Abstract
Treatment of malignant tumors, such as rhabdomyosarcoma (RMS), can improve overall survival (OS). It is time-consuming and expensive for patients to obtain benefits from randomized controlled trials (RCTs) with OS as the primary end-point. Therefore, another surrogate end-point is necessary; however, there is no report on the surrogacy analysis of RMS. In this study, we performed a systematic review of RCTs, involving patients with newly diagnosed RMS, and 11 RCTs were identified. We performed a meta-analysis to assess the surrogacy of intermediate end-points for OS. The correlations between surrogate end-points and OS were investigated using Spearman's rank correlation coefficient (ρ). The coefficient of determination (R2) was employed to measure the strength of the association. A total of 5183 patients were randomly allocated to 34 treatment groups. A marginal correlation (R2 = 0.281, ρ = 0.445) between the hazard ratios (HRs) for event-free survival (EFS) and OS was observed. In patients with localized RMS, the EFS HR had a weaker correlation with OS HR in the sensitivity analysis than that in the primary analysis. Overall, the surrogacy of EFS for OS cannot be confirmed.
Similar content being viewed by others
Introduction
Rhabdomyosarcoma (RMS) is one of the most common soft tissue sarcomas (STS) in children and adolescents. It accounts for 10.3% of STS in Japanese children, and adolescents and young adults (AYA) aged between 15 and 39 years1; the incidence rate of RMS is 4 per 100,000 individuals for the 0–19-year age group in Europe2. Moreover, RMS in children and AYA accounts for approximately 64% of all RMS cases1. In children and adolescents, the two common RMS subtypes are embryonal RMS (ERMS), accounting for approximately 57% of RMS and alveolar RMS (ARMS), accounting for approximately 26% of RMS3. Children and AYA with ERMS present a better prognosis than those with ARMS3. Furthermore, approximately 66% of ARMS patients with the paired box 3/7-forkhead box O1 (PAX3/7-FOXO1) fusion genes are regarded as a high-risk population4.
Since the 1970s, the Intergroup Rhabdomyosarcoma Study Group (IRSG) has performed certain clinical trials that elucidated prognostic factors such as histology, tumor location, and presence of metastasis, and a multi-disciplinary treatment comprising chemotherapy, radiotherapy, and surgery, which have led to a better prognosis5,6,7,8,9. Based on these results, in the 2000s, some investigational chemotherapy regimens were established for each risk group by the Children's Oncology Group (COG), USA, European pediatric Soft Tissue Sarcoma Study Group (EpSSG), and Japan Rhabdomyosarcoma Study Group (JRSG). Currently, alkylating agents, including cyclophosphamide and ifosfamide, are used in combination with vincristine and dactinomycin, regarded as standard chemotherapy regimens5,6,7,8,9. Additionally, camptothecin-11 (CPT-11) is considered to improve chemotherapy efficacy in children and adults with relapsed RMS10. Nevertheless, the difference in overall survival (OS) of patients with ARMS and those with intermediate-risk ERMS between the standard and experimental arms in phase III randomized controlled trials (RCTs) is not significant. Moreover, a few effective chemotherapies are available for patients with relapsed and metastatic RMS. Therefore, future RCTs with high-quality evidence should be conducted to find favorable chemotherapy regimens, including drug selection, administration sequence, dosage, and dosing intervals. It is definitive that treating malignant tumors, such as RMS, can improve OS. OS is the clearest end-point in which an exact point in time can be defined. However, if OS is set as the primary end-point, the clinical trial requires a very large population, huge cost, high follow-up rate, and long-term follow-up. OS is affected by the subsequent treatments. Furthermore, even if there is no treatment effect on survival post-progression (SPP), the OS comparison is affected11. New treatments and drugs will be introduced in the future owing to medical science developments. In such an era, it would take a long time for children and AYA with malignant tumors to receive benefits from RCTs defining the primary end-point as OS, verifying the effectiveness of the new treatment. Thus, another surrogate end-point must be defined as the primary end-point in RCTs of such diseases. In osteosarcoma occurring in children and AYA, event-free survival (EFS) or pathological response rate (RR) is frequently set as a primary end-point12. However, in RMS, EFS or disease-free survival (DFS) is often set as a primary end-point (Table 1). On the contrary, there are no studies on the relationship between such intermediate end-points and OS focusing on the RCTs of RMS. We performed a meta-analysis including all RCTs that involved chemotherapy for newly diagnosed RMS patients and assessed the surrogacy of intermediate end-points for OS (Table 1)5,6,7,8,9,13,14,15,16,17,18.
Methods
Study selection
We systematically searched databases including PubMed, EBSCOhost MEDLINE, Scopus, and the Cochrane Central Register of Controlled Trials (CENTRAL) based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement19. We used keywords “rhabdomyosarcoma,” “chemotherapy,” “randomized controlled trial,” and “randomized, clinical trials” to identify RCTs on RMS reported in English between January 1974 and January 2022. This search was conducted by two authors independently (KT and MK).
Data and outcome
The date of publication, study title, study duration, inclusion criteria, phase of a clinical trial, primary and secondary outcome measures, intervention and treatment used in each arm, sample size, sex, age, histological type, number and proportion of patients with metastasis, application of intention-to-treat (ITT) analysis, post-protocol therapy, pathological response to chemotherapy, adverse events, and survival data were extracted. For OS, EFS, and RR, the median, hazard ratio (HR) with 95% confidence interval (CI), and P-values were recorded whenever available. DFS and failure-free survival (FFS) were regarded as EFS and were analyzed in this study. The definition of EFS in each trial was adopted. Eight trials defined EFS as relapse or death; six trials defined EFS as disease progression, death, or relapse; one trial defined EFS as disease progression, second malignancy, or death; one trial defined EFS as disease progression, relapse, second malignancy, or death; and one trial defined EFS as death, relapse, disease progression, appearance of a new tumor, change to second-line chemotherapy, or the date of last follow-up. The RR was defined as the proportion of responders (complete or partial) among the assessed patients. Complete and partial definitions were followed for each trial.
The EFS and OS rates for 1-, 3-, and 5-year were extracted from the data using the Kaplan–Meier method. In case these data were not mentioned in the report, they were estimated as binary proportions from each Kaplan–Meier plot for EFS or OS. Toxicity was scored based on the Common Terminology Criteria for Adverse Events (CTCAE) v.5.0, and the focus of this study was CTCAE grade 3 or 4. All information was retrieved and confirmed by two authors (K.T. and M.K.), and the differences among them were arbitrated by Y.K. and T.I.
Statistical analyses
Pooled HRs with 95% CIs for EFS and OS were calculated using a meta-analysis. Additionally, pooled odds ratios (ORs) with the corresponding 95% CIs for EFS and OS at 1, 3, and 5 years were obtained from a meta-analysis. The Mantel–Haenszel method was applied using the review manager software (version 5.4; Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). All remaining statistical analyses were performed by applying the Statistical Analysis System (SAS) (version 9.4; SAS Institute, Cary, NC, USA). P-values were obtained from two-sided tests, and results with P < 0.05 were considered significant. Cochrane's Q-test and I2 statistics were applied to quantify heterogeneity.
As recommended by Schürmann et al. 20, we utilized random effects meta-regression to estimate the relationship between surrogate end-points and OS. Spearman's rank correlation coefficient (ρ) between the surrogate end-points and OS was also estimated. This association strength was assessed by the adjusted coefficient of determination (R2). We defined squared coefficient values as excellent (> 0.9), very good (0.9 to < 0.75), good (0.75 to < 0.5), moderate (0.5 to < 0.25), and poor (0.25 ≥).
Results
Characteristics of the RCTs included in the meta-analysis
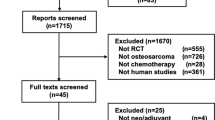
A systematic literature search was conducted, and 278 articles were initially identified for screening. A total of 106 articles were excluded because of duplication, and 172 were screened for eligibility. After screening the abstracts, 158 articles were excluded (not RCT, n = 126; not RMS, n = 32). After 14 full-text screenings, we obtained phase II or III RCTs of chemotherapy that involved chemotherapy-naive patients with newly diagnosed RMS. Ultimately, the remaining 11 RCTs were eligible, and a meta-analysis of such RCTs was performed (Fig. 1). Table 1 shows the main encapsulated features of the included trials. No trial had described the post-protocol treatment in detail.
Collectively, 11 eligible RCTs randomized 5183 patients to 34 treatment arms. Each arm received two to six cytotoxic agents, including vincristine and actinomycin. A total of 27 treatment arms received radiotherapy, and 645 patients (12.4%) diagnosed with metastatic disease were enrolled in seven treatment arms. Neo-adjuvant chemotherapy, adjuvant chemotherapy, and chemotherapy without surgery were administered to 6, 21, and 7 treatment arms, respectively. EFS, DFS, or FFS was adopted to be the primary end-point in four RCTs. Both OS and EFS (or DFS and FFS) were used in five RCTs, and two of them also chose the primary end-point as RR. Another RCT defined the primary end-point as 3-year OS, and another defined the primary end-point as RR and toxicity.
Except one RCT, either EFS or OS was considered the primary or secondary end-point in all RCTs. Histology subtypes included ARMS (n = 1534, 29.6%), ERMS (n = 2794, 53.91%), pleomorphic/other RMS (n = 97, 1.87%), and Ewing/undifferentiated pleomorphic sarcoma (n = 356, 6.87%).
Meta-analysis of treatment effects and adverse events
We performed a meta-analysis of OS HR using the random-effect model and found that the experimental arms failed to differ significantly from the control arms (HR 0.97, 95% CI 0.86–1.09, P = 0.60) (Fig. 2). This study showed no evidence of statistical heterogeneity in OS HR (P = 0.28; I2 = 14%) (Fig. 2). There was also no publication bias from the funnel plot (Supplementary Fig. 1). In contrast, the experimental arms were significantly more favorable than the control arms in terms of 1-year EFS (OR 0.76, 95% CI 0.63–0.91; P = 0.003) (Table 2, Supplementary Fig. 2) and RRs (CR + PR) (OR 1.35, 95% CI 1.03–1.77; P = 0.03) (Table 2, Supplementary Fig. 3). The control arms were significantly more favorable than the experimental arms in terms of overall severe adverse events (OR 1.55, 95% CI 1.20–1.99; P = 0.0007) (Table 2, Supplementary Fig. 4). The former showed a slight publication bias, but the latter two showed no publication bias in the funnel plots (Supplementary Figs. 5–7). The remaining intermediate end-points, ORs for 3- and 5-year EFS and 1-, 3-, and 5-year OS, and HR for EFS, did not show significant differences between the control and experimental arms (Table 2, Supplementary Figs. 8–13).
Correlations between EFS and OS
The HR for EFS was moderately correlated to the HR for OS (Spearman’s rho = 0.445, 95% CI − 0.140 to 0.800, P = 0.1275). The equation for the regression line (Fig. 3) is as follows:
This equation indicated that the risk reduction of OS HR was approximately 6.6% smaller than that of EFS HR. The trial-level R2 was 0.281 (95% CI 0.000–0.637), indicating slight association (Table 3, Fig. 3). Considering the 1-, 3-, 5-year EFS, the 3-year EFS had the strongest correlation with OS HR (R2 = 0.444; 95% CI 0.098–0.790; ρ = 0.593; 95% CI 0.063–0.862; P = 0.0325) (Table 3). Nevertheless, among the 1-, 3-, 5-year OS, the 3-year OS was well correlated to OS HR (R2 = 0.727, 95% CI 0.535–0.919; ρ = 0.781, 95% CI 0.495–0.915, P = 0.0001) and the 5-year OS was excellently correlated to OS HR (R2 = 0.911, 95% CI 0.839–0.983; ρ = 0.953, 95% CI 0.873–0.983, P < 0.0001) (Table 3). The other correlations were moderate or poor (Table 3).
Sensitivity analysis
Subsequently, we performed a sensitivity analysis, excluding the treatment arms of the metastatic populations (Tables 4, 5). After excluding seven treatment arms (Carli 1988, Maurer 1988_Group IV, Maurer 1993_Group IV, Crist 1995_Clinical group IV, Breitfield 2001, Arndt 2009, Oberlin 2012), the correlation between the EFS HR and OS HR became poorer (R2 = 0.267, 95% CI 0.000–0.671; ρ = 0.400, 95% CI − 0.360 to 0.841, P = 0.2861) than that of the primary analysis (Table 5). The correlation between the EFS at 1, 3, and 5 years to OS HR deteriorated, whereas that between OS at 1, 3, and 5 years and OS HR improved (Table 5).
Discussion
In RCTs, EFS, defined as the primary end-point, has some advantages over OS, as it requires a small sample size, shorter follow-up period for assessment, and low cost12,21. Moreover, EFS is not generally affected by post-progression treatments. Nonetheless, there is a disadvantage of being affected by the method used to evaluate and ascertain bias, such as knowledge of the therapy received22. It is possible that new diagnostic methods or anti-tumoral agents would dilute the surrogacy relationship in the future, even if the present surrogacy is reportedly strong23. Some reasons for the expectation of EFS as a surrogate end-point for OS are the social demand for early approval of new drugs and the theory that EFS is not only a surrogate end-point for OS but also makes positive contributions to the patients’ quality of life21. Although many surrogate end-point analyses have evaluated the EFS surrogacy for OS, a true end-point in RCTs for various malignant tumors, there has been no such study for RMS. This is the first report about surrogate end-point analyses for RMS and surrogacy analysis of intermediate end-points for OS.
The control arms of the studies were administered several cytotoxic agents, including vincristine and actinomycin, whereas the experimental arms received more toxic and multi-disciplinary treatments, additional radiotherapy, or anti-tumor agents. In the meta-analysis, the experimental arms were more favorable than the control arms in terms of 1-year EFS. However, longer endpoints such as 3- and 5-year EFS and 3- and 5-year OS did not show the superiority of experimental arms in RCTs conducted from 1974 were gathered. In other words, for EFS at a very early point, the experimental arms can be more favorable than the control arms, but for EFS at a later point, treatment effect between the arms would diminish. None of the new drug treatments have been highly successful. Overall, for RMS, there was only one RCT included in Table 1 showing that OS of the experimental arm was superior to that of the control arm. It is difficult to conduct clinical trials successfully due to a lack of effective drugs.
There are distinct prognoses and clinical courses between patients with localized RMS and those with metastatic RMS, even though these two groups of patients usually receive chemotherapy based on VAC. Therefore, we performed a sensitivity analysis excluding patients with metastatic RMS. After excluding metastatic patients with RMS, the HR of EFS was more poorly correlated with the HR of OS in the sensitivity analysis than in the primary analysis (Table 5). Likewise, the correlation between EFS at 1, 3, and 5 years and OS HR became progressively poor. In contrast, the correlation between 1-, 3-, and 5-year OS and OS HR improved accordingly. This result is similar to that previously reported for osteosarcoma12 and Ewing sarcoma24.
In general, it is considered that the longer the survival term following recurrence, the weaker the surrogacy of DFS for OS25. Broglio et al.11 reported that if there was a little difference in SPP between the two treatment arms, longer periods of SPP will weaken the association between PFS HR and OS HR and make it difficult to show a statistically significant difference in the OS between the treatment arms. Zer et al.26 evaluated published RCTs on locally advanced/metastatic STS, with systemic therapy in at least one arm. They finally identified 52 RCTs as eligible for analysis, including phase II and III studies, as in this study26. There were some differences from our study, that is, they included different treatment lines and various soft tissue sarcomas, and allowed control arms such as placebo or best supportive care26. The statistical analysis of the association between two endpoints was assessed using linear regression weighted by study sample size and association strength was assessed using the standardized β coefficient, not using coefficient of determination R226. Their result showed significant correlations between PFS HR and OS HR and between RR OR and OS HR. The authors considered that shorter SPP, about 12 months, accounted for a large portion of eligible studies, which likely led to significant correlations between PFS HR and OS HR26. In contrast, as recommended by Schürmann et al.20, we utilized random effects meta-regression. The study mainly included patients with ERMS, who usually present longer SPP than those with other soft tissue sarcomas because the 5-year survival rate of patients with ERMS after relapse is 20–52%27. For this reason, the surrogacy of EFS for OS HR might have become weak in this study.
The relapse rate in patients with RMS who achieved complete remission or stable mass was 31.1–36.2%28,29. Additionally, recurrence occurred within 18 months after the first diagnosis in 50.4%–67.5% of relapsed patients28,29 and within 5 years after first diagnosis in 95% of relapsed patients28. The 5-year post-relapse survival rates in patients with group I and II/III ERMS were 52% and 20%, respectively27. However, the 5-year post-relapse survival rates in patients with groups I and II–IV ARMS or undifferentiated sarcoma were 40% and 3%, respectively27. In addition, the time to relapse after the end of the primary treatment has a significant influence on prognosis. Four-year survival rates after relapse within 6, 6–12, and more than 12 months were 12%, 21%, and 41%, respectively30. According to these results, ERMS with post-relapse survival was dominant because 11 RCTs used in this study comprised 53.9% of ERMS and 29.6% of ARMS; therefore, surrogacy of EFS for OS HR turned out to be weak. The 5-year EFS was more weakly correlated to OS HR than 3-year EFS, possibly because patients with early relapse RMS tend to die early and patients with late relapse patients tend to have long post-relapse survival. Another reason is that individual post-relapse treatments were heterogeneous. Some relapse patients may be able to undergo complete surgical excision and have long-term post-relapse survival29.
DFS has still not been validated to be a surrogate measure of OS in patients with breast cancer. Nevertheless, when patients with HER2-positive early breast cancer are selected as the object, DFS could be an acceptable surrogate for OS 31. Goldberg et al. 22 suggested that an appropriate biomarker, clinically defined patient selection, and receiving effective treatment would likely lead to restoration of PFS surrogacy for OS. In the case of RMS, which is known to be an ultra-rare sarcoma32, it seemed difficult to apply patient selection and specific treatment for RCTs of RMS. Thus, in terms of malignant tumors, it is important to discover or develop new biomarkers that can be acceptable surrogates for OS.
Our study has several limitations. First, we did not use individual data but published data. Second, most of the eligible RCTs did not describe the study phase. Third, only two RCTs described the ITT analysis. Finally, there were differences between each follow-up examination or later RCT treatment because eligible patients were registered for a long period (1979–2016).
This study concludes that when EFS is regarded as the primary end-point for an RCT of RMS, at least a three-year follow-up period is needed. Additionally, this result shows that the association between EFS and OS was modest, and EFS surrogacy for OS in RCTs of RMS was not confirmed. Therefore, it is necessary to discover or develop new biomarkers for RMS that can be an acceptable surrogate for OS.
Data availability
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Fukushima, T., Ogura, K., Akiyama, T., Takeshita, K. & Kawai, A. Soft tissue sarcoma in adolescent and young adult patients: A retrospective study using a nationwide bone and soft tissue tumor registry in Japan. Jpn. J. Clin. Oncol. 51, 1080–1087. https://doi.org/10.1093/jjco/hyab044 (2021).
Ferrari, A. et al. Defining and listing very rare cancers of paediatric age: Consensus of the Joint Action on Rare Cancers in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors. Eur. J. Cancer 110, 120–126. https://doi.org/10.1016/j.ejca.2018.12.031 (2019).
Sultan, I., Qaddoumi, I., Yaser, S., Rodriguez-Galindo, C. & Ferrari, A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2600 patients. J. Clin. Oncol. 27, 3391–3397. https://doi.org/10.1200/JCO.2008.19.7483 (2009).
Gallego, S. et al. Fusion status in patients with lymph node-positive (N1) alveolar rhabdomyosarcoma is a powerful predictor of prognosis: Experience of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancer 124, 3201–3209. https://doi.org/10.1002/cncr.31553 (2018).
Maurer, H. M. et al. The intergroup rhabdomyosarcoma study-I. A final report. Cancer 61, 209–220. https://doi.org/10.1002/1097-0142(19880115)61:2%3c209::aid-cncr2820610202%3e3.0.co;2-l (1988).
Maurer, H. M. et al. The intergroup rhabdomyosarcoma study-II. Cancer 71, 1904–1922. https://doi.org/10.1002/1097-0142(19930301)71:5%3c1904::aid-cncr2820710530%3e3.0.co;2-x (1993).
Crist, W. et al. The third intergroup rhabdomyosarcoma study. J. Clin. Oncol. 13, 610–630. https://doi.org/10.1200/JCO.1995.13.3.610 (1995).
Crist, W. M. et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J. Clin. Oncol. 19, 3091–3102. https://doi.org/10.1200/JCO.2001.19.12.3091 (2001).
Breitfeld, P. P. et al. Ifosfamide and etoposide are superior to vincristine and melphalan for pediatric metastatic rhabdomyosarcoma when administered with irradiation and combination chemotherapy: A report from the Intergroup Rhabdomyosarcoma Study Group. J. Pediatr. Hematol. Oncol. 23, 225–233. https://doi.org/10.1097/00043426-200105000-00010 (2001).
Defachelles, A. S. et al. Randomized phase II trial of vincristine-irinotecan with or without temozolomide, in children and adults with relapsed or refractory rhabdomyosarcoma: A European paediatric soft tissue sarcoma study group and innovative therapies for children with cancer trial. J. Clin. Oncol. 39, 2979–2990. https://doi.org/10.1200/JCO.21.00124 (2021).
Broglio, K. R. & Berry, D. A. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl. Cancer Inst. 101, 1642–1649. https://doi.org/10.1093/jnci/djp369 (2009).
Tanaka, K. et al. Surrogate endpoints for overall survival in randomised controlled trials of localised osteosarcoma: A meta-analytic evaluation. Sci. Rep. 10, 8573. https://doi.org/10.1038/s41598-020-65591-z (2020).
Carli, M. et al. Tumor response and toxicity after single high-dose versus standard five-day divided-dose dactinomycin in childhood rhabdomyosarcoma. J. Clin. Oncol. 6, 654–658. https://doi.org/10.1200/JCO.1988.6.4.654 (1988).
Arndt, C. A. et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J. Clin. Oncol. 27, 5182–5188. https://doi.org/10.1200/JCO.2009.22.3768 (2009).
Oberlin, O. et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J. Clin. Oncol. 30, 2457–2465. https://doi.org/10.1200/JCO.2011.40.3287 (2012).
Hawkins, D. S. et al. Addition of vincristine and irinotecan to vincristine, dactinomycin, and cyclophosphamide does not improve outcome for intermediate-risk rhabdomyosarcoma: a report from the children’s oncology group. J. Clin. Oncol. 36, 2770–2777. https://doi.org/10.1200/JCO.2018.77.9694 (2018).
Bisogno, G. et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 19, 1061–1071. https://doi.org/10.1016/S1470-2045(18)30337-1 (2018).
Bisogno, G. et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 20, 1566–1575. https://doi.org/10.1016/S1470-2045(19)30617-5 (2019).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Schürmann, C. & Sieben, W. Differences in surrogate threshold effect estimates between original and simplified correlation-based validation approaches. Stat. Med. 35, 1049–1062. https://doi.org/10.1002/sim.6778 (2016).
Goldberg, R. M. et al. Clinical trial endpoints in metastatic cancer: Using individual participant data to inform future trials methodology. J. Natl. Cancer Inst. djab218 (2021). https://doi.org/10.1093/jnci/djab218.
Di, L. A., Bleiberg, H. & Buyse, M. Overall survival is not a realistic end point for clinical trials of new drugs in advanced solid tumors: A critical assessment based on recently reported phase III trials in colorectal and breast cancer. J. Clin. Oncol. 21, 2045–2047. https://doi.org/10.1200/JCO.2003.99.089 (2003).
Xie, W. et al. A Systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr. 3, pkz002 (2019). https://doi.org/10.1093/jncics/pkz002
Tanaka, K., Kawano, M., Iwasaki, T., Itonaga, I. & Tsumura, H. A meta-analytic evaluation of the correlation between event-free survival and overall survival in randomized controlled trials of newly diagnosed Ewing sarcoma. BMC Cancer 20, 379. https://doi.org/10.1186/s12885-020-06871-9 (2020).
Sargent, D. et al. Adjuvant Colon Cancer End-points (ACCENT) Group. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur. J. Cancer 47, 990–996 (2011). https://doi.org/10.1016/j.ejca.2010.12.015.
Zer, A., Prince, R. M., Amir, E. & AbdulRazak, A. Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: End Point selection, surrogacy, and quality of reporting. J. Clin. Oncol. 34, 1469–1475. https://doi.org/10.1200/JCO.2015.64.3437 (2016).
Pappo, A. S. et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J. Clin. Oncol. 17, 3487–3493. https://doi.org/10.1200/JCO.1999.17.11.3487 (1999).
Mazzoleni, S. et al. Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer 104, 183–190. https://doi.org/10.1002/cncr.21138 (2005).
Chisholm, J. C. et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: A nomogram to better define patients who can be salvaged with further therapy. J. Clin. Oncol. 29, 1319–1325. https://doi.org/10.1200/JCO.2010.32.1984 (2011).
Mattke, A. C. et al. Does the time-point of relapse influence outcome in pediatric rhabdomyosarcomas?. Pediatr. Blood Cancer. 52, 772–776. https://doi.org/10.1002/pbc.21906 (2009).
Saad, E. D. et al. Disease-free survival as a surrogate for overall survival in patients with HER2-positive, early breast cancer in trials of adjuvant trastuzumab for up to 1 year: A systematic review and meta-analysis. Lancet Oncol. 20, 361–370. https://doi.org/10.1016/S1470-2045(18)30750-2 (2019).
Stacchiotti, S. et al. Ultra-rare sarcomas: A consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer 127, 2934–2942. https://doi.org/10.1002/cncr.33618 (2021).
Acknowledgements
This work was supported in part by the National Cancer Center Research and Development Fund (2020-J-3) and the Japan Agency for Medical Research and Development (AMED) under grant numbers JP21ck0106614 and JP21ck0106507. We would like to acknowledge Satista Co., Ltd. (Kyoto, Japan) for its assistance in performing the statistical analysis. We would like to thank Editage (www.editage.com) for English language editing by native English-speaking editors.
Author information
Authors and Affiliations
Contributions
K.T. and H.T. conceived and designed the study. Y.K., K.T., M.K., and T.I. performed the systematic literature search and data extraction. Y.K., K.T., M.K., I.I., and H.T. performed the analysis and interpreted the data. Y.K., K.T., M.K., T.I., I.I., and H.T drafted and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubota, Y., Tanaka, K., Kawano, M. et al. Surrogacy analysis of intermediate end-points for overall survival in randomized controlled trials of rhabdomyosarcoma. Sci Rep 12, 19381 (2022). https://doi.org/10.1038/s41598-022-23944-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23944-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.