Abstract
The aim of this cross-sectional study was to objectively assess the salivary flow rate and composition and periodontal inflammation in obstructive sleep apnoea (OSA) patients. The subjects, who underwent whole-night polysomnography or polygraphy, were referred for saliva sampling and periodontal examination. According to the severity of OSA based on the Apnoea Hypopnea Index (AHI) value, the subjects were classified into groups: no OSA (AHI < 5; N = 17), mild to moderate OSA (AHI 5–29.9; N = 109), and severe OSA (AHI > 30; N = 79). Salivary flow rate, pH, salivary electrolytes, and cortisol were measured from collected saliva samples. Periodontal examination included assessment of the number of teeth, dental plaque, bleeding on probing and periodontal measurements: gingival recession, probing pocket depth, clinical attachment level (CAL) and periodontal inflamed surface area (PISA) score. There were no significant differences in salivary flow rate, salivary pH, salivary electrolyte concentrations or electrolyte ratios among the groups classified according to the severity of OSA. However, subjects without OSA had higher salivary cortisol concentrations than OSA groups (p < 0.001). Increased plaque scores were associated with a higher AHI (r = 0.26; p = 0.003). According to the salivary flow rate, subjects with hyposalivation and reduced salivation had higher concentrations of salivary electrolytes and lower salivary pH than subjects with normal salivation. Subjects with hyposalivation had an increased Mg/PO4 ratio (p < 0.001) and a reduced Ca/Mg ratio (p < 0.001). Furthermore, subjects with severe OSA tended to have higher CALs and plaque volumes. In conclusion, under pathological conditions, such as OSA, multiple interactions might impact salivary flow and electrolyte composition. Complex interrelationships might affect the integrity of oral health, especially considering OSA severity, inflammation, concomitant diseases and medications.
Similar content being viewed by others
Introduction
Saliva is responsible for the protection of intraoral structures, maintaining healthy hard and soft oral tissues while taking part in digestion and articulation of speech. Because of the buffer systems, saliva helps to maintain an acceptable pH range, whereas its components can provide information about caries formation or inflammation of the oral cavity1,2,3.
Salivary flow rate can be measured by objective examination techniques as previously described by Navazesh and Kumar4, providing insight into oral and general health2. More precisely, salivary flow rate may be modulated by many local factors affecting the oral cavity, such as mouth breathing and smoking, as well as therapies and systemic diseases, such as diabetes mellitus5. Waking up with a dry mouth is often reported by patients with obstructive sleep apnoea (OSA), a sleep-related breathing disorder characterized by sleep fragmentation and recurrent collapses of the upper airway during sleep6. Furthermore, it has been shown that hyposalivation is more frequent in OSA patients than in healthy subjects7. However, these findings were based on reports of the subjective feeling of dry mouth in OSA patients. Makeeva et al., using both subjective evaluation and objective measurement of salivary flow rate, found that patients with severe OSA indeed had a reduction in their salivary flow rate8. A reduced salivary flow rate leads to changes in saliva composition that could increase plaque accumulation and the risk for caries, mucosal and gingival infection, and inflammation9.
One of the most common immune-inflammatory diseases of the oral cavity is periodontitis10. Periodontal clinical parameters such as the clinical attachment level (CAL) are important to evaluate the severity of periodontitis, whereas bleeding on probing (BoP), probing pocket depth (PPD) and periodontal inflammation surface area (PISA) provide information on the severity of periodontal inflammation11. However, in addition to the clinical estimation, periodontal inflammation could also be determined by biochemical markers12. The determination of saliva composition and salivary flow rate could be considered an option for extended diagnostics of local and systemic inflammation12,13,14. More specifically, increased levels of salivary calcium and phosphate, as well as an imbalance in the Mg/Ca ratio and alterations in the salivary flow rate, may be associated with periodontitis15,16.
Evidence from the literature indicates that saliva should be considered a useful and noninvasive diagnostic tool, but it has still been underutilized for routine diagnosis of respiratory diseases17. Furthermore, recent systematic reviews and meta-analyses18,19 confirmed the association of OSA and periodontitis. One of the possible explanations might be disorders of the immune system, since sleep fragmentation, as one of the key features of OSA, has a deep impact on immunity and as such might contribute to periodontal inflammation20. An assessment of saliva composition, flow rate and periodontal inflammation may facilitate diagnosis and monitoring of the progression of oral diseases that might be enhanced in OSA patients. Thus, the aim of this study was to objectively assess the salivary flow rate and composition, along with periodontal inflammation, in OSA patients.
Results
Out of 220 recruited patients, 209 (135 men and 70 women) agreed to participate in this study, leading to a response rate of 95%.
The demographic characteristics of the subjects, as well as their subjective assessment of dry mouth and oral hygiene habits, are shown in Table 1. Out of 205 subjects, 17 (35.3% male, 64.7% female) did not have OSA (Apnoea Hypopnea Index [AHI] score < 5), 109 (62.4% male, 37.6% female) subjects were diagnosed with mild to moderate OSA (AHI 5–29.9) and 79 (77.2% male, 22.8% female) had severe OSA (AHI > 30) (Table 1).
Among subjects with no OSA, the median age was 37 (31–51) years, and the average body mass index (BMI) was 25.1 kg/m2. Among subjects with mild to moderate OSA, the median age was 58 (50–67) years, and the average BMI was 28.7 kg/m2, whereas among subjects with severe OSA, the median age was 61 (51–68) years, and the average BMI was 31.4 kg/m2.
Subjects with severe OSA were older (p < 0.001) and had a higher body mass index (p < 0.001) than those in the two other groups. Regarding concomitant diseases, 17.6% of subjects with no OSA had hypertension, whereas 57.8% of subjects with mild to moderate OSA and 63.3% of subjects with severe OSA had hypertension (p = 0.002). There was no significant difference in the frequency of diabetes mellitus type 2 among the studied groups (5.9% in the no OSA, 12.8% in the mild to moderate OSA, and 21.5% in the severe OSA groups; p = 0.139) (Table 1). The investigated groups differed significantly only in the use of antihypertensives (p = 0.010), which is in accordance with the data collected on systemic diseases (Supplementary Table 1).
Saliva composition
There was no statistically significant difference between the groups in terms of subjective dry mouth upon awakening (p = 0.233) and during the daytime. However, subjects with mild to moderate OSA most frequently reported difficulties swallowing dry food (p < 0.005). Although the subjects with no OSA did not report dry mouth while eating, there was no statistically significant difference between the groups (Table 1).
The subjects without OSA and those with mild to moderate OSA had more frequent dental check-ups during the year (p = 0.020) and had better oral hygiene habits, such as dental flossing (p = 0.004) and using mouthwash (p = 0.001), than the subjects with severe OSA (Table 1).
The salivary flow rate and salivary parameters in the investigated groups are summarized in Table 2.
There were no significant differences in salivary flow rate, salivary pH, or salivary calcium, phosphate, or magnesium concentrations or their ratios among the groups. However, subjects without OSA had higher salivary cortisol concentrations than those who had OSA (p = 0.011).
Periodontal assessment
Regarding periodontal status, the subjects with severe OSA had fewer teeth than subjects without OSA (p = 0.019). Furthermore, subjects with OSA had more plaque than those without OSA (p < 0.001). There was no significant difference in BoP, CAL, PISA score or stage of periodontitis among the groups (Table 2).
Increased plaque scores were associated with a higher AHI (r = 0.26; p = 0.003) (Supplementary Table 2).
Salivary flow rate
Regarding the salivary flow rate, the following categories were defined: hyposalivation (flow rate less than 0.1 mL/min, N = 25), reduced salivation (flow rate 0.1–0.3 mL/min, N = 97) and normal salivation (flow rate > 0.3 mL/min, N = 66) (Table 3).
There was no significant difference in sex, age or body mass index between the groups.
Subjects with hyposalivation and reduced salivation had significantly lower pH levels (p < 0.001) and higher salivary calcium (p < 0.001), salivary phosphate (p < 0.001), and salivary cortisol concentrations (p = 0.003) than subjects with normal salivation (Table 3).
The highest concentrations of salivary magnesium (p < 0.001) were found in subjects with hyposalivation. Consequently, subjects with hyposalivation had a significantly higher Mg/PO4 ratio (p < 0.001) and lower Ca/Mg ratio (p < 0.001) (Table 3).
There were no significant differences in periodontal status among the groups; however, subjects with hyposalivation tended to have higher PISA scores than those in the other two groups.
The linear regression analysis indicated an association between a higher salivary flow rate and male respondents (β = − 0.154, p = 0.016), lower salivary calcium concentrations (β = − 0.215, p = 0.027), salivary cortisol concentrations (β = − 0.176, p = 0.008), and a higher Ca/Mg ratio (β = 0.329, p = 0.001) (Table 4). When multivariable regression analysis was performed with 142 subjects who underwent periodontal examination, predictors recognized as relevant for the salivary flow rate were male sex and salivary phosphate concentration, indicating higher salivary flow with lower phosphate levels in saliva (Supplementary Table 3).
Discussion
In our study, subjects with hyposalivation or a reduced salivary flow rate had decreased values of salivary pH and a Ca/Mg ratio, whereas concentrations of calcium, magnesium, and phosphate and the Mg/PO4 ratio were higher compared to subjects with normal salivation. There was no statistically significant difference between the groups in terms of subjective dry mouth upon awakening and during the daytime. In addition, subjects with severe OSA had less frequent dental check-ups and poor interdental oral hygiene habits.
Dry mouth as a subjective sensation of oral dryness was very commonly reported among OSA patients. Previous studies among patients with OSA have used questionnaires to subjectively assess the presence of dry mouth and have reported that the prevalence of hyposalivation/xerostomia increased with the severity of OSA6,7,21. Similarly, self-reported dry mouth symptoms were found among patients at risk for OSA. However, when objective measurements of morning hyposalivation were performed using the Schirmer test, there was no correlation with the risk for OSA22.
Regarding the objective measurements of oral dryness, our study, along with that by Makeeva et al., collected saliva and measured salivary flow rate, as previously described by Navazesh4, among patients who underwent diagnostic polysomnography or polygraphy procedures in a sleep laboratory. In the study by Makeeva et al., hyposalivation was diagnosed in patients with severe OSA, and the salivary pH value was decreased. Possibly due to the larger sample size in our study, we found no differences in the salivary flow rate or pH value between the OSA groups. There is a reason to believe that changes in sympathetic outflow as seen in OSA patients might affect the regulation of the salivary flow rate23,24. When we used that approach and analysed the results regarding salivary flow rate, we found that subjects with hyposalivation had a lower pH than subjects with normal salivation. Although we found no significant difference in the severity of OSA concerning the salivary flow rate, subjects with hyposalivation had a tendency to have higher AHI values that require further investigation.
Some studies have shown that a decrease in the salivary flow rate can lead to an alteration of saliva composition in the context of different chronic diseases. Mata et al. showed a decreased salivary flow rate along with a higher salivary calcium concentration in subjects with diabetes mellitus14, whereas Ponciano et al. recorded a low concentration of salivary calcium and a high phosphate concentration along with hyposalivation in subjects with mucopolysaccharidosis25. In our study, salivary flow rate, salivary pH, and calcium, magnesium and phosphate concentrations did not differ according to the severity of OSA among subjects referred to a sleep laboratory. To the best of our knowledge, this is the first study that measured salivary electrolytes in patients referred to a sleep laboratory due to sleep-related breathing disorders. This study indicated no difference in salivary calcium, magnesium or phosphate concentrations associated with the severity of OSA. However, when we analysed the results with regard to salivary flow rate, we found that concentrations of electrolytes and salivary cortisol significantly increased with reduced salivary flow rate.
The salivary flow rate might be influenced by age and sex among healthy subjects26. More specifically, the salivary flow rate was greater in males than in females, and older age was associated with decreased unstimulated flow27. In our study, which was performed among predominantly OSA subjects, no significant difference was found in sex, age or body mass index between the groups according to the salivary flow rate. However, regression analysis revealed a significant effect of male sex on the increased salivary flow rate with concomitant diseases included in the analysis. In addition, concomitant diseases of OSA, such as hypertension and diabetes mellitus type 2, might be considered conditions that affect the salivary flow rate28. One might speculate that in OSA, an interplay of underlying pathophysiological mechanisms and specific phenotype markers could substantially modulate the salivary flow rate as well as the salivary composition.
The salivary cortisol concentration was higher in subjects without OSA than in those who were diagnosed with OSA. Plasma cortisol concentration could be used as an important marker for pathophysiological changes in sleep disorders29, and some studies yielded inconsistent results regarding plasma cortisol concentration and the severity of OSA30. In addition to plasma cortisol concentration, salivary cortisol concentration could be used in the assessment of OSA severity31, even though previous studies yielded inconsistent results regarding the association between salivary cortisol and the severity of OSA32,33.
The results from periodontal examination showed that subjects with OSA had more plaque and increased plaque scores that were associated with a higher AHI. A reduced salivary flow rate can lead to altered salivary pH and saliva composition, resulting in the accumulation of an increased number of microorganisms that form the oral biofilm, which is one of the most important aetiological factors for periodontitis5,34,35. Furthermore, in this study, an increased salivary calcium concentration was associated with the CAL, which might contribute to dental plaque formation. Salivary electrolytes may be involved in processes in periodontitis16, although some studies yielded inconsistent results10,36,37. Additionally, in this study, periodontal stages did not differ significantly with regard to OSA severity. However, subjects with severe OSA had a tendency to have higher CALs and plaque volumes than other subjects. Previous research showed that OSA severity is associated with severe forms of periodontitis18,19,38 and found that both diseases share similar risk factors that are associated with systemic inflammation39,40,41. Our study included a calculation of the PISA score as a novel method for determining periodontal inflammation in patients with sleep-related breathing disorders. The results of our study did not show a significant difference in the PISA score regarding OSA severity. However, subjects with hyposalivation had a tendency to have higher PISA scores than those in the other groups, indicating that hyposalivation and OSA might affect periodontal inflammation.
Among the limitations of this study, the cross-sectional design prevented conclusions on the causal association between saliva parameters and OSA. Furthermore, we lacked information on the precise wake time before sampling, which might have contributed to the concentrations measured. Although we tried to control for this effect by inviting subjects within a limited time frame in the morning, given the possible additional impact of the exact time between waking and saliva sampling, future studies are needed to more precisely elucidate the influence of saliva sampling time on salivary flow rate and composition.
In our study, subjects referred to a sleep laboratory with hyposalivation or a reduced salivary flow rate had altered saliva composition. In addition, higher CAL and plaque values were found in subjects with severe OSA. In conclusion, we argue that under pathological conditions, such as OSA, multiple interactions might impact salivary flow and electrolyte composition. Complex interrelationships might affect the integrity of oral health, especially considering OSA severity, inflammation, concomitant diseases and medications.
Methods
Subjects
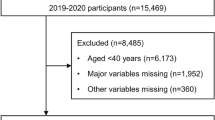
This cross-sectional study was conducted between November 2018 and October 2019. The subjects, who underwent whole-night polysomnography or polygraphy at the Split Sleep Medicine Centre (SMC), were invited to participate in this study. All 209 subjects included in this study signed an informed consent form for the use of personal data when accessing the survey and were provided with written information about the study. Subjects older than 18 years who underwent whole-night polysomnography (PSG, N = 51) or polygraphy (PG, N = 154) and refrained from consuming any food or beverage 2 h before saliva sample collection were included in this study (N = 205). Subjects younger than 18 years and those who did not adhere to the recommendation of fasting for 2 h prior to saliva sample collection were excluded from this study (N = 4) (Fig. 1). This study was approved by the Ethics Committee of the University of Split School of Medicine (Class: 003–08/14-03/0001, No: 2181–198-03-04-14-0027) and is in accordance with the Declaration of Helsinki. This study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies.
Sleep assessment
Whole-night PSG (Alice 5LE, Philips Respironics, Eindhoven, the Netherlands) or whole-night PG (Alice NightOne, Philips Respironics, Eindhoven, the Netherlands; SOMNOcheck2, Weinmann, Germany) was performed in the SMC. All data were stored on a computer, manually scored, and evaluated according to the published American Academy of Sleep Medicine (AASM) and European Sleep Research Society (ESRS) guidelines by a certified sleep physician and technician42.
According to the results of whole-night PSG/PG, the severity of OSA was based on the AHI value, in accordance with the AASM diagnostic criteria and ESRS guidelines42. Following the whole-night polysomnography and/or polygraphy, we divided the total number of events (apnoeas and hypopneas) by the total number of hours the patient was asleep. Apnoea was defined as a complete cessation of air flow for 10 s or more, while hypopnea was defined as a decrease in air flow by more than 50% for 10 s or more, both followed by desaturation of 3% or more42. Therefore, according to the severity of OSA, the subjects were classified into groups: no OSA (AHI < 5; N = 17), mild to moderate OSA (AHI 5–29.9; N = 109), and severe OSA (AHI > 30; N = 79). Following the sleep assessment, subjects who consented to participate in this study were referred for saliva collection (N = 205) and periodontal examination (N = 142) (Fig. 1).
Questionnaire
All of the subjects were interviewed by one examiner who was blinded to the PSG/PG reports. Data on medications (antidepressants, diuretics, antihypertensives, sedatives, bronchodilators, analgesics, antihistamines, anticonvulsants, antiparkinsonian drugs, anticholinergics, retinoids, anorexics, muscle relaxants, and decongestants) and concomitant diseases were collected from the patients’ medical records.
The questionnaire collected demographic data and an evaluation of the subjective assessment of dry mouth upon awakening with the responses on a Likert scale including never, rarely, sometimes, often, and almost always, as well as 5 more questions related to the subjective assessment of dry mouth during the daytime with yes/no answers and questions regarding oral hygiene habits43,44.
Saliva assessment
Collection, pH measurement and storage
Unstimulated saliva was collected from all subjects between 9 and 12 am. The subjects were instructed not to eat food, drink beverages, smoke or chew gum 2 h prior to testing. During the test, subjects were advised to rinse out their mouth with water and relax for 1 min, swallow to void the mouth of saliva and then minimize movements for the next 15 min while collecting the saliva and gently spitting into the preweighted test tubes, as previously described by Navazesh and Kumar4. Immediately after collection, salivary pH was determined by the PICCOLO Plus pH tester with ± 0.01 accuracy and replaceable HI1295 16-cm (6.3") electrode with a temperature sensor (Hanna Instruments HI98113/Spectrum 240-73492, Smithfield, RI, United States). Prior to measuring the pH, the electrode was calibrated using standards at pH 4.0 and pH 7.0. After that, saliva samples were weighed with an analytical balance (KERN ALJ 220-4M). The weight of the preweighted empty test tubes was subtracted from the total weight of the saliva and the test tube to calculate only the mass of the collected saliva that was measured in g/min, which was considered equal to mL/min4. All saliva samples were frozen at − 18 °C and stored until biochemical analysis.
Biochemical measurements
Calcium, phosphate and magnesium concentrations were measured at the Clinical Institute for Laboratory Diagnostics, Clinical Hospital Centre Zagreb with an Agilent 7500 cx (Agilent Technologies, Waldbronn, Germany) and inductively coupled plasma mass spectrometry (ICP‒MS). Saliva samples (400 μL) were digested with nitric acid (2 mL of 65% HNO3 and 1 mL of H2O) using high-pressure microwave digestion (UltraCLAVE, Milestone, Italy). After cooling, the samples were diluted with 1% (v/v) HNO3 to a total volume of 15 mL, and calcium, magnesium, and phosphates were analysed by ICP‒MS. All standard solutions were prepared from a 1-g/L PlasmaCAL standard (SCP Science, Canada). Seronorm® TraceElements Serum Control Level I and Level II (Sero AS, Billingstad, Norway) were used to control for the accuracy of the measurements. Free salivary cortisol was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) produced by Demeditec Diagnostics GmbH, Kiel, Germany. Specifically, free cortisol from the tested saliva sample competed for binding with a cortisol-enzyme conjugate to a polyclonal antibody on a precoated microtiter well. The addition of a substrate caused colour development, which was measured spectrophotometrically at 450 nm and was inversely proportional to the concentration of free salivary cortisol in the saliva sample.
Periodontal examination
Periodontal status was assessed immediately after saliva collection by two experienced periodontists (MR, PS). After examination of the same 10 participants, the achievement of an intraexaminer and extraexaminer reliability greater than 95% allowed the examiners to independently collect data. Out of 205 subjects, 63 were excluded from the periodontal examination: those who were edentulous or had fewer than two teeth (N = 13) and those who refused to participate in this study due to lack of time/interest (N = 50) (Fig. 1). The examination included assessment of the number of teeth, dental plaque, bleeding on probing and periodontal measurements: gingival recession (GR), PPD and CAL expressed in millimetres. GR was defined as the distance between the cement-enamel junction and the gingival margin, and the distance between the gingival margin and the bottom of the gingival sulcus was defined as the PPD. The sum of GR and the PPD was calculated as the CAL. These periodontal parameters were measured following previously published studies for periodontal assessment and diagnosis45,46,47. Based on those results, PISA scores were calculated using an online calculator available at www.parsprototo.info as previously described48.
Statistics
The analysis was performed in SPSS (SPSS 14.0 Student Version for Windows) and MS Excel (Microsoft Corporation, 2018, Microsoft Excel). Data were tested for normality with the use of the Kolmogorov‒Smirnov test or the Shapiro‒Wilk normality test. Data are presented as the medians with interquartile ranges for continuous variables. Categorical variables are reported as frequencies and percentages for each investigated category. All reported differences were assessed with the use of the appropriate test for statistical significance. The names of all tests are described in the footnotes according to their use in the tables. When more than two comparisons were performed, the nonparametric Kruskal‒Wallis test was calculated for group differences, followed by the Mann‒Whitney test to assess the specific differences between groups. Categorical data reported as frequencies (percentages) were compared with the chi square test or Fisher’s exact test, depending on the variable.
Multiple linear regression was performed when salivary flow rate was included as a dependent variable, whereas independent variables included in the model were AHI (events/h), age, sex, BMI, salivary calcium, salivary phosphate, salivary magnesium, Ca/Mg, Ca/PO4, Mg/PO4 and salivary cortisol. Statistical significance was set at p < 0.05. The sample size was calculated following an analysis in MedCalc (MedCalc for Windows, version 19.1.2.) based on the correlation coefficient of the mean AHI and salivary flow rate (r = − 0.273) assessed in a pilot sample of 23 respondents, of whom 16 had a diagnosis of OSA (mild to severe) and 7 had no OSA. In the reported analysis, the α-level was 0.05, and power was set at 90%. The final sample size calculation was 136 respondents, which was increased towards the end of the study with the aim of having as many control subjects as possible.
Data availability
The data that support the findings of this study are available from the corresponding author, [KP], upon reasonable request.
References
Edgar, M. Saliva and Oral Health 4th edn. (British Dental Association, 2012).
Wozniak, M., Paluszkiewicz, C. & Kwiatek, W. M. Saliva as a non-invasive material for early diagnosis. Acta Biochim. Pol. 66, 383–388. https://doi.org/10.18388/abp.2019_2762 (2019).
Gao, X., Jiang, S., Koh, D. & Hsu, C. Y. Salivary biomarkers for dental caries. Periodontology 2000 70, 128–141. https://doi.org/10.1111/prd.12100 (2016).
Navazesh, M. & Kumar, S. K. Measuring salivary flow: Challenges and opportunities. J. Am. Dent. Assoc. 139 (Suppl), 35S-40S. https://doi.org/10.14219/jada.archive.2008.0353 (2008).
Tschoppe, P., Wolgin, M., Pischon, N. & Kielbassa, A. M. Etiologic factors of hyposalivation and consequences for oral health. Quintessence Int. 41, 321–333 (2010).
Oksenberg, A., Froom, P. & Melamed, S. Dry mouth upon awakening in obstructive sleep apnea. J. Sleep Res. 15, 317–320. https://doi.org/10.1111/j.1365-2869.2006.00527.x (2006).
Pico-Orozco, J., Carrasco-Llatas, M., Silvestre, F. J. & Silvestre-Rangil, J. Xerostomia in patients with sleep apnea-hypopnea syndrome: A prospective case-control study. J. Clin. Exp. Dent. 12, e708–e712. https://doi.org/10.4317/jced.5659356593 (2020).
Makeeva, I. M. et al. Xerostomia and hyposalivation in patients with obstructive sleep apnoea. Clin. Otolaryngol. 46, 782–787. https://doi.org/10.1111/coa.13735 (2021).
Almstahl, A. & Wikstrom, M. Oral microflora in subjects with reduced salivary secretion. J. Dent. Res. 78, 1410–1416. https://doi.org/10.1177/00220345990780080601 (1999).
Ramenzoni, L. L. et al. Oral diagnostic methods for the detection of periodontal disease. Diagnostics https://doi.org/10.3390/diagnostics11030571 (2015).
Nesse, W. et al. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 35, 668–673. https://doi.org/10.1111/j.1600-051X.2008.01249.x (2008).
Kuboniwa, M. et al. Prediction of periodontal inflammation via metabolic profiling of saliva. J. Dent. Res. 95, 1381–1386. https://doi.org/10.1177/0022034516661142 (2016).
Yoshizawa, J. M. et al. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 26, 781–791. https://doi.org/10.1128/CMR.00021-13 (2013).
Mata, A. D. et al. Effects of diabetes mellitus on salivary secretion and its composition in the human. Mol. Cell Biochem. 261, 137–142. https://doi.org/10.1023/b:mcbi.0000028748.40917.6f (2004).
Rajesh, K. S., Zareena, H. S. & Arun Kumar, M. S. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 6, 461–465. https://doi.org/10.4103/0976-237X.169846CCD-6-461 (2015).
Meisel, P. et al. Magnesium/calcium ratio in serum predicts periodontitis and tooth loss in a 5-year follow-up. JDR Clin. Trans. Res. 1, 266–274. https://doi.org/10.1177/2380084416663458 (2016).
Li, C. X. et al. A narrative review of exploring potential salivary biomarkers in respiratory diseases: Still on its way. J. Thorac. Dis. 13, 4541–4553. https://doi.org/10.21037/jtd-21-202jtd-13-07-4541 (2021).
Stazic, P. et al. Periodontitis severity in obstructive sleep apnea patients. Clin. Oral Investig. https://doi.org/10.1007/s00784-021-04012-5 (2022).
Al-Jewair, T. S., Al-Jasser, R. & Almas, K. Periodontitis and obstructive sleep apnea’s bidirectional relationship: A systematic review and meta-analysis. Sleep Breath 19, 1111–1120. https://doi.org/10.1007/s11325-015-1160-8 (2015).
Mullington, J. M., Simpson, N. S., Meier-Ewert, H. K. & Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 24, 775–784. https://doi.org/10.1016/j.beem.2010.08.014 (2010).
Lopez-Jornet, P. et al. Sleep quality in patients with xerostomia: a prospective and randomized case-control study. Acta Odontol. Scand. 74, 224–228. https://doi.org/10.3109/00016357.2015.1099730 (2016).
Apessos, I., Andreadis, D., Steiropoulos, P., Tortopidis, D. & Angelis, L. Investigation of the relationship between sleep disorders and xerostomia. Clin. Oral Investig. 24, 1709–1716. https://doi.org/10.1007/s00784-019-03029-1 (2020).
Mese, H. & Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 34, 711–723. https://doi.org/10.1111/j.1365-2842.2007.01794.x (2007).
Cowie, M. R. Sleep apnea: State of the art. Trends Cardiovasc. Med. 27, 280–289. https://doi.org/10.1016/j.tcm.2016.12.005 (2017).
Ponciano, S. Hyposalivation, acidic saliva, decayed teeth and oral yeast prevalence in children with mucopolysaccharidosis. MedicalExpress https://doi.org/10.5935/MedicalExpress.2015.05.02 (2015).
Firat, F., Cermik, T. F., Sarikaya, A. & Berkarda, S. Effects of gender and age on the quantitative parameters of [99mTc]pertechnetate salivary gland scintigraphy in normal subjects. Nucl. Med. Commun. 27, 447–453. https://doi.org/10.1097/00006231-200605000-00006 (2006).
Fenoll-Palomares, C. et al. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Rev. Espanola Enfermedades Dig. 96, 773–783. https://doi.org/10.4321/s1130-01082004001100005 (2004).
Dodds, M. W., Yeh, C. K. & Johnson, D. A. Salivary alterations in type 2 (non-insulin-dependent) diabetes mellitus and hypertension. Commun. Dent. Oral Epidemiol. 28, 373–381. https://doi.org/10.1034/j.1600-0528.2000.028005373.x (2000).
Tomfohr, L. M., Edwards, K. M. & Dimsdale, J. E. Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med. Rev. 16, 243–249. https://doi.org/10.1016/j.smrv.2011.05.003 (2012).
Bozic, J. et al. Morning cortisol levels and glucose metabolism parameters in moderate and severe obstructive sleep apnea patients. Endocrine 53, 730–739. https://doi.org/10.1007/s12020-016-0925-6 (2016).
Raff, H., Ettema, S. L., Eastwood, D. C. & Woodson, B. T. Salivary cortisol in obstructive sleep apnea: The effect of CPAP. Endocrine 40, 137–139. https://doi.org/10.1007/s12020-011-9474-1 (2011).
Ghiciuc, C. M. et al. Restoring the salivary cortisol awakening response through nasal continuous positive airway pressure therapy in obstructive sleep apnea. Chronobiol. Int. 30, 1024–1031. https://doi.org/10.3109/07420528.2013.795155 (2013).
Yan, Y. R. et al. The association of salivary biomarkers with the severity of obstructive sleep apnea and concomitant hypertension. Am. J. Med. Sci. 357, 468–473. https://doi.org/10.1016/j.amjms.2019.03.004 (2019).
Wikner, S. & Soder, P. O. Factors associated with salivary buffering capacity in young adults in Stockholm, Sweden. Scand. J. Dent. Res. 102, 50–53. https://doi.org/10.1111/j.1600-0722.1994.tb01152.x (1994).
Bradshaw, D. J., McKee, A. S. & Marsh, P. D. Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J. Dent. Res. 68, 1298–1302. https://doi.org/10.1177/00220345890680090101 (1989).
Sewon, L. & Makela, M. A study of the possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch. Oral Biol. 35 (Suppl), 211S-212S. https://doi.org/10.1016/0003-9969(90)90160-c (1990).
Sewon, L. A., Karjalainen, S. M., Sainio, M. & Seppa, O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J. Clin. Periodontol. 22, 267–270. https://doi.org/10.1111/j.1600-051x.1995.tb00146.x (1995).
Seo, W. H. et al. The association between periodontitis and obstructive sleep apnea: A preliminary study. J. Periodontal Res. 48, 500–506. https://doi.org/10.1111/jre.12032 (2013).
Young, T., Skatrud, J. & Peppard, P. E. Risk factors for obstructive sleep apnea in adults. JAMA 291, 2013–2016. https://doi.org/10.1001/jama.291.16.2013291/16/2013 (2004).
Timmerman, M. F. & van der Weijden, G. A. Risk factors for periodontitis. Int. J. Dent. Hyg. 4, 2–7. https://doi.org/10.1111/j.1601-5037.2006.00168.x (2006).
Latorre, C. et al. Association between obstructive sleep apnea and comorbidities with periodontal disease in adults. J. Indian Soc. Periodontol. 22, 215–220. https://doi.org/10.4103/jisp.jisp_38_18JISP-22-215 (2018).
AAoSM. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual (American Academy of Sleep Medicine, 2001).
Sutej, I., Bozic, D., Peros, K. & Plancak, D. Cigarette smoking and its consequences on periodontal health in teenagers: A cross-sectional study. Cent. Eur. J. Public Health 29, 311–316. https://doi.org/10.21101/cejph.a6671 (2021).
Basic, K., Peros, K., Bosnjak, Z. & Sutej, I. Subgingival microbiota profile in association with cigarette smoking in young adults: A cross-sectional study. Dent. J. https://doi.org/10.3390/dj9120150 (2021).
Ainamo, J. & Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 25, 229–235 (1975).
O’Leary, T. J., Drake, R. B. & Naylor, J. E. The plaque control record. J. Periodontol. 43, 38. https://doi.org/10.1902/jop.1972.43.1.38 (1972).
Tonetti, M. S., Greenwell, H. & Kornman, K. S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 89 (Suppl 1), S159–S172. https://doi.org/10.1002/JPER.18-0006 (2018).
KrizanSmojver, B., Altabas, K., Knotek, M., Basic Jukic, N. & Aurer, A. Periodontal inflamed surface area in patients on haemodialysis and peritoneal dialysis: A Croatian cross-sectional study. BMC Oral Health 20, 95. https://doi.org/10.1186/s12903-020-01086-7 (2020).
Acknowledgements
The authors would like to thank Dr. Petra Stazić, DMD, for her help in data acquisition and Shelly Pranić, MSPH, PhD for help in language editing. This study was supported by the Croatian Ministry of Science and Education (grant for university institutional science funding) and by the Croatian Science Foundation project “Translational research on neuroplasticity of breathing and effect of intermittent hypoxia in anesthesia and sleep-TIHO2_SLEEP_BREATH” (grant number IP-11-2013-5935; principal investigator Z. Dogas).
Author information
Authors and Affiliations
Contributions
M.T.D. contributed to conception, design, data acquisition, and analysis and interpretation and drafted and critically revised the manuscript; R.P. contributed to conception, design, and data interpretation and critically revised the manuscript; L.L.K. contributed to design, performed all statistical analyses and critically revised the manuscript; I.P.D. contributed to design and data analysis and critically revised the manuscript; M.V. contributed to design, data analysis, and interpretation and critically revised the manuscript; M.R. contributed to design, analysis and interpretation; D.R. contributed to laboratory processing work, analysis and interpretation; I.L. contributed to laboratory processing work, analysis and interpretation; K.G. contributed to laboratory processing work, analysis and interpretation; K.P. contributed to conception, design, analysis, and interpretation and critically revised the manuscript; and Z.D. contributed to conception, design, and data interpretation and critically revised the manuscript. All authors gave final approval and agree to be held accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author (s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tranfić Duplančić, M., Pecotić, R., Lušić Kalcina, L. et al. Salivary parameters and periodontal inflammation in obstructive sleep apnoea patients. Sci Rep 12, 19387 (2022). https://doi.org/10.1038/s41598-022-23957-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23957-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.