Abstract
We aimed to explain the role of mesenchymal stem cells (MSC-exosomes) on gene expressions of epithelial to mesenchymal transition (EMT), angiogenesis, and apoptosis. Four different cell lines were employed, including ACHN, 5637, LNCaP, and PC3, as well-known representatives for renal, bladder, hormone-sensitive, and hormone-refractory prostate cancers, respectively. Cell lines were exposed to diverse concentrations of mesenchymal stem cells-derived exosomes to find IC50 values. Percentages of apoptotic cells were evaluated by Annexin/P.I. staining. Micro Culture Tetrazolium Test assessed proliferative inhibitory effect; and prostate biomarker (KLK2), EMT (E-cadherin and Snail), angiogenesis genes (VEGF-A/VEGF-C), apoptosis genes (BAX/BCL2, P53) and Osteopontin variants (OPNa/b, and c) mRNA levels were studied by realtime PCR method. All 5637, LNCaP, and PC3 following treatment with exosomes illustrated specific responses with changes in expression of different genes. The increased TP53 and decreased BCL2 expressions were seen in 5637, LNCaP, and PC3. In PC3, OPNb and OPNc have raised more than P53; in LNCap, the increase was in VEGF-c. In 5637 cells, more than TP53 and BCL2 changes, two other genes, VEGFa and B.A.X., have decreased, suggesting exosomes’ anti-apoptotic and anti-angiogenic effects. The kidney tumor cell line saw no significant gene expression change in ten targeted genes. MSC-exosomes therapy has augmented some interesting antitumor effects on prostate, bladder, and kidney cancer cell lines. This effect which originates from exosomes’ potency to persuade apoptosis and prevent the proliferation of cancer cells simultaneously, was more substantial in bladder cancer, moderate in prostate cancer, and mild in renal cancer.
Similar content being viewed by others
Introduction
The National Cancer Institute’s report shows that three urological cancers are among the top ten frequent cancers. Prostate, bladder, kidney, and renal pelvis cancers ranked second, sixth, and eighth common cancer types1. Approximately 248,500 new prostate cancer cases are estimated in 2021, accounting for about 13% of all new cancer cases in the U.S.A.1. The other two urological cancers are less common. However, their incidence is still high, with 83,730 new bladder cancers and 76,080 new kidney and renal pelvis cancers1. Of every three male individuals, one is diagnosed with one of these three cancers in their lifetime.
Although some developments in cancer treatments have occurred in the past decade, cancer treatment is still challenging, and advanced urological cancers require more effective therapies2,3. Cell-based treatments have been promising, but the unfavorable immunological reaction could complicate their usage4. Recent growing evidence showed that stem cells could play a therapeutic role via secreting various paracrine factors, mediated by some tiny functional vesicles5,6,7. In 1981, it was shown that the small vesicles released by different cells are isolable8. Several biologic components and chemicals are anticancer reagents9,10,11. These tiny vesicles were later named exosomes by Johnstone et al.12,13. Exosomes showed advantages over other cellular therapies, which resulted in graft versus host disease (GVHD) and emboli formation14. Exosomes are a subgroup of extracellular products with diameters ranging from 50 to 120 nm and cup-shaped morphology14. It has been shown that cancer cells secrete more exosomes than healthy cells15. These small vesicles generally contribute to tumor formation, growth, progression, invasiveness, and the ability to escape from the host immune system3,16. Exosomes can change epithelial-mesenchymal transition (E.M.T.), angiogenesis, and apoptosis17. E.M.T. process is a crucial step in cancer invasion and metastasis. E.M.T. phenotype is related to dysregulation of E-cadherin and Snail genes18,19,20. Angiogenesis provides more blood flow enriched in oxygen and nutritious substances required for progression21.
Blocking cancer crosstalk via depleting its exosomes from the circulatory system is outlined in many studies as an advanced method for tumor treatment8. Diluting these troublesome vesicles by adding healthy exosomes could be another possible creative technique. This manuscript evaluates the effect of MSC-exosomes on ACHN, 5637, LNCaP, and PC3 as representatives for renal, bladder, hormone-sensitive prostate, and hormone-refractory prostate cancers, respectively, as used in former studies22,23,24.
Materials and methods
Cell lines
5637 Cell line as primary bladder tumor, ACHN Cell line as metastatic renal adenocarcinoma, LNCaP Cell line as metastatic prostate cancer, and PC3 Cell line as prostate adenocarcinoma (grade IV) with ATCC number HTB-9, CRL-1611, CRL-10995, and CRL-1435 respectively prepared and cultured the (National Bank of Iran Cell Reserves under the name of Pasteur Institute-Tehran). All four cell lines cultured in Gibco DMEM medium (Carlsbad, CA) supplemented with 10% Gibco F.B.S. (Carlsbad, CA), Gibco penicillin (1000 U/ml), and Gibco streptomycin (100 µg/ml) (B.R.L.) and then incubated at 37 °C, 90% humidity with 5% CO2 incubator. During our testing, we confirmed that all methods followed the ABCAM’s protocol (https://www.abcam.com/) according to relevant guidelines and regulations.
Preparation, isolation, and culture of adipose tissue-derived mesenchymal cells
In this in vitro study, 10 g of abdominal adipose tissue was used to isolate mesenchymal stem cells (M.S.C.) from a referred patient for liposuction after signing the written informed consent. The research was run under the Tehran University of Medical Sciences ethical committee (IR.TUMS.MEDICINE.REC.1400.159). The fat sample taken in saline buffer phosphate solution containing the antibiotic penicillin–streptomycin was transferred to a cell culture laboratory under sterile conditions. After several washing steps with saline buffer phosphate and the physiological serum, the tissue was cut into small pieces. By collagenase I, adipose tissue was digested, and mesenchymal stem cells were extracted from this tissue.
First, 1.5 mg of collagenase I enzyme was added for each gram of fat and incubated for 60 min at 37 °C. Then, it was centrifuged for 10 min at 2000 rpm. Finally, the cell sediment in DMEM (Dulbecco’s Modified Eagle’s Medium) culture medium with 1% penicillin and streptomycin, 10% F.B.S. (fetal bovine serum) was moved to a T75 cell culture flask and incubated at 37 °C with 5% carbon dioxide (CO2) and 90% humidity. The cell culture medium was changed every four days. The trypsin cells were then isolated from the flask and ready for use when they reached the third or fourth passage stage25.
Cell characterization based on cell markers
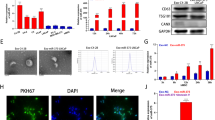
Flow cytometry was used to determine M.S.C.s isolated from adipose tissue. In this method, 250,000 cells were used. The 75 T flask containing these cells in passage three was centrifuged after rinsing with PBS and trypsinized. After counting with 2–3 ml of PBS solution, centrifuged again, and the supernatant discarded. The cells were homogenized using 250 μl PBS. 50 μl of the suspension and 5 μl of each conjugated monoclonal antibodies to the fluorescent dyes were added to each tube. In parallel, the cells were exposed to the isotype control antibody. Then, the tubes were incubated for 40 min at 4 °C and finally analyzed by using flow cytometry26.
Antibodies used for fat stem cell examination checked the expression of CD90-FITC and CD105-FITC proteins and the lack of expression of CD45-FITC and HLA-DR proteins. Antibodies used to examine exosomes target CD9, CD63, and CD81 proteins. After evaluating cells, the DMEM culture medium was replaced with free serum exosome. After 48 h, the supernatant of the cells was collected, and the EXOCIB kit (Cib Biotech Co. Iran) was used to extract the exosomes.
Exosome isolation and determination
We isolated the Exosomes from the M.S.C.s media by using the Exocib isolation kit. Exocib isolation kit had two reagents, A and B. According to the manufacturer’s protocols, first of all, we removed cellular debris, then collected the cell media and centrifuged the falcon (10 min, 3500 rpm by SIGMA 3-18KHS heated, refrigerated table top centrifuge, Germany). Then the collected supernatant was vortexed with reagent A (ratio 5:1), then vortexed for 10 min, and incubated for 24 h at refrigerator temperature. The combination was centrifuged (45 min, 2800 rpm), and the exosome plate was washed with 100 µl of reagent B. Ultimately, the exosome plate was saved at − 80 °C. Exosomes’ determination was carried on via employing flow cytometry and scanning electron microscopy.
To confirm the extracted Exosome, we used electron microscope imaging (MIRA3 TESCAN) with the following characteristics: S.E.M. magnification and resolution: 200kx, Det.in beam (detector is inside the column), B.I.: 7.00, working distance: 5.14 mm, view filed: 1.04 µm and Scale bar: 200 nm (Fig. 1).
Micro culture tetrazolium test
LNCaP, ACHN, 5637, and PC3 cell lines were implanted in a plate (4 × 103 cells/well-96 well). All four cells were exposed to extracted exosomes and incubated for 3different times, 24/48 and 72 h. The rate of cell proliferation was assessed using the M.T.T. method. After these times, Hiperion NanoDrop (Medizintechnik GmbH & Co.KG, Germany) read the OD of cell viability at 570 nm following the manufacturer's protocol. The MTT test (three replications) was completed to evaluate IC50 (half-maximum inhibitory concentration).
Apoptosis assay and D.N.A. cell cycle flow cytometry analysis
To evaluate the Apoptosis assay, PC3, LNCaP, 5637, and ACHN cancer cell lines were implanted in the six-well plates separately and incubated for 48 h in the presence and absence of exosomes. Induction of apoptosis by exosomes evaluated by Annexin V-FITC staining test. To assess the D.N.A. cell cycle assay, after 48 h, PC3, LNCaP, 5637, and ACHN cancer cell lines were exposed to exosomes, and the cells were washed with cold PBS and fixed with cold ethanol (70%). After one night, washed with PBS twice, all four cancer cell lines and incubated with RNase I, then stained with 500μL propidium iodide dye (PI) By BD Flow cytometer, separated PC3, LNCaP, 5637, ACHN cancer cell lines, and Flowjo software 7.6.1. (https://flowjo.software.informer.com) analyzed data according to the manufacturer’s procedure. The calculated values of sub-G1 are considered apoptotic values.
R.N.A. isolation, cDNA synthesis, and real-time PCR
All four cancer cell lines, ACHN, LNCaP, 5637, and PC3, were exposed to exosomes and investigated for expression of some essential genes like BCL2, B.A.X., and P53 (apoptosis genes), Osteopontin (O.P.N.), and vascular endothelial growth factor or VEGF (angiogenesis genes) isoforms, KLK2 (prostate biomarker), and expression of Snail, E-cadherin (E.M.T. marker). R.N.A. was extracted via the Roche TRIzol, and complementary D.N.A.s (cDNAs) were reverse transcribed using a Takara cDNA synthesis kit (According to the kit manufacturer’s instructions). To estimate the expression of mentioned genes, the 2−ΔΔCT method is with the RT-PCR cycler QIAGEN’s machine. The nucleotide sequence of the primers in this study is consistent with the previous study20,27,28.
Statistical analysis
All documents were available as means ± S.D. of the three duplicate measurements. T-test and ANOVA analyses were used to evaluate the outcomes.
Ethical considerations
All patients signed the written informed consent, and the Tehran University of Medical Sciences ethical committee (IR.TUMS.MEDICINE.REC.1400.159) approved the study.
Results
The extracted exosomes were confirmed by the relevant antibodies (CD9, CD63, and CD81) through flow cytometry and apparently, through electron microscope images (Figs. 1, 2). The morphology of prostate, bladder, and kidney cancer cell lines treated with exosomes after 48 h have been shown using light microscopy (Fig. 3).
Light microscopic images of prostate, bladder, and kidney cancer cell lines. Untreated and treated PC3 cells with exosomes (A, B), untreated and treated LNCaP cells with exosomes (C, D), untreated and treated 5637 cells with exosomes (E, F), and finally, untreated and treated 5637 cells with exosomes (G, H) are shown.
Exosomes inhibit cell proliferation
The cytotoxic effect of exosomes (0–25 × 106 Par) was investigated in PC3, LNCaP, 5637, and ACHN cell lines for 24/48 and 72 h (Fig. 4). According to the results obtained in this study, IC50 values of exosomes were 15 × 106 Particles for PC3, LNCaP, and 5637 cell lines; and 20 × 106 Particles for ACHN cell line. The results presented that exosomes had a significant cytotoxic effect on all four target cell lines in a dose-dependent and time-dependent manner.
Effects of exosomes in different concentrations (0–25 × 106 Par) on cell proliferation. The anti-growth effect of exosomes was measured by M.T.T. assay following 24, 48, and 72 h in 5637, ACHN, LNCaP, and PC3 cell lines. IC50 pharmaceutical doses of 15 × 106 Particle in PC3, LNCap, and 5637 cell line and 15 × 106 Particle in ACHN cell line were determined. Data showed that exosomes' anti-proliferative impact reduces cell viability with a dose- and time-dependent manner.
Exosomes can stimulate apoptosis
To investigate the effect of exosomes on apoptosis of PC3, ACHN, LNCaP, and 5637 cancer cell lines, we used Annexin/P. I method with flow cytometry. The outcomes were an apoptotic increase in four cancer cell lines treated with exosomes compared to the control group (Fig. 5). We recognized an induction in total apoptosis (early and late apoptotic cells), Annexin V-positive/PI-negative as Annexin+/P.I, and the lowest percentage of necrosis (Annexin-/PI+) in cured cells while at the end-stage of apoptotic cells are Annexin V/PI++ showed as Annexin V-FITC positive/PI-positive, compared with the control group in all four targeted cancer cell lines.
Flow cytometric analysis of LNCaP, PC3, ACHN, and 5637 cells apoptosis using Annexin-V-Flous. The lower left quadrant shows live cells, the lower right quadrant shows early apoptotic cells, the upper right quadrant shows late apoptotic cells, and the upper left quadrant shows necrotic cells. Results regarding induced apoptosis of PC3, LNCaP, 5637 and ACHN cancer cells untreated and treated with Exosome. Statistical significance was defined at *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the corresponding control.
Exosomes encourage sub-G1 and G1 arrest in prostate, bladder, and kidney cancer cell lines
Flow cytometry was used to study the induction of cell cycle arrest and the cell cycle of PC3, ACHN, LNCaP, and 5637 cancer cell lines treated with exosomes (Fig. 6).
Cell cycle analysis of LNCaP, PC3, 5637, and ACHN cancer cell lines. DNA content of LNCaP, PC3, 5637, and ACHN cancer cell lines was assessed after exposed with exosomes by PI staining. The cell cycle analysis detected G1 area increased in LNCaP, PC3, 5637, and ACHN cell lines when treated with exosomes. The percentages of cells at the G2 phase were concurrently reduced in all treated cells. Therefore, exosomes apparently induce sub G1/G1 arrest in LNCaP, PC3, 5637, and ACHN cell lines.
The 5637, PC3, LNCap, and ACHN cells had a rise in sub-G1 phase cells (as apoptotic phase) following the exposure of cells with exosomes (5.25–12.82%), (5.03–18.93%), (3.14–10.22%) and (9.12–10.20%) besides the reduction in the following cell cycle phases, respectively. The outcomes of 5637, PC3, ACHN, and LNCap cancer cells showed that exosome-treated cells arrest the cell cycle in the G1 phase compared to the control group (71.48–62.43%), (59.16–59.04%), (68.65–55.72%) and (75.72–70.62%), respectively (Fig. 6).
The impact of exosomes on gene expression
It was observed that exosomes had the same synergistic apoptotic effect in LNCaP, PC3, and 5637 but not in ACHN cells by an increase of P53 and a decrease of the BCL2 gene expression. In PC3 cell lines, OPNa, OPNc, and P53 gene expression increased after exosome treatment, contrary to the BCL2 decrease. In another cell line of prostate cancer, LNCaP, the impact of exosomes was highlighted as the increase of VEGF-c and P53 gene expression and reduction of BCL2 and BAX gene expression. The most significant antitumor effect of exosomes was in bladder tumor cell line 5637 through TP53 gene expression increase simultaneously with a reduction in VEGF-a/BAX/BCL2 genes. No significant gene expression changes of the ten investigated genes were seen for the kidney tumor cell line in ACHN cells (Fig. 7).
Results of PC3, LNCaP, 5637, and ACHN cancer cells exposed with exosomes on expression of selected genes after 48 h. Values are given as mean ± S.D. of three independent experiments. Statistical significance was defined at *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the corresponding control.
Discussion
Our result indicated that exosomes could have an anticancer effect in PC3, LNCaP, ACHN, and 5637 by increasing cell apoptosis, necrosis and inhibiting cell proliferation. We showed that exosomes had the same synergistic apoptotic effect in LNCaP, PC3, and 5637 but not in ACHN cells by an increase of P53 and decrease of the BCL2 gene expression in exosomes-treated PC3, 5637, and LNCaP cancer cell lines.
The anticancer therapeutic prospects of MSC-exosomes in several tumors have been considered recently29. Recent studies have defined contradictory observations of MSC-derived exosomes' effect on cancer cells in which some suggested tumor growth and metastasis inducer, but others reported preventing tumor cell growth. Our data showed MSC-derived exosomes as an apoptotic factor in tumor cell lines of PC3, LNCaP, ACHN, and 5637. At the same time, it was demonstrated that secreted exosomes from cancer cell lines HeLa, PC3, MCF-7, Panc-1, and DLCL2 contain inhibitors of apoptosis. MSC-derived exosomes can fight with the tumor cells against modifications that frequently happen in the environment around a tumor30.
Furthermore, exosomes carry small noncoding RNAs called micro-RNAs playing role in posttranscriptional gene expression modulation31. It is shown that noncoding micro-RNA (mir-143) present in human bone marrow M.S.C. (BMSC)-derived exosomes can stop cell migration and metastasis in human prostate cancer. The mir-143 is a down regulator of the Trefoil factor 3 (TFF3) gene32. A study by Lázaro‐Ibáñez E. and colleagues showed that extracellular vesicles (E.V.s) originated from prostate cancer cell lines. Human plasma samples contain double-stranded genomic D.N.A. fragments with some mutations be considered as the biomarkers in both cancer detection and prognosis. Different fragmented genomic D.N.A. is found in subpopulations of prostate cancer E.V.s like apoptotic bodies, microvesicles, and exosomes33,34,35. Provided preclinical data indicates that Adipose-derived stromal cells (A.S.C.) prevent prostate cancer cell growth, causing prostate cancer cell apoptosis with reduced activity of BCL2L1 by miR-145, so it can be a new and favorable therapeutic strategy in patients with prostate cancer36. It has also been suggested that BMSC-derived exosomal miR-9-3p repressed bladder cancer progression through Endothelial cell-specific molecule 1 (ESM1) downregulation, presenting a novel therapeutic target for bladder cancer therapy37.
In our study, the Annexin V-FITC staining assay indicated the increasing apoptosis, necrosis, and inhibition of cell proliferation in all four target tumor cell lines when exposed to MSC-derived exosomes. Bot gene expression profile showed that 5637 was the most responsive tumor cell line to exosome therapy because both TP53 gene expression increased and VEGF-a gene expression decreased simultaneously. Zheng et al. showed that exosomal lncRNA brain cytoplasmic 200, brain cytoplasmic R.N.A. 1, was substantially upregulated in urinary exosomes from patients with a bladder tumor linked to lymph node metastasis38. Also, BCYRN1 increases VEGFC and its receptor (VEGF‐C/VEGFR3) signaling in bladder cancer patients with lymph node metastatic.
So, BCYRN1 can be a therapeutic target for patients with bladder cancer. Treatment of low (5637) and high (T24) grade bladder cancer cell lines with T24 tumor cell-derived exosomes can trigger cell proliferation and activation of Akt and ERK pathways39. A long noncoding R.N.A. is called Urothelial cancer associated 1 (RNA-UCA1) increases in bladder cancer and regulates the expression of several genes participating in tumorigenesis. It was shown by Xue M. et al. that RNA-UCA1 can increase bladder tumor growth and development40. MKP1 is a transcriptional target of p53. Cai X and colleagues showed that Exosome–transmitted microRNA‐133b reduced bladder cancer proliferation by an increase of DUSP141.
Our data indicated both TP53 increase and BCL2 decrease in two prostate cancer cells, PC3 and LNCaP. The potential for creating new blood vessels from the pre-existing vessels of menstrual stem cells (MenSCs)-secreted exosomes on the PC3 cell line was assessed, and it was shown that MenSCs-derived exosomes could block tumor angiogenesis42. Yu et al. unexpectedly discovered that prostate cancer-derived exosomes are essential facilitators of bone homeostasis and osteoclastic lesions that stimulate tumor growth in bone43. Prostate-specific G-protein coupled receptors (PSGR) in prostate cancer are related to poor survival (O.S.). Exosomal PSGR triggers tumor cell migration, metastasis, stemness, and E.M.T. and reshapes LNCaP and RWPE-1 cells44. Elham et al. in vitro study showed that exosomes could enhance tumor size and serum P.S.A. level when xenograft-bearing mice have processed DU145 cell-derived exosomes intravenously45. They suggest that irrespective of androgen receptor phenotype, prostate tumor cells-derived exosomes considerably boost various mechanisms promoting prostate cancer progression45.
Yang Lin et al. reported that exosomes could stimulate tumor expansion and proliferation and prevent apoptosis in ACHN cell lines. Removal of the exosomes from the microenvironment of renal cancer or inhibition of its function can be new strategies for the treatment of renal cancer46. Several genetic biomarkers are presented in kidney tumors47,48. However, no significant change was seen in our study in all ten genes investigated. Based on Zhang L et al. study, renal cancer 786-0 cells-derived exosomes significantly promoted angiogenesis via upregulation of VEGF expression in human umbilical vein endothelial cells (HUVECs), which may be prompted by the decreasing of hepatocyte cell adhesion molecule49. It was shown that exosomes secreted by HEK293 (human embryonic kidney cells) and HT-1080 (fibrosarcoma) could suppress the growth and proliferation of p53-deficient cells50. So, Exosomes transfer p53 proteins between cells and can decrease cell proliferation and cell growth in p53-negative cells50. However, our data showed no significant gene expression change of the P53 gene in ACHN cells as the kidney tumor cell line.
Conclusion
We have employed mesenchymal stem cells derived exosomes for treating four urology human cancer cell lines. Our results demonstrated that this novel, safe method of cancer therapy could be effective in vitro, mainly on gene expression bases. In other words, exosome treatment ultimately can trigger various pathway modulation in apoptosis, angiogenesis, and E.M.T. processes, which could efficiently hinder tumor progression in bladder, prostate, and renal cancers, with bladder cancer as the best responder to this new cancer therapy.
Data availability
All information, data, and photos are all provided through the manuscript, and additional will be provided if requested.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2021. CA Cancer J. Clinic. 71(1), 7–33 (2021).
Arrighetti, N. et al. Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 26(33), 6132–6148 (2019).
Urabe, F., Kosaka, N., Kimura, T., Egawa, S. & Ochiya, T. Extracellular vesicles: Toward a clinical application in urological cancer treatment. Int. J. Urol. 25(6), 533–543 (2018).
Gilligan, K. E. & Dwyer, R. M. Engineering exosomes for cancer therapy. Int. J. Mol. Sci. 18(6), 1122 (2017).
Chang, Y.-H., Wu, K.-C., Harn, H.-J., Lin, S.-Z. & Ding, D.-C. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transpl. 27(3), 349–363 (2018).
Matthay, M. A. & Abman, S. H. Exosome-Based Therapy for Bronchopulmonary Dysplasia 10–12 (American Thoracic Society, 2018).
Hu, G.-W. et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 6(1), 1–15 (2015).
Fan, Q. et al. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 414, 107–115 (2018).
Mirzaei, A., Zareian Baghdadabad, L., Khorrami, M. H. & Aghamir, S. M. K. Arsenic Trioxide (ATO), a novel therapeutic agent for prostate and bladder cancers. Transl. Res. Urol. 1(1), 1–6 (2019).
Aghamir, S. M. K. et al. Comparison on diagnostic accuracy of prostate cancer detection tools: A systematic review and meta-analysis. Transl. Res. Urol. 1(1), 31–44 (2019).
Khatami, F. & Hasanzad, M. Circulating tumor cells as a novel prostate cancer diagnostic tool. Transl. Res. Urol. 2(3), 93–95 (2020).
Zhang, J. et al. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genomics Proteomics Bioinform. 13(1), 17–24 (2015).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in exosome isolation techniques. Theranostics 7(3), 789 (2017).
Qin, Y., Sun, R., Wu, C., Wang, L. & Zhang, C. Exosome: A novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int. J. Mol. Sci. 17(5), 712 (2016).
Zhang, L. & Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochimica et Biophysica Acta (BBA)-Rev. Cancer 1871(2), 455–468 (2019).
Pierini, I. Immune-Derived Exosomes Mimetics (IDEM) as an Innovative, Versatile and Biomimetic Nanoparticle System for Cancer Treatment (Politecnico di Torino, 2020).
Abak, A., Abhari, A. & Rahimzadeh, S. Exosomes in cancer: Small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ 6, e4763 (2018).
Serrano-Gomez, S. J., Maziveyi, M. & Alahari, S. K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer 15(1), 1–14 (2016).
Aban, C. et al. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci. Rep. 11(1), 1–11 (2021).
Mirzaei, A., Rashedi, S., Akbari, M. R., Khatami, F. & Aghamir, S. M. K. Combined anticancer effects of simvastatin and arsenic trioxide on prostate cancer cell lines via downregulation of the VEGF and OPN isoforms genes. J. Cell. Mol. Med. 26, 2728–2740 (2022).
Li, X. et al. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 3(1), 011503 (2019).
Sinha, R. et al. Analysis of renal cancer cell lines from two major resources enables genomics-guided cell line selection. Nat. Commun. 8(1), 1–10 (2017).
Quentmeier, H., Zaborski, M. & Drexler, H. G. The human bladder carcinoma cell line 5637 constitutively secretes functional cytokines. Leuk. Res. 21(4), 343–350 (1997).
Tai, S. et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71(15), 1668–1679 (2011).
Alabdulkarim, Y. et al. Recent advances in bone regeneration: The role of adipose tissue-derived stromal vascular fraction and mesenchymal stem cells. J. Limb Lengthening Reconstr. 3(1), 4 (2017).
Li, H., Ghazanfari, R., Zacharaki, D., Lim, H. C. & Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 1370(1), 109–118 (2016).
Mirzaei, A. et al. Novel combination therapy of prostate cancer cells with arsenic trioxide and flutamide: An in-vitro study. Tissue Cell 74, 101684 (2022).
Mirzaei, A., Jahanshahi, F., Khatami, F., Reis, L. O. & Aghamir, S. M. K. Human prostate cancer cell epithelial-to-mesenchymal transition as a novel target of arsenic trioxide and curcumin therapeutic approach. Tissue Cell 76, 101805 (2022).
Chulpanova, D. S., Kitaeva, K. V., James, V., Rizvanov, A. A. & Solovyeva, V. V. Therapeutic prospects of extracellular vesicles in cancer treatment. Front. Immunol. 9, 1534 (2018).
Valenzuela, M. M. A. et al. Exosomes secreted from human cancer cell lines contain inhibitors of apoptosis (IAP). Cancer Microenviron. 8(2), 65–73 (2015).
Rezaeian, A., Tarki, F. E. & Taheri, K. K. Exosomes and Urological Cancers (2022).
Che, Y. et al. Exosomes derived from miR-143-overexpressing MSCs inhibit cell migration and invasion in human prostate cancer by downregulating TFF3. Mol. Ther. Nucleic Acids 18, 232–244 (2019).
Lázaro-Ibáñez, E. et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: Apoptotic bodies, microvesicles, and exosomes. Prostate 74(14), 1379–1390 (2014).
Saadati, M., Tamehri, S., Pour Kamali, M. & Taheri, D. Phosphatase and tensin gene associated with features of aggressive prostate cancer. Transl. Res. Urol. 3(1), 32–37 (2021).
Rashedi, S. Landscape of circular ribonucleic acids in urological cancers. Transl. Res. Urol. 3(2), 45–47 (2021).
Takahara, K. et al. microRNA-145 mediates the inhibitory effect of adipose tissue-derived stromal cells on prostate cancer. Stem Cells Dev. 25(17), 1290–1298 (2016).
Cai, H. et al. Exosomal microRNA-9-3p secreted from BMSCs downregulates ESM1 to suppress the development of bladder cancer. Mol. Ther. Nucleic Acids 18, 787–800 (2019).
Zheng, H. et al. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin. Transl. Med. 11(7), e497 (2021).
Yang, L., Wu, X.-H., Wang, D., Luo, C.-L. & Chen, L.-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol. Med. Rep. 8(4), 1272–1278 (2013).
Xue, M. et al. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol. Cancer 16(1), 1–13 (2017).
Cai, X. et al. Exosome–transmitted microRNA-133b inhibited bladder cancer proliferation by upregulating dual-specificity protein phosphatase 1. Cancer Med. 9(16), 6009–6019 (2020).
Alcayaga-Miranda, F. et al. Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget 7(28), 44462 (2016).
Yu, L. et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J. Extracell. Vesicles 10(3), e12056 (2021).
Li, Y. et al. Exosome carrying PSGR promotes stemness and epithelial-mesenchymal transition of low aggressive prostate cancer cells. Life Sci. 264, 118638 (2021).
Hosseini-Beheshti, E. et al. Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget 7(12), 14639 (2016).
Yang, L. et al. Effects of renal carcinoma cell line ACHN-derived exosomes on ACHN cell proliferation and apoptosis. Nan Fang yi ke da xue xue bao= J. South. Med. Univ. 32(10), 1498–1502 (2012).
Aghamir, S. M. K. et al. The impact of succinate dehydrogenase gene (SDH) mutations in renal cell carcinoma (RCC): A systematic review. Onco Targets Ther. 12, 7929 (2019).
Khatami, F., Aghamir, S. M. K. & Tavangar, S. M. Oncometabolites: A new insight for oncology. Mol. Genet. Genomic Med. 7(9) (2019).
Zhang, L. et al. The 786-0 renal cancer cell-derived exosomes promote angiogenesis by downregulating the expression of hepatocyte cell adhesion molecule. Mol. Med. Rep. 8(1), 272–276 (2013).
Burdakov, V. et al. Exosomes transfer p53 between cells and can suppress growth and proliferation of p53-negative cells. Cell Tissue Biol. 12(1), 20–26 (2018).
Acknowledgements
Special thanks to Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
S.M.K.A. and M.A. are principal investigators, A.R.R. and F.K.H. wrote the manuscript, A.M. collected data, K.G.H. analyzed the data, and S.H.K. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezaeian, A., Khatami, F., Heidari Keshel, S. et al. The effect of mesenchymal stem cells-derived exosomes on the prostate, bladder, and renal cancer cell lines. Sci Rep 12, 20924 (2022). https://doi.org/10.1038/s41598-022-23204-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23204-x
This article is cited by
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Molecular Cancer (2024)
-
Tissue-derived extracellular vesicles in cancer progression: mechanisms, roles, and potential applications
Cancer and Metastasis Reviews (2023)
-
HP1α promotes the progression of prostate cancer
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.