Abstract
In order to investigate the reliability of correcting GAT formulas in comparison with dynamic contour tonometry (DCT), this study included 112 right eyes of 112 healthy subjects aged from 21 to 77 years, whose eyes underwent to a full ophthalmologic exam. IOP was measured in each eye with DCT and then with GAT. IOP values obtained with GAT were corrected with 10 equations and then compared with those provided by DCT. Participants mean age was 42.24 ± 14.08 years; mean IOP measured with DCT was 17.61 ± 2.87 and 15.50 ± 2.47 mmHg, measured with GAT. The mean discordance between DCT and GAT measurements was 2.11 ± 2.24 mmHg. All the correcting formulas, but Srodka one (p ˂ 0.001), tend to increase the difference between GAT and DCT. According to these results Śródka equation provides the best correction, reducing the difference between the two IOP measurement methods of − 0.03 ± 0.85 mmHg. Other equations do not provide a valid improvement of the agreement between the two methods or they provide a worsening of the agreement.
Similar content being viewed by others
Introduction
The intraocular pressure (IOP) measurement is crucial not only in the diagnosis and in management of glaucoma, as IOP represents the only treatable risk factor in clinical practice, but it is also essential in the post-operative management of corneal, cataract and vitreo-retinal surgeries and to help the detection of some rare diseases1.
Goldmann applanation tonometry (GAT), based on the so called Imbert-Fick law2,3, is the gold standard for IOP measurement, but unfortunately several factors, such as central corneal thickness (CCT), curvature (Km) and structure can influence its accuracy4.
It is well known that in case of cataract surgery after corneal refractive procedures, the intraocular lens (IOL) power to be implanted is underestimated5,6,7,8, as well as the GAT measurements are absolutely unreliable9.
To obtain more reliable GAT values, several experimental and theoretical calculation-based studies elaborated correcting formulas, based upon different parameters influencing GAT readings, such as corneal CCT, Km and subject age10,11,12,13,14,15,16,17,18.
The problem of testing the accuracy of these formulas is that results have to be compared with the true IOP value, measurable in vivo with an invasive technique, which is inexecutable in ordinary clinical practice.
Quite a few years ago, a new tonometer has been launched into the market, namely the dynamic contour tonometry (DCT); it is based on the Pascal law19, as an alternative of applanation principle, and it seems to be not influenced by CCT, Km, stiffness, properties and morphology20,21,22.
As DCT is assumed to be independent from corneal characteristics, the aim of this study is to compare DCT measurements to GAT, corrected according to 10 different formulas (which take into account some corneal parameters) in healthy subjects, to verify if these formulas effectively provide GAT values not influenced by corneal properties.
Methods
One hundred and twelve right eyes of 112 healthy patients with no history of corneal diseases, previous corneal/ocular surgery or trauma, were included in this retrospective study, performed in 2021 on data previously obtained. Patients’ age ranged from 21 to 77 (mean = 42.24 ± 14.08) years old. The present study adhers to the ethical principles of the Declaration of Helsinki. Informed consent was obtained from each patient, and Institutional Review Board (IRB) approval was also obtained from the ComEtico Campania Sud (CECS). All the patients underwent a complete ophthalmic examination including, among the others, a CCT and Km measurement with a Pentacam (Oculus, Germany); the examination was considered to be reliable when the quality factor was 95% or higher, as suggested by the factory. Corneas were anesthetized with oxybuprocaine eye drops [Novesina, Novartis, Italy] before IOP measurements. During the measurement, subjects were asked to keep both eyes opened, breathe quietly, and fixate into the distance behind the examiner.
IOP measurement were taken in the same order on the same day, between 3:00 and 5:00 p.m. DCT was performed first, followed 10–15 min later by GAT and each of them was performed once, to avoid IOP reduction due to repeated measurement4. DCT head consists in a cylindrical tip with a surface that resembles the corneal contour when the pressure on both corneal sides is equal, so there is no need to apply additional forces that could modify the corneal profile and to influence the following GAT IOP measurements19.
When the tip of the DCT tonometer contacts the cornea, an audible signal, that changes in pitch with variations in pressure detected, indicates the correct positioning. The tip generates an electrical signal, proportional to the IOP value, which is calculated automatically and displayed on the digital screen along with the ocular pulse amplitude and the quality of each reading with a scale classified from Q1 (optimum) to Q5 (unacceptable). Only good quality DCT readings (Q1, Q2, and Q3) were used for this study. Regarding GAT measurements, a fluorescein strip was used in each eye and the patient was asked to blink several times before the measurement was performed, to obtain a fluorescein ring thickness similar to the one shown in the Goldmann tonometer manual, that should be between 0.25 and 0.3 mm. Patient comfort was ensured and the examiner did not hold the lids open, to avoid any pressure on the globe. DCT and GAT measurements were obtained by 2 different examiners who were not aware of results obtained with the other technique.
IOP measurements provided by GAT have been corrected by using the 10 formulas shown in Table 1, derived from manometric studies in vivo16,17, from retrospective studies11 and methanalisys15, and from studies based on mathematical calculation10,12,13,14.
Statistical analysis was performed with SPSS 26.0 (SPSS, Inc., Chicago, IL). The normality of data was examined by the exact Kolmogorov–Smirnov. The Student T-test was used for pair-wise comparisons of IOP obtained with DCT and GAT and also with DCT and all IOP obtained after applying 10 correcting formulas. This latter test was also used for pair-wise comparison of differences between measurement obtained with and without IOP correction.
A p-value less than 0.05 was considered statistically significant. Therefore, Bland–Altman evaluation, was performed.
Required sample size was calculated with a power calculation software (G*Power, Version 3.1.9.6, Faul, Erdfelder, Lang, & Buchner, 2020. Available at https://www.gpower.hhu.de). It was estimated that with a significance level of 5% and a test power of 80%, a sample size of 73 eyes would be necessary to detect a mean IOP difference of 0.05 mmHg, given a within-subject SD for IOP equal to 1.50 mmHg.
The differences were evaluated both as absolute and non-absolute error utilizing SPSS.
Results
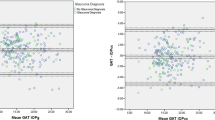
All analyzed data were normally distributed (all p > 0.050). The obtained results are summarized in Tables 2, 3, 4. The correlation between uncorrected and corrected GAT with DCT is shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11.
Both fixed bias and proportional bias were found only with Foster correction (Fig. 11). There was no evidence of fixed bias or proportional bias with all other correction formulas (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10).
According to these results, IOP measurements provided by GAT without any correction are lower than those provided by DCT.
Utilizing formulas shown in Table 1, an increase of mean difference (DCT-GATcorrected) is obtained with formulas number 3, 4, 7, 8 and 10; in particular, formulas 3, 4, 7, 10 tend to underestimate the IOP measured by GAT, providing a further reduction of IOP value in comparison to DCT. Formulas 6 and 8, instead, tend to overestimate the IOP measured by GAT: in these cases, the difference DCT-GATcorrected is negative, since GAT corrected by equations 6 and 8 is higher than DCT.
As shown in Table 2, a reduction of mean difference between DCT and GATcorrected is obtained with formulas 1, 2, 5, 7 and 9 formulas; Śródka (formula 5) provides the highest reduction of mean difference (DCT-GATSrodka = 1.86 ± 2.48).
If we consider the absolute error (Table 3) we can clearly see that all the formulas, but Śródka one, tend to increase the difference between GAT and DCT. Śródka equation provides a statistically significant decrease of the difference between these two methods.
Discussion
IOP role in etiopathogenesis and development of glaucoma has been recently questioned, in fact it is not included in primary open-angle glaucoma definition given by European Glaucoma society (EGS) guidance. Nevertheless, several studies have established the importance of its reduction in glaucomatous patient management23. Therefore, tonometry remains mandatory in all the patient over 50 years old in all the subjects considered to be at risk of glaucoma development.
Even if GAT is considered to be the gold standard, but unfortunately it cannot be used to measure the IOP in different body’s position24 and several factors make it not reliable; in fact it is based on the so-called Imbert-Fick law that is not a physical law, or even an engineering principle, but it was just invoked as an explanation of how applanation tonometers worked2,3.
The validity of the law needs the structure applanated by the flat surface to be thin, elastic, flexible, and the pressure of the applanating surface to be the only force acting against it. Unfortunately, the cornea is aspherical, wet and neither perfectly flexible nor infinitely thin.
Moreover, tonometer tips do not contact the cornea alone, but come into contact with the precorneal tear film, which creates surface tension and produces capillary attraction (or repulsion) between it and objects in contact with its meniscus.
For this reason, it is clear that corneal thickness influences IOP measurement; with thin corneas causing wrong low readings and thick corneas wrong high measurement if the thickness is the result of increased collagen fibrils; low readings if the thickness is a result of corneal edema4,25.
Several authors tried to overcome these problems, proposing different correcting formulas.
We decided to compare the results obtained with these formulas with DCT because this tonometry instrument is based on Blaise Pascal’s law of hydrostatic pressure, in which a hypothetical corneal shape (contour) is achieved when the pressure on either side of the cornea is equal.
The force distribution that is needed to gently fit the corneal surface to that hypothetical contour counterbalances the force distribution generated by the IOP. A tonometer tip, equipped with this hypothetical contour and that touches the cornea, alters the corneal shape into the desired contour. The distribution of interface forces between the tip and the cornea equals the force distribution generated by the IOP. Hence, a pressure sensor that is centrally and concavely embedded into the tonometer tip should precisely measures the pressure of the eye.
Moreover, several authors found a good agreement between intra-camerural IOP and IOP provided by DCT, with a lower influence of central corneal thickness on DCT readings in comparison to GAT readings20,22.
DCT seems to provide IOP readings that should be closer to the “real” pressure, but it is not widely spread and it requires an higher patient compliance.
Several papers compared the results obtained with GAT and DCT26,27,28, but only few tried to correlate the IOP readings with correcting formulas.
Our results are in line with previous studies where has been established that DCT provides higher value of IOP in comparison to GAT24,27,28. In particular the mean difference between DCT and GAT (2.11 mmHg) and the range (from − 4 to 9 mmHg) are consistent with Cook et al.26, who found a mean difference of 1.8 mmHg and a range from − 2.9 to 6.5 mmHg.
Concerning the comparison between DCT and GAT correcting formulas29,30,31, our results aren’t always in agreement.
Results disagreement could be due to the different characteristics of the examined population, in particular it could be related to the different range of IOP, in fact in our study healthy subjects were considered, in the previous studies patient with glaucoma or with glaucoma suspect were examined.
For example, Asejczyk-Widlicka et al.30 found Śródka equation and Elsheikh2009 equation to be useful in IOP measurements, opposite to other formulas which did not provide a better agreement. In our study, just Śródka equation is associated with a statistically significant decrease in the differences between GAT and DCT, providing a better correction. These differences could be related to the different considered GAT IOP values interval, that in our study ranged between 11 and 22 mmHg, while in Asejczyk-Widlicka et al.30 study it ranged between 19 and 42 mmHg.
This study has some limitations: both DCT and GAT measurements were performed once, to avoid IOP reduction due to repeated measurement4, even if in comparative studies of IOP, it is common practice to take the average of three measurements. The equations used in this study come from different theoretical models experimental and manometric studies, but they do not provide an important improvement of the agreement between GAT and DCT in fact some of them provide a worsening of the agreement. Just Śródka equation slightely reduces the difference between DCT and GAT, but this is just a statistically significant result which doesn’t seem to justify its use and application in clinical practice.
Despite the results disagreement between the studies, the general tendency is a poor utility of the equations in all the studies.
This poor efficacy of equation could be due to the fact that the parameters considered by the equations could be not sufficient to explain the disagreement between the two methods of tonometry. Moreover, this study is based on the assumption that DCT provides values that are closer to the real IOP, assumption that is supported by some manometric studies. Nevertheless, it is also necessary to assume that DCT could not reflect the real intraocular pressure.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
De Bernardo, M. & Rosa, N. Fuchs’ endothelial and myotonic dystrophies: Corneal dystrophy in myotonic patients. Invest. Ophthalmol. Vis. Sci. 58(13), 5838. https://doi.org/10.1167/iovs.17-23171 (2017).
Markiewitz, H. H. The so called Imbert-Fick law. Arch. Ophthalmol. 64, 159. https://doi.org/10.1001/archopht.1960.01840010161018 (1960).
Gloster, J. & Perkins, E. S. The validity of the Imbert-Fick law as applied to applanation tonomery. Exp. Eye Res. 44, 274–283. https://doi.org/10.1016/S0014-4835(63)80048-2 (1963).
Whitacre, M. C. & Stein, R. Sources of error of Goldmann type tonometers. Surv. Ophthalmol. 38(1), 1–30. https://doi.org/10.1016/0039-6257(93)90053-A (1993).
De Bernardo, M., Capasso, L., Caliendo, L., Paolercio, F. & Rosa, N. IOL power calculation after corneal refractive surgery. Biomed. Res. Int. 2014, 658350. https://doi.org/10.1155/2014/658350 (2014).
De Bernardo, M., Iaccarino, S., Cennamo, M., Caliendo, L. & Rosa, N. Corneal anterior power calculation for an IOL in post-PRK patients. Optom. Vis. Sci. 92(2), 190–195. https://doi.org/10.1097/OPX.0000000000000458 (2015).
Rosa, N., Cione, F., Pepe, A., Musto, S. & De Bernardo, M. An advanced lens measurement approach (ALMA) in post refractive surgery IOL power calculation with unknown preoperative parameters. PLoS ONE 15, e0237990. https://doi.org/10.1371/journal.pone.0237990 (2020).
De Bernardo, M., Borrelli, M., Imparato, R. & Rosa, N. Calculation of the real corneal refractive power after photorefractive keratectomy using pentacam, when only the preoperative refractive error is known. J. Ophthalmol. 1, 1916369. https://doi.org/10.1155/2020/1916369 (2020).
De Bernardo, M., Capasso, L., Caliendo, L., Vosa, Y. & Rosa, N. Intraocular pressure evaluation after myopic refractive surgery: A comparison of methods in 121 eyes. Semin. Ophthalmol. 31, 233–242. https://doi.org/10.3109/08820538.2014.962156 (2016).
Elsheikh, A., Alhasso, D. & Pye, D. Goldmann tonometry correction factors based on numerical analysis. J. Biomech. Eng. 131(11), 111013. https://doi.org/10.1115/1.4000112 (2009).
Shimmyo, M., Ross, A. J., Moy, A. & Mostafavi, R. Intraocular pressure, Goldmann applanation tension, corneal thickness, and corneal curvature in Caucasians, Asians, Hispanics, and African Americans. Am, J, Ophthalmol. 136(4), 603–601. https://doi.org/10.1016/S0002-9394(03)00424-0 (2003).
Elsheikh, A., Alhasso, D., Gunvant, P. & Garway-Heath, D. Multiparameter correction equation for Goldmann applanation tonometry. Optom. Vis. Sci. 88(1), E102–E112. https://doi.org/10.1097/OPX.0b013e3181fc3453 (2011).
Śródka, W. Applanation pressure function in Goldmann tonometry and its correction. Acta Bioeng. Biomech. 15(3), 97–106 (2013).
Chihara, E. Assessment of true intraocular pressure: The gap between theory and practical data. Surv. Ophthalmol. 53(3), 203–218. https://doi.org/10.1016/j.survophthal.2008.02.005 (2008).
Doughty, M. J. & Zaman, M. L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta analysis approach. Surv. Ophthalmol. 44(5), 367–408. https://doi.org/10.1016/S0039-6257(00)00110-7 (2000).
Foster, P. J. et al. Accuracy of clinical estimates of intraocular pressure in Chinese eyes. Ophthalmology 107(10), 1816–1821. https://doi.org/10.1016/S0161-6420(00)00343-2 (2000).
Kohlhaas, M. et al. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch Ophthalmol. 124(4), 471–476. https://doi.org/10.1001/archopht.124.4.471 (2006).
Ehlers, N., Bramsen, T. & Sperling, S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 53(1), 34–43. https://doi.org/10.1111/j.1755-3768.1975.tb01135.x (1975).
Kanngiesser, H. E., Kniestedt, C. & Robert, Y. C. Dynamic contour tonometry: Presentation of a new tonometer. J. Glaucoma 14(5), 344–350 (2005).
Kniestedt, C., Nee, M. & Stamper, R. L. Dynamic contour tonometry: A comparative study on human cadaver eyes. Arch. Ophthalmol. 122(9), 1287–2129. https://doi.org/10.1001/archopht.122.9.1287 (2004).
Kaufmann, C., Bachmann, L. M. & Thiel, M. A. Intraocular pressure measurements using dynamic contour tonometry after laser in situ keratomileusis. Invest. Ophthalmol. Vis. Sci. 44, 3790–3794. https://doi.org/10.1167/iovs.02-0946 (2003).
Boehm, A. G., Weber, A., Pillunat, L. E., Koch, R. & Spoerl, E. Dynamic contour tonometry in comparison to intracameral IOP measurements. Invest. Ophthalmol. Vis. Sci. 49(6), 2472–2477. https://doi.org/10.1167/iovs.07-1366 (2008).
Gordon, M. O. & Kass, M. A. The Ocular Hypertension treatment study: Design and baseline description of the partecipants. Arch. Ophthalmolol. 117(5), 573–583. https://doi.org/10.1001/archopht.117.5.573 (1999).
De Bernardo, M., Borrelli, M., Cembalo, G. & Rosa, N. Intraocular pressure measurements in standing position with a rebound tonometer. Medicina 55(10), 701. https://doi.org/10.3390/medicina55100701 (2019).
Rosa, N. & De Bernardo, M. Central corneal thickness determination in corneal edema. Graephes Arch. Clin. Exp. Ophthlamol. 255, 1251–1252. https://doi.org/10.1007/s00417-017-3634-9 (2017).
Cook, J. A. et al. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology 119(8), 1552–1557. https://doi.org/10.1016/j.ophtha.2012.02.030 (2012).
Kaufmann, C., Bachmann, L. M. & Thiel, M. A. Comparison of dynamic contour tonometry with Goldmann applanation tonometry. Invest. Ophthalmol. Vis. Sci. 45(9), 3118–3112. https://doi.org/10.1167/iovs.04-0018 (2004).
Kniestedt, C. et al. Correlation between intraocular pressure, central corneal thickness, stage of glaucoma, and demographic patient data: Prospective analysis of biophysical parameters in tertiary glaucoma practice populations. J. Glaucoma 15(2), 91–97. https://doi.org/10.1097/00061198-200604000-00003 (2006).
Ang, G. S., Nicholas, S. & Wells, A. P. Poor utility of intraocular pressure correction formulae in individual glaucoma and glaucoma suspect patients. Clin. Exp. Ophthalmol. 39(2), 111–118. https://doi.org/10.1111/j.1442-9071.2010.02445.x (2011).
Asejczyk-Widlicka, M., Srodka, W. & Pierscionek, B. K. A comparative analysis of Goldmann tonometry correction. J. Glaucoma 26(3), 233–240. https://doi.org/10.1097/IJG.0000000000000588 (2017).
Wachtl, J., Töteberg-Harms, M., Frimmel, S., Roos, M. & Kniestedt, C. Correlation between dynamic contour tonometry, uncorrected and corrected Goldmann applanation tonometry, and stage of glaucoma. JAMA Ophthalmol. 135(6), 601–608. https://doi.org/10.1001/jamaophthalmol.2017.1012 (2017).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
Author information
Authors and Affiliations
Contributions
N.R. conceived the study. M.D.B. wrote the original draft. C.C., and I.D.P. performed data acquisition and analysis. F.C. performed statistical analysis. L.C. interpretated the data. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Bernardo, M., Casaburi, C., De Pascale, I. et al. Comparison between dynamic contour tonometry and Goldmann applanation tonometry correcting equations. Sci Rep 12, 20190 (2022). https://doi.org/10.1038/s41598-022-24318-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24318-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.