and fabrications16. Many research labs have been used to manufacture copper nanostructure with different shapes by various methods. More precisely, crystal structure could be considered as one of the important effective factors on the properties and applications of NPs. Synthesis of Nano-metal oxides often involves toxic compounds especially when using surface capping and reducing agent via chemical methods17. The disadvantage of preparing nano-metal oxides in chemical methods vanished by using Arc discharge method in the presence of deionized water which is an alternative, not expensive, effective and environmentally friendly method avoiding toxicity that may cause cell damage18. In general, Arc discharge routines usually used to create metal nanoparticles with spherical shape under normal conditions. The unique properties of Copper oxide (CuO-NPs) and Copper Nano wires (CuO-NWs) piqued the interest of researchers, who have devoted special attention to Nano research and applications14,15,16,17. The properties of the produced Nano material were remarkably influenced by the technique of production11. Indeed, Arc discharge method successfully produced Nano-metals from the metal electrodes12. The parameters variance such as temperature14,19, pressure19,20, electrode shape12, the gap between electrodes21, current14, the voltage applied22, type of power supply22, and dielectric medium have great effect on the nanoparticle’s size and shape yields18,23. On the other hand, numerous studies showed that copper oxides have a great effect on bacterial inhibition against Escherichia coli, and a mild inhibitory effect on other bacterial strains9. Moreover, the antimicrobial activity of CuO-NPs has also been reported against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis, and methicillin-resistance Staphylococcus aureus (MRSA)9. Recently, few reports studied the bactericidal activity of CuO-NPs which was found to generate reactive oxygen species (ROS) causing bacterial cell death24. The CuO-NPs size greatly affects the antibacterial activity (the smaller nanoparticles size, the higher antibacterial activity)24. CuO-NPs directly bind to the bacterial cell wall, interact with the membranes’ proteins that lead to membrane perforation and releasing intracellular materials25. Some researches mentioned that Cu ions may interact with the sulfur and phosphorus-containing biomolecules (DNA and proteins) that lead to structure distortion26,27.
Thus, the aim of this study was to produce CuO-NPs and CuO-NWs with low toxicity by both solid–liquid or solid–gas Arc discharge methods due to their high biological activity against bacteria. The study also aimed to examine the yield morphology produced using a high resolution transmission electron microscope (HR-TEM), to investigate the nanoparticles crystallinity using the X-ray diffraction analyzer (XRD), inductively coupled plasma optical emission spectrometry (ICP-OES) to know the exact amount produced, and the sample stability was measured through Zeta potential. On the top of that, the growing urge to find new therapy in the combat against colistin resistant strains obligate us to explore the potential antibacterial activity of CuO-NPs and CuO-NWs against some colistin-resistant Acinetobacter baumannii strains. Hence, this is the first work to emphasize the antibacterial activity of Copper in different shapes against colistin-resistant bacterial strains.
Materials and methods
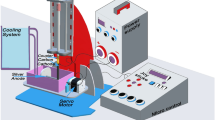
High purity CuO-NPs and CuO-NWs were synthesized by the Arc discharge method under fixed conditions of electrode dimensions, alternating current, power supply, voltage used, vessel capacity, rotational speed, pH, electrode gap, and discharge duration as tabulated in Table 1. A voltage of 70 V with reasonable current 15 A is used to maintain a steady Arc discharge that would improves the yield quality and quantity in both experiments. Another key component is the cathode's rotational speed at 950 rpm that accelerates metal clustering and prevents it from condensation on cathode surface. This parameter has a significant impact on the particle size properties and stability. The cathode is taken in large dimension with respect to the anode to increase the yield. The experiment was carried twice by changing dielectric material. Copper electrodes dispersed in deionized water (conductivity = 0.8–0.9 µS) were used to produce CuO-NPs at temperature about 20 °C as shown in Fig. 1a, then copper electrodes were used to get CuO-NWs using argon (Ar) gas as dielectric media with a temperature of 100 °C as shown in Fig. 1b. The process was free of chemical agents. The transition of metal vapors into Nano yield may be divided into three stages: nucleation, cluster growth, and condensation in deionized water2.
Filtration and characterization
Filtration was carried out by using a cooling ultracentrifuge model (Hettich MIKRO, German) separation of the nanoparticles was according to their mass where the lower layer contains massive particle. The relative centrifugal force (RCF) was then calculated by applying Eq. (1) 21.
where RCF is the relative centrifugal force (cm/sec2), r is the rotational radius (cm), and N is the rotating speed (revolution per minute, rpm). Then each sample was characterized by JEOL JEM-2100 HR-TEM, XRD, and Zetasizer (nano 90 Malvern, UK).
In vitro studies
Evaluation of cytotoxic effects of the prepared nanoparticles
Human lung fibroblast (WI-38 cells) normal cell line (ATCC® number: CCL-75™) was obtained from the American Type Culture Collection (ATCC, Rockville, MD). MTT assay is a quantitative method that can be used to measure the cells’ viability. Precultured cell line (5 × 104 cell/well in Corning® 96-well tissue culture plate) was exposed to eight serially diluted concentrations, incubated for 24 Hrs then the numbers of viable cells were determined by the MTT test. The relation between surviving cells and drug concentration was plotted to get the survival curve of WI-38 cell line after treatment with CuO-NPs and CuO-NWs one at a time according to Eq. (2). The cytotoxic concentration (CC50), the concentration required to cause toxic effects in 50% of intact cells, was estimated from graphical plots of the dose response curve for each tested concentration using Origin Pro6.8 software28.
Antibacterial activity
Disc diffusion method
The antibacterial activities of CuO-NPs and CuO-NWs were evaluated against different Acinetobacter baumannii colistin resistant strains. 1.5 × 106 CFU/ml (0.5 McFarland) bacterial suspensions were prepared, 100 μl of each was swabbed over the surface of Müeller-Hinton agar (MHA) plate. Disc-diffusion method was carried out to assess the antibacterial activity; each sterilized disc (Whatman No. 1 with 6 mm diameter) was saturated with 25 μl of 20 mg CuO-NPs and CuO-NWs individually then placed over the inoculated MHA plates. Incubation for 18 Hrs at 37 ± 2 °C was done29,30. Colistin resistant strains of Acinetobacter baumannii under test were kindly identified and provided by the Microbiology Department’s Strain Bank, et al.-Shatby Pediatric Hospital, Alexandria, Egypt.
Minimum inhibitory concentration (MIC)
MIC test was done by adding a mixture of 20 μl tween 80, 80 μl of sterile Müeller-Hinton broth and 100 μl of CuO-NPs and CuO-NWs individually. The mixture was serially diluted using a two-fold dilution in 96-well microtiter plate. 100 μl of 0.5 McFarland bacterial suspensions was inoculated in each well. MIC is known as the minimum inhibitory concentration of the tested drug to inhibit the bacterial growth29,30.
Bacterial lethality curve
Time-kill curve was investigated to estimate the optimum time required for CuO-NPs and CuO-NWs to kill the bacterial vegetative cells. CuO-NPs and CuO-NWs were added individually to 1 ml Müeller-Hinton broth by using the nanoparticles MIC values then 1 ml of the bacterial inoculum was added. Aliquots from each tube were taken to assess the bacterial growth through different incubation time (0, 2, 4, 6, 8, 12 and 24 Hrs) at OD 600 nm29.
Transmission electron microscope study
Transmission electron microscopic (TEM) examination (JEM-100 CX, JOEL, Japan), has a resolution of 3 nm at 30 kV. Buffers and dehydration protocol were used by graded acetone series and epoxy resin embedding. Ultrathin sections were prepared on grids then stained with 3% uranyl acetate29,31.
Reactive oxygen species content measurement
Reactive oxygen species (ROS) was measured by a HPF (3′-(phydroxyphenyl fluorescein) where 5 mM HPF was added to 200 μL bacterial suspension treated with 100 μg/mL of the most potent nanoparticles. The plate was incubated in dark at 37 °C, and fluorescence of each well was measured at 0, 1, 2, and 3 h, respectively, with excitation/emission at 490/515 nm32.
Results and discussions
Preparation and characterization of the Nano yields
Uniform cuprous oxides with different morphologies have been successfully synthesized using different pressure and temperature during Arc discharge process. After solid–liquid phase Arc discharge process for CuO-NPs preparation, the vessel contained suspended nanoparticles dispersed in deionized water. To extract the nanoparticles from the solution, the radius and rotational speed of the ultracentrifuge were adjusted to 7.5 cm and 10,000 rpm for 30 min, respectively. HR-TEM study was performed for each sample (Fig. 2b). The results showed that CuO-NPs have spherical shape with average diameter 41 ± 5 nm.
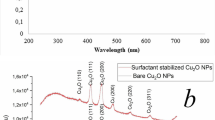
On the other hand, HR-TEM depicts CuO-NWs (Fig. 2a) as a thin wire shape in the Nano range with diameter 9 ± 3 nm and length from 3 to 5 µm which were easily collected after 10 min from switching off the Arc discharge unit. It’s clear that the surface area of CuO-NWs was greater than CuO-NPs. CuO-NPs and CuO-NWs were prepared in different conditions which resulted in different shapes. Figure 3a illustrated the XRD pattern of each sample. The pattern assigned to intensities of three major peaks revealed the presence of CuO-NWs. The high intense peak for face centered cubic (fcc) structure was in good agreement with (JCPDS #03-1018). On the other hand, XRD (Fig. 3b) revealed sharp diffraction peaks at 2θ indexed for highly pure CuO-NPs using JCPDS file number 01-080-0076. The XRD data and HR-TEM results confirmed that the prepared samples were pure (without impurities). Both CuO-NWs and CuO-NPs were crystalline materials. When nanoparticles were immersed in deionized water, they created an area of electrical inhomogeneity at the solid–solution interface.
As a result, an apparent excess of charge at the solid surface was precisely balanced by a diffuse area of another opposite charge. This diffused area, which originated in the aquatic environment, was known as the electrical double layer. This process may cause nanoparticles’ aggregation; therefore, it is important to measure the electrical potential of the interface between the aqueous solution and the stationary layer which is known as zeta potential. Zeta potential was measured for CuO-NWs & CuO-NPs (−47 ± 1.4 and −34 ± 1.8 mV, respectively). The high zeta potential confirmed the stability of the prepared samples.
EDX analysis CuO-NWs results were displayed in Fig. 4-a, which declared that the prepared nanoparticles were almost pure Cu2O. On the other hand Fig. 4-b showed a uniform distribution of copper to oxygen with atomic ratio of 1:1 in CuO-NPs which had higher presence of Oxygen atoms compared to CuO-NWs. This result ensured the formation of pure nano-yields.
ICP-OES Device was used to examine exactly the amount of produced Nano copper yields prepared with different temperature and pressure. According to these measurements the masses of the prepared samples namely CuO-NWs or CuO-NPs were found to be 72 ± 2 mg/min and 54 ± 1 mg/min respectively. According to the abovementioned investigations, to successfully make the gliding Arc between two electrode gaps, strongly apply field with sufficient energy must done to eliminate the electron from an electrode (and behind metal ions) in gas or liquid. It was found that the Arc discharge preparation method for the two Nano-systems resulted in different yield shape which may be related to the change of pressure and temperature that affect the work function which was responsible for eliminating the electron forming metal ions nanoparticles.
Cytotoxic effects of the prepared nanoparticles
In a trial to study the in vitro cytotoxic effect of the prepared CuO-NWs & CuO-NPs the cell proliferation using human normal lung fibroblast cell line (WI-38 cells) was tested. Lung fibroblast cell line was chosen due to the possible biomedical application of the prepared CuO nanoparticles as a potent treatment against Acinetobacter baumannii infection. It was found that at 620 µg/mL of CuO-NWs, and CuO-NPs, the fibroblast cell viability were 45 and 15% respectively. CuO-NWs & CuO-NPs CC50 values were 458.8 and 155.6 µg/mL respectively (Fig. 5). Fahmy et al.33 studied the Cu/CuO-NPs effect on normal lung cells and reported IC50 value reached 201.26 µg/mL.
Antibacterial activity
Antibacterial activity of CuO-NWs & CuO-NPs were evaluated against Colistin resistant strains of Acinetobacter baumannii through various techniques namely disc diffusion method, minimum inhibitory concentration (MIC), bacterial lethality curve, HR-TEM study, ROS content measurement and NADH content measurement. Data revealed that CuO-NWs had higher antibacterial activity than the nanoparticles spherical form. This may be explained by the smaller size with larger surface area of the prepared nanowires compared to the nanoparticles form. A. baumannii strain number 10 was the most susceptible strain while A. baumannii strain number 1 was the most resistant one (Table 2, Fig. 6a). The bacterial lethality curve was assessed against the most resistant strain (A. baumannii strain number 1). It was noticed that CuO-NWs completely eradicated the bacterial growth after 4 h contact time while CuO-NPs inhibited the bacterial growth after 8 h (Fig. 6b). This test confirmed the superior antibacterial activity of CuO-NWs. The antibacterial mechanism of CuO-NWs was studied through different techniques against the most resistant strain (A. baumannii strain number 1). It has been previously verified that metal nanoparticles usually inhibit the bacterial growth through disrupting the bacterial cell membrane and metal ions releasing that enters the bacterial cytoplasm and disrupt the protein functions. This assumption was confirmed through the HR-TEM study of the treated bacterial cells which revealed the CuO precipitation and adsorption on the bacterial cell surface leading to cell deformation (Fig. 7c). The bacterial cell response to such stimuli has usually resulted in a reactive oxygen species (ROS) increment. ROS content measurement was assessed with a commercial fluorescent probe, it was observed that the ROS content increased by 23% after 4 h incubation with the potent CuO-NWs (Fig. 7a). Moreover, the observed red fluorescent dead cells were observed which confirmed the CuO antibacterial mechanism (cytoplasmic membranes inactivation and bacterial cells apoptosis due to cellular membrane disruption) (Fig. 7b).
Antibacterial effect of CuO-NWs & CuO-NPs against Acinetobacter baumannii strains represented in ROS generation with a fluorescent HPF probe (a), fluorescent images of Acinetobacter baumannii cells after treatment with live/dead fluorescent dyes to indicate cell membrane permeability (b) and transmission electron microscopic (TEM) study (c).
Lv et al.34 stated that Cu-NPs inhibited the bacterial growth of Staphylococcus aureus and Escherichia coli isolates by cytoplasmic membranes inactivation. The Cu-induction of bacterial ROS leads to stimulation of DNA strand cleavage34. Chatterjee et al.35 revealed that Cu-NPs can inhibit the bacterial growth via various mechanisms namely lipid peroxidation, ROS generation, protein oxidation and DNA degradation in E. coli cells. Other studies mentioned that the antibacterial mechanism of action of Cu-NPs was due to the reactive complex formation between the cellular medium organics and CuO-NPs36. Gunawan et al.37 also confirmed the antibacterial mechanism of CuO-NPs due to copper-peptide complex formation that lead to multiple fold increase of the bacterial ROS resulted in the overall inhibition of biomass growth. Kumar et al.38 reported that the CuO nanostructures with high surface area showed higher antimicrobial activity.
The mechanism of action of cupric (Cu2+) and cuprous (Cu1+) ions in inhibiting the bacterial growth was discussed by Midander et al.39 who stated that the Cu2+ ions liberation from CuO nanostructures could disrupt the bacterial cell membrane and finally deactivate the microbial growth. Jadhav et al.40 proposed the antibacterial activity of CuO-NPs can be attributed to the induction of ROS originated from the electron–donating nature of copper oxides. Meghana et al.41 mentioned that the antibacterial activity of CuO and Cu2O nanoparticles is highly and closely related to the metal oxidation states which follow the independent pathway of the cell membrane disruption and bactericidal/bacteriostatic activity. It was also reported that the ROS pathway can be applied to CuO nanoparticles than Cu2O. The antibacterial effect of cupper oxides against E.coli was proposed as that the Cu2O usually form copper(I)–peptide complex while CuO cause free radical generation. CuO nanoparticles produced significant ROS in terms of super oxides while Cu2O did not. Another study investigated the antibacterial activity of CuNPs against multispecies biofilm, and it was proved that CuNPs have an immediate action with gradual increasing through time reaching their highest antibiofilm effect after 7 days incubation42. Moreover, a time-kill curve kinetics of Cu-NPs declared that the complete microbial growth eradication can be observed within 4 Hrs incubation43.
Conclusion
In summary, CuO-NWs with diameter 9 ± 3 nm and length from 3 to 5 µm showed higher stability compared with spherical shape of CuO-NPs with average diameter 41 ± 5 nm . Different copper oxide shapescan be selectively synthesized by the choice of a suitable pressure and temperature. The difference between energy applied and the work function to eliminate electrons may be transferred to heat energy in dielectric media and/or play a vital role in reshaping the crystal order of the formed ions to produce specific shape. In vitro cytotoxic effect of the prepared CuO-NWs & CuO-NPs using human normal lung fibroblast cell line (WI-38 cells) revealed that CuO-NWs & CuO-NPs CC50 values were 458.8 and 155.6 µg/mL respectively. The antibacterial activity of CuO-NWs was higher than CuO-NPs and that could be explained by that the cupric (Cu2+) and cuprous (Cu1+) ions inhibited the bacterial growth by disrupting the bacterial cell membrane and ROS induction. According to the high antibacterial activity and relatively low toxicity, Cu-NWs could be used as a new potent treatment against colistin and multi-drug resistant infections.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Anirudh, S. & Pavan Kumar, G. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnology Reports 25, e00427 (2020).
Saravanan, M., Ramachandran, B. & Barabadi, H. The prevalence and drug resistance pattern of extended spectrum β–lactamases (ESBLs) producing Enterobacteriaceae in Africa. Microb. Pathog. 114, 180–192 (2018).
Yu, Z., Rabiee, H. & Guo, J. Synergistic effect of sulfidated nano zerovalent iron and persulfate on inactivating antibiotic resistant bacteria and antibiotic resistance genes. Water Res. 198, 117141 (2021).
Lee, H. & Lee, H. Clinical and economic evaluation of multidrug-resistant Acinetobacter baumannii colonization in the intensive care unit. Infect. Chemother. 48(3), 174–180 (2016).
Mody, L. et al. Prevalence of and risk factors for multidrug-resistant Acinetobacter baumannii colonization among high-risk nursing home residents. Infect. Control Hosp. Epidemiol. 36(10), 1155–1162 (2015).
Hua, X. et al. Colistin resistance in Acinetobacter baumannii MDR-ZJ06 revealed by a multiomics approach. Front. Cell. Infect. Microbiol. 7, 45 (2017).
Asif, M., Alvi, I. A. & Rehman, S. U. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Res. 11, 1249 (2018).
Rezaie, A. B., Montazer, M. & Rad, M. M. Low toxic antibacterial application with hydrophobic properties on polyester through facile and clean fabrication of nano copper with fatty acid. Mater. Sci. Eng., C 97, 177–187 (2019).
Alavi, M. & Karimi, N. Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int. J. Biol. Macromol. 128, 893–901 (2019).
Ferrag, C. et al. Polyacrylamide hydrogels doped with different shapes of silver nanoparticles: Antibacterial and mechanical properties. Coll. Surf. B 197, 111397 (2021).
El-khatib, A. M., Elsafi, M., Sayyed, M. I., Abbas, M. I. & El-Khatib, M. Impact of micro and nano aluminium on the efficiency of photon detectors. Results Phys. 30, 104908 (2021).
El-Khatib, A. M., Badawi, M. S., Ghatass, Z. F., Mohamed, M. M. & Elkhatib, M. Synthesize of silver nanoparticles by arc discharge method using two different rotational electrode shapes. J. Cluster. Sci. 29(6), 1169–1175 (2018).
Timerkaev, B. A., Shakirov, B. R. & Timerkaeva, D. B. Creation of silicon nanostructures in electric arc discharge. High Energy Chem. 53(2), 162–166 (2019).
Mbewana-Ntshanka, N. G., Moloto, M. J., Mubiayi, P. K. Antimicrobial activity of the synthesized of copper chalcogenide nanoparticles. J. Nanotechnol. 2021 (2021).
Rai, M. & Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 94(2), 287–293 (2012).
Nishant, V. & NikhilKumar, N. Synthesis and biomedical applications of copper oxide nanoparticles: an expanding horizon. ACS Biomater. Sci. Eng. 5(3), 1170–1188 (2019).
Siddiqi, K. S. & Husen, A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: A review. Biomater. Res. 24, 11. https://doi.org/10.1186/s40824-020-00188-1 (2020).
Tseng, K. H. et al. Study on the characteristics of zinc oxide nanocolloid prepared using electrical spark discharge method. J. Clust. Sci. https://doi.org/10.1007/s10876-020-01949-7 (2021).
Zhao, T., Liu, Y. & Zhu, J. Temperature and catalyst effects on the production of amorphous carbon nanotubes by a modified arc discharge. Carbon 43(14), 2907–2912 (2005).
Su, Y., Yang, Z., Wei, H., Kong, E. S. W. & Zhang, Y. Synthesis of single-walled carbon nanotubes with selective diameter distributions using DC arc discharge under CO mixed atmosphere. Appl. Surf. Sci. 257(7), 3123–3127 (2011).
Samy Yousef, A., Khattab, T. A. & Osman, MZi. Effects of Increasing electrodes on CNTs yield synthesized by using arc-discharge technique. J. Nanomater. https://doi.org/10.1155/2013/392126 (2013).
Neha, A. & Sharma, N. N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 50, 135–150. https://doi.org/10.1016/j.diamond.2014.10.001 (2014).
Rahnemai, H. & Poursalehi, R. The effect of liquid environment on composition, colloidal stability and optical properties of nickel nanoparticles synthesized by arc discharge in liquid. Proc. Mater. Sci. 11(2015), 347–351. https://doi.org/10.1016/j.mspro.2015.11.119 (2015).
Bhattacharjee, Rahul. The emergence of metal oxide nanoparticles (NPs) as a phytomedicine: A two-facet role in plant growth, nano-toxicity and anti-phyto-microbial activity. Biomed. Pharmacother. 155, 113658 (2022).
Mallick, S. et al. Iodine-stabilized Cu nanoparticle chitosan composite for antibacterial applications. ACS Appl. Mater. Interfaces 4(3), 1313–1323 (2012).
Nisar, P., Ali, N., Rahman, L., Ali, M. & Shinwari, Z. K. Antimicrobial activities of biologically synthesized metal nanoparticles: An insight into the mechanism of action. J. Biol. Inorg. Chem. 24(7), 929–941 (2019).
Raffi, M. et al. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 60(1), 75–80 (2010).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method 65, 55–63 (1983).
Elnaggar, Y. S. et al. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: elaboration; in vitro and in vivo appraisal. Nanomedicine 15(13), 1269–1284 (2020).
Aljohani, F. S. et al. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 12(1), 1–20 (2022).
Hartmann, M. et al. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 54(8), 3132–3142 (2010).
Kwun, M. S. & Lee, D. G. Investigation of distinct contribution of nitric oxide and each reactive oxygen species in indole-3-propionic-acid-induced apoptosis-like death in Escherichia coli. Life Sci. 285, 120003 (2021).
Fahmy, H. M., Ebrahim, N. M. & Gaber, M. H. In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J. Trace Elem. Med. Biol. 60, 126481 (2020).
Lv, P. et al. Effect of NaOH concentration on antibacterial activities of Cu nanoparticles and the antibacterial mechanism. Mater. Sci. Eng., C 110, 110669 (2020).
Chatterjee, A. K., Chakraborty, R. & Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25(13), 135101 (2014).
Chakraborty, R. et al. A simple, fast and cost-effective method of synthesis of cupric oxide nanoparticle with promising antibacterial potency: Unraveling the biological and chemical modes of action. Biochim. et Biophys. Acta (BBA) Gen. Subj. 1850(4), 845–856 (2015).
Gunawan, C., Teoh, W. Y., Marquis, C. P. & Amal, R. Cytotoxic origin of copper (II) oxide nanoparticles: Comparative studies with micron-sized particles, leachate, and metal salts. ACS Nano 5(9), 7214–7225 (2011).
Kumar, S. et al. Facile synthesis of CuO nanowires and Cu2O nanospheres grown on rGO surface and exploiting its photocatalytic, antibacterial and supercapacitive properties. Physica B Condens. Matter 558, 74–81 (2019).
Midander, K. et al. Surface characteristics, copper release, and toxicity of nano-and micrometer-sized copper and copper (II) oxide particles: A cross-disciplinary study. Small 5(3), 389–399 (2009).
Jadhav, S., Gaikwad, S., Nimse, M. & Rajbhoj, A. Copper oxide nanoparticles: synthesis, characterization and their antibacterial activity. J. Clust. Sci. 22(2), 121–129 (2011).
Meghana, S., Kabra, P., Chakraborty, S. & Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 5(16), 12293–12299 (2015).
Rojas, B. et al. Antibacterial activity of copper nanoparticles (Cunps) against a resistant calcium hydroxide multispecies endodontic biofilm. Nanomaterials 11(9), 2254 (2021).
Sacoto-Figueroa, F. K. et al. Molecular characterization and antibacterial activity of oral antibiotics and copper nanoparticles against endodontic pathogens commonly related to health care-associated infections. Clin. Oral Invest. 25(12), 6729–6741 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
B.H.E. and M.E. Conceptualization, Methodology, Investigation, and Wrote the main manuscript text. M.T. Revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elwakil, B.H., Toderas, M. & El-Khatib, M. Arc discharge rapid synthesis of engineered copper oxides nano shapes with potent antibacterial activity against multi-drug resistant bacteria. Sci Rep 12, 20209 (2022). https://doi.org/10.1038/s41598-022-24514-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24514-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.