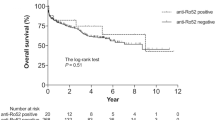
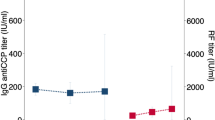
Abstract
Anti-cyclic citrullinated peptide antibody testing is used to diagnose rheumatoid arthritis and associated with interstitial lung disease in RA. Herein, we investigate the relationship between anti-CCP antibody and ILD in SSc. We performed a retrospective analysis at a tertiary medical center between 2005 and 2019. Patients with SSc, systemic lupus erythematosus, and polymyositis/dermatomyositis (PM/DM) were evaluated for anti-CCP antibody and ILD. Additionally, medical records of SSc patients with ILD were reviewed. SSc patients had the highest anti-CCP antibody positivity rate compared to those with SLE and PM/DM. The incidence of ILD was higher in SSc patients with anti-CCP antibody than in those without. The usual interstitial pneumonia (UIP) incidence was higher in the anti-CCP antibody-positive group than in the anti-CCP antibody-negative group. The DLCO was lower in the anti-CCP antibody-positive group than in the anti-CCP antibody-negative group. On multivariable analysis, factors associated with SSc-ILD were anti-CCP antibody or rheumatoid factor (β coefficient, 2.652 [95% CI 1.472 to 4.776]) and anti-Scl70 antibody (β coefficient, 4.011 [95% CI 2.142 to 7.508]). Anti-CCP antibody may be associated with a higher incidence of ILD in SSc. SSc patients with anti-CCP antibody may have more UIP pattern and lower DLCO.
Trial Registration Retrospectively registered.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc) is a chronic, heterogeneous, multisystem disease characterized by widespread vascular dysfunction with fibrosis of the skin and internal organs, such as the lungs. According to the 2013 Classification Criteria for Systemic Sclerosis, there are three hallmarks of SSc: fibrosis of the skin and/or internal organs, production of specific autoantibodies, and evidence of vasculopathy1. Evaluating disease staging and organ involvement is important to guide effective treatment implementation and predict outcomes in SSc. Interstitial lung disease (ILD) is an important internal organ involvement in SSc, which accounts for 33% of deaths occurring in patients with SSc2.
Connective tissue disease-associated interstitial lung disease (CTD-ILD) is characterized by inflammation and/or fibrosis of the lungs in patients with systemic rheumatic diseases, such as rheumatoid arthritis (RA), SSc, systemic lupus erythematosus (SLE), and polymyositis/dermatomyositis (PM/DM)3. Based on a histopathologic analysis, CTD-ILD can be classified into the following subtypes: nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), organizing pneumonia (OP), apical fibrosis, diffuse alveolar damage (DAD), and lymphoid interstitial pneumonia (LIP)4. The two most common subtypes of CTD-ILD are NSIP and UIP3. UIP is characterized by non-uniform fibrosis with honeycomb change, fibroblast foci, and mild inflammation, while NSIP is characterized by uniform fibrosis with a varying proportion of interstitial inflammation4. The dominant pattern of ILD is UIP in RA, and NSIP in SSc3.
For detection and characterization of CTD-ILD, high-resolution computed tomography (HRCT) is more sensitive than chest radiography and conventional computed tomography (CT)5. The HRCT pattern is associated with the histopathologic process6. A reticular pattern with traction bronchiectasis on HRCT is associated with a fibrotic process, whereas a ground-glass pattern is associated with an inflammatory process5. A radiographic and/or histologic pattern in ILD can be a risk factor for progression because the prognosis of UIP is worse than that of non-UIP7,8,9.
Anti-cyclic citrullinated peptide (CCP) antibody is a group of autoantibodies directed against the citrullinated epitopes specific to RA and these appear years before the onset of clinically apparent disease10. Anti-CCP antibody is associated with more erosive joint diseases and extra-articular manifestations, such as ILD10,11,12. In a study of 230 RA-ILD patients in the UK, anti-CCP antibody was the strongest predictor of RA-ILD13. Interestingly, recent studies have suggested that the anti-CCP antibody positivity rate is increased in patients with SSc14,15,16,17,18. However, the role of anti-CCP antibody in SSc-ILD has not been well studied. Therefore, we examined the relationship between anti-CCP antibody and ILD in patients with SSc at our hospital, using HRCT and pulmonary function tests (PFT).
Methods
Patient selection and study design
This was a retrospective observational study conducted at a single tertiary care institution (Severance Hospital, Yonsei University Health System, Seoul, South Korea) between January 2005 and May 2021. Patients with lupus, PM/DM, or SSc who had been tested for anti-CCP antibody were included in our study. A total of 936 patients diagnosed with SLE based on the 1997 American College of Rheumatology (ACR) criteria or the 2012 Systemic Lupus Erythematosus International Collaborating Clinics criteria, were included19,20. Additionally, 156 patients diagnosed with PM/DM based on the Bohan and Peter criteria, were included21,22. Furthermore, 260 patients diagnosed with SSc based on the 2013 ACR/European League Against Rheumatism classification criteria, were included1. We further evaluated the diagnosis of RA based on the 2010 ACR classification criteria23.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Severance Hospital (IRB approval number: 2021-1213-001) and conducted in accordance with the principles set forth in the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study by the Institutional Review Board of Severance Hospital.
Assessment of the clinical manifestations and laboratory findings associated with SSc
Clinical manifestations of SSc, including thickening of the skin of the fingers extending to the metacarpophalangeal joints (MCP), puffy fingers, sclerodactyly, fingertip lesions, telangiectasia, Raynaud’s phenomenon, and joint pain, were reviewed. Nail fold capillary abnormalities were assessed by capillary microscopy. The results of anti-nuclear antibody (ANA), anti-Scl-70 antibody, anti-centromere antibody, white blood cells, hemoglobin level, platelets, serum creatinine level, aspartate aminotransferase level, total protein level, albumin level, urine protein-creatinine ratio, right ventricular systolic pressure on echocardiography, and erosive changes in hand radiography were reviewed. These test results were based on the time SSc was diagnosed at our hospital or the time of referral when SSc was diagnosed at another hospital.
Measurement of anti-CCP antibody
Serum anti-CCP antibody levels were measured using the QUANTA Flash CCP3 (Werfen, San Diego, CA, USA), according to the manufacturer’s instructions. The cut-off point was set to 5 units/mL, and the results of the anti-CCP antibody test were reviewed retrospectively.
Evaluation of SSc-ILD by HRCT
For patients with SSc-ILD, two chest radiologists (C.H.P. and Y.J.H) with over 10 years of experience in chest CT interpretation, independently analyzed HRCT findings while blinded to the laboratory results and clinical information. HRCT was evaluated according to the guidelines for idiopathic pulmonary fibrosis24,25. The presence or absence of honeycombing, traction bronchiectasis, consolidation, ground-glass opacity, and other abnormalities was carefully evaluated, and the distribution of abnormalities was categorized as no abnormality or subpleural, peribronchial, or basal predominance to determine the pattern of ILD. HRCT findings of UIP comprise irregular linear hyperattenuating areas, honeycombing, traction bronchiectasis or bronchiolectasis, architectural distortion, and focal ground-glass attenuation, while HRCT findings of NSIP comprise ground-glass attenuation, irregular linear hyperattenuating areas, and consolidation5.
Evaluation of SSc-ILD by pulmonary function test
PFT results, including forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) at the time of ILD diagnosis in patients with SSc-ILD, were analyzed. DLCO was calculated using the following equation; DLCO predicted corrected = DLCO predicted * (1.7 * hemoglobin/(Age-Sex-Factor + hemoglobin)). For females of any age and children less than 15 years old, the Age-Sex-Factor is 9.38. For males 15 years old or older, the Age-Sex-Factor is 10.2226.
Statistical analysis
Data analysis was conducted using IBM SPSS Statistics for Windows, v. 26 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as medians and inter-quartile ranges, and categorical variables are expressed as frequencies and percentages. Continuous variables were compared using Student’s t-test, and categorical data were compared using the Chi-square test or Fisher’s exact test, as appropriate. In all statistical analyses, a two-tailed p value of < 0.05 was considered statistically significant. For multiple comparisons problem, p values Bonferroni corrected were applied. The risk factors were analyzed by using the univariate and multivariate logistic regression analysis.
Results
Association between anti-CCP antibody and ILD in patients with CTD
We evaluated the positivity rate of the anti-CCP antibody and rheumatoid factor (RF) in patients diagnosed with CTD at our hospital. Among patients with SLE, PM/DM, and SSc, those with SSc had the highest incidence of anti-CCP antibody (9.2% vs. 11.5% vs. 16.2%, respectively; p = 0.006) while there was no statistical significance in RF single positivity among patients with SLE, PM/DM, and SSc (Table 1). Among 42 SSc patients with anti-CCP antibody, 37 patients had both anti-CCP antibody and RF, while 5 patients had only anti-CCP antibody. In patients with SLE and SSc, the incidence of ILD was higher in the RF single-positive group and the anti-CCP-positive group than in the anti-CCP/RF-negative group (Table 2). In patients with SLE and SSc, the incidence of RA was higher in the anti-CCP-positive group than in the group with RF single positivity (SLE 57.0% vs. 25.2%; p < 0.001, SSc 42.9% vs. 16.4%; p = 0.006) (see Supplementary Table S1). We also evaluated the relationship between the diagnosis of RA and ILD in CTDs. In patients with SLE, the incidence of ILD was higher in the RA group than in the group not diagnosed with RA (17.4% vs. 6.5%, p < 0.001) (see Supplementary Table S2). In patients with SSc, there was a trend that the incidence of ILD was higher in the RA group than in the group not diagnosed with RA (65.5% vs. 42.45%, p = 0.054), but there was no statistical significance. Additionally, there was no difference in incidence of ILD between anti-CCP-positive SSc patients with RA vs without RA (see Supplementary Table S3).
Baseline characteristics of the patients with SSc based on anti-CCP antibody and RF positivity
We compared baseline clinical characteristics of the patients with SSc based on anti-CCP antibody and RF positivity (Table 3). Abnormal nail fold capillaries were more frequent in the anti-CCP/RF-negative group and the RF single-positive group than in the anti-CCP-positive group. Joint pain and radiographic erosion were more frequent in the anti-CCP-positive group than in the anti-CCP/RF-negative group and the RF single-positive group. Hemoglobin and albumin levels were lower in the anti-CCP-positive group than in the anti-CCP/RF-negative group and the RF single-positive group. Platelet counts and anti-Scl-70 antibody positivity rate was higher in the anti-CCP-positive group than in the anti-CCP/RF-negative group and the RF single-positive group. Although longer disease duration can be associated with ILD severity, there was no difference in disease duration between anti-CCP-positive and -negative groups. Some medications can be confounding factors for ILD severity because steroid, immunosuppressants, and proton pump inhibitors may have a protective effect on ILD progression. In our study, anti-CCP-positive group used steroid, immunosuppressants, and proton pump inhibitors more frequently than did anti-CCP-negative groups (Table 3, see Supplementary Table S4). However, these factors may not increase the risk of ILD because these are not risk factors but protective factors.
Comparison of HRCT pattern based on anti-CCP antibody in patients with SSc-ILD
The anti-CCP-positive group had a higher UIP incidence than the anti-CCP-negative group (55.6% vs. 31.1%, p = 0.021) (Table 4). RF single, anti-Scl-70 and anti-centromere antibodies were not associated with UIP in patients with SSc-ILD. The anti-CCP-positive group had a lower incidence of ground-glass opacities than the anti-CCP -negative group (63.0% vs. 84.4%, p = 0.015) (see Supplementary Table S5. Figure 1A,B show the representative NSIP pattern in anti-CCP-negative SSc patients. They also show extensive ground-glass opacities and traction bronchiectasis, with basal predominance. Figure 1C,D show the representative UIP pattern in anti-CCP -positive SSc patients. They also show predominant lower lobe pulmonary fibrosis and traction bronchiectasis with exuberant honeycombing sign.
Representative HRCT images of patients with SSc-ILD. Axial (A) and coronal (B) HRCT images of an anti-CCP antibody-negative patient with SSc-ILD. They show extensive ground-glass opacities and traction bronchiectasis with basal predominance. These findings are typical of a non-specific interstitial pneumonia pattern. Axial (C) and coronal (D) HRCT images of an anti-CCP antibody-positive patient with SSc-ILD. They show predominant lower lobe pulmonary fibrosis and traction bronchiectasis with exuberant honeycombing sign. These findings are consistent with the usual interstitial pneumonia pattern. HRCT: high resolution computed tomography; SSc: systemic sclerosis; ILD: interstitial lung disease.
Comparison of PFT results based on anti-CCP antibody and RF positivity in patients with SSc-ILD
The PFT results demonstrated that the DLCO was lower in the anti-CCP antibody-positive group than in the anti-CCP antibody-negative group (57.2% vs. 67.8%, respectively; p = 0.006) (Table 5). Because DLCO is low in anti-CCP-positive patients, we further evaluate the presence of PAH. In SSc patients, DLCO was lower in patients with PAH than in patients without PAH (see Supplementary Table S6). However, there is no difference of RVSP between anti-CCP-positive vs. anti-CCP-negative groups with low DLCO (see Supplementary Table S7). There was no significant difference in the FVC between the anti-CCP-positive and -negative groups. Furthermore, there was no significant difference in the DLCO or FVC between the antibody-positive and -negative groups for other autoantibodies such as the RF single, anti-Scl-70, and anti-centromere antibodies.
Univariate and multivariate regression analysis for identification of biomarkers for ILD in SSc
To further validate the association between ILD and anti-CCP/RF, we performed univariate and multivariate regression analysis. We compared several factors associated with arthritis phenotypes such as anti-CCP antibody, RF, diagnosis of RA, joint pain, and joint erosion. Anti-CCP antibody or RF positivity is the most statistically significant when univariate regression analysis was performed (Table 6). Therefore, we used anti-CCP antibody or RF positivity for multivariate regression analysis. Other parameters such as abnormal nailfold capillaries, anti-Scl70 antibody, white blood cell count, platelet, and albumin were significant in univariate regression analysis and were used for multivariate regression analysis. When multivariate regression analysis on factors associated with ILD was performed, anti-CCP antibody or RF, anti-Scl70 antibody, white blood cell count, and albumin were statistically significant (Table 7). The association between anti-Scl70 antibody and ILD has been well known, while the association between anti-CCP antibody or RF and ILD in SSc has not been addressed before.
Discussion
Although the anti-CCP antibody is highly specific for RA, it has been reported to be positive in other diseases, such as tuberculosis (32%), SLE (17%), psoriatic arthritis (15.6%), autoimmune hepatitis (9%), idiopathic pulmonary fibrosis (6.5%), and primary biliary cholangitis (2.7%)27,28,29,30,31. Indeed, the anti-CCP antibody positivity rate is higher in patients with CTDs, including SLE, PM/DM, and SSc, than in the general population32. Interestingly, our results demonstrated that the anti-CCP antibody positivity rate was higher in patients with SSc (16.2%) than in those with SLE (9.2%) or PM/DM (11.5%), using data from a single tertiary center. We also observed that anti-CCP antibody positivity was associated with a higher incidence of ILD in SSc. Our observation is consistent with that of a previous meta-analysis, which showed that anti-CCP antibody may be associated with pulmonary fibrosis in patients with SSc33.
We further evaluated the subtype and severity of ILD using HRCT and PFT in SSc patients, based on anti-CCP antibody positivity. Among patients with SSc-ILD, the anti-CCP antibody-positive group had a predominantly UIP pattern, while the anti-CCP antibody-negative group had a predominantly NSIP pattern. Furthermore, SSc patients with anti-CCP antibody demonstrated a lower DLCO. This is consistent with the results of a study on idiopathic pulmonary fibrosis, which found that UIP was less reversible and had a worse prognosis than NSIP24,34,35. In our study, anti-CCP antibody positivity is associated with lower frequency of ground-glass opacities in HRCT. Therefore, it may suggest that anti-CCP antibody positivity is associated with poor response to treatment. Alternatively, it may be the result of a delay in detection of ILD which can be associated with more fibrotic changes. However, there was no difference in disease duration between anti-CCP-positive and -negative groups. Therefore, the anti-CCP antibody might be helpful to predict the prognosis of ILD in SSc patients. It is unknown whether the anti-CCP antibody plays a pathologic role in CTD-ILD or is merely a biomarker associated with RA. Interestingly, our data demonstrated an association between anti-CCP antibody positivity and ILD in patients with SSc and SLE. It is also known that anti-CCP antibody positivity is associated with CTD-ILD in patients with anti-synthetase syndrome36. Therefore, the anti-CCP antibody could have played a role not only in RA but also in CTD-ILD.
In the disease progression of RA, the appearance of the anti-CCP antibody precedes the symptoms of arthritis37. This suggests that anti-CCP antibodies may occur outside the joints. Indeed, the anti-CCP antibody was found in the sputum of asymptomatic first-degree relatives of patients with RA38. Furthermore, citrullination of proteins has been found in the lungs of patients with early RA, and the anti-CCP antibody was observed in their bronchoalveolar lavage fluid39. It is possible that lung inflammation plays a role in the development of the anti-CCP antibody by inducing peptidylarginine deiminase to produce citrullinated epitopes40. The anti-CCP antibody was also detected in non-RA patients with UIP41. The presence of subclinical HRCT abnormalities has been reported in anti-CCP antibody positive patients without arthritis42. Further studies are needed to determine the role that anti-CCP antibody plays in CTD-ILD.
Alternatively, these findings may suggest that there are overlap syndromes with features of both RA and other CTDs, such as SSc, SLE, or PM/DM43. The term “overlap syndrome” has been used if anti-CCP antibodies are present in patients with non-RA rheumatologic disorder. The presence of the anti-CCP antibody appears to be a good marker for joint synovitis and erosive changes in patients with SSc-RA overlap syndrome30. In our study, radiographic evidence of erosive change and joint pain was also more frequently observed in SSc patients with the anti-CCP antibody than in SSc patients without the anti-CCP antibody. However, radiography detected join erosion in only 30% of anti-CCP positive SSc patients. Furthermore, based on the ACR classification criteria for RA, 42.9% of anti-CCP positive SSc patients can be classified as RA overlap syndrome. Although anti-CCP antibody positivity is associated with RA overlap syndrome in SSc, more than 50% of anti-CCP-positive SSc patients did not have clear features of RA in our study population. When anti-CCP antibody is positive in SSc patients, it is important to monitor not only joint symptoms but also development of ILD.
It is interesting to find that RF is also associated with ILD in SSc. In our study, 25.8% of SSc patients were RF single positive. The pattern of ILD is different between RF single positivity and anti-CCP antibody positivity. RF single positivity is more frequent in non-UIP while anti-CCP antibody is more frequent in UIP. Our data demonstrated that anti-CCP antibody or RF is associated with ILD in SSc independent of anti-Scl70 antibodies. Therefore, it will be helpful to evaluate anti-CCP antibody or RF in SSc for the early detection of ILD.
This study has several limitations. First, the data for this study were collected retrospectively; this may have led to bias in patient selection and analysis. Second, since this was an observational, cross-sectional study, the long-term prognostic outcomes were not evaluated. Recently, several immunosuppressants such as mycophenolate mofetil, cyclophosphamide, rituximab, abatacept, and calcineurin inhibitors demonstrated therapeutic effects in CTD-ILD. Therefore, it is important to identify serologic markers to guide treatment strategies for SSc-ILD. Further research is needed to evaluate treatment response according to anti-CCP antibody positivity in SSc. Third, a variety of cofounding factors can affect the frequency of anti-CCP antibody and ILD in SSc. However, some of them are lacking due to the retrospective nature of the study such as the modified Rodnan skin score or the types of skin involvement (limited vs. diffuse). Therefore, we can only draw modest conclusion.
Conclusions
Anti-CCP antibody may be associated with a higher incidence of ILD in SSc. SSc patients with anti-CCP antibody may have more UIP pattern and lower DLCO. An anti-CCP antibody test may be helpful for early detection of ILD in SSc patients.
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional supporting files.
Abbreviations
- CTD-ILD:
-
Connective tissue disease-associated interstitial lung disease
- PM:
-
Polymyositis
- DM:
-
Dermatomyositis
- NSIP:
-
Nonspecific interstitial pneumonia
- UIP:
-
Usual interstitial pneumonia
- OP:
-
Organizing pneumonia
- HRCT:
-
High-resolution computed tomography
- CT:
-
Computed tomography
- PAH:
-
Pulmonary arterial hypertension
- SSc:
-
Systemic sclerosis
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
- ACR:
-
American College of Rheumatology
- PFT:
-
Pulmonary function test
- FVC:
-
Forced vital capacity
- DLCO:
-
Diffusing capacity of the lung for carbon monoxide
- SLE:
-
Systemic lupus erythematous
References
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum Dis. 72(11), 1747–1755 (2013).
Steen, V. D. & Medsger, T. A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum Dis. 66(7), 940–944 (2007).
Korsten, P., Konig, M. F., Tampe, B. & Mirsaeidi, M. Editorial: Interstitial lung disease in the context of systemic disease: Pathophysiology Treatment and Outcomes. Front. Med. (Lausanne) 7, 644075 (2020).
Yoo, H. et al. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): Evolving concept of CT findings, pathology and management. Eur. J. Radiol. Open 8, 100311 (2021).
Kim, E. A. et al. Interstitial lung diseases associated with collagen vascular diseases: Radiologic and histopathologic findings. Radiographics 22(suppl_1), S151–S165 (2002).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT in patients with little or no radiological evidence of honeycombing: Secondary analysis of a randomised, controlled trial. Lancet Respir. Med. 2(4), 277–284 (2014).
Travis, W. D., Matsui, K., Moss, J. & Ferrans, V. J. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: Survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am. J. Surg. Pathol. 24(1), 19–33 (2000).
Oldham, J. M. et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur. Respir. J. 47(6), 1767–1775 (2016).
Wong, A. W., Ryerson, C. J. & Guler, S. A. Progression of fibrosing interstitial lung disease. Respir. Res. 21(1), 32 (2020).
van Venrooij, W. J., van Beers, J. J. & Pruijn, G. J. Anti-CCP antibodies: The past, the present and the future. Nat. Rev. Rheumatol. 7(7), 391–398 (2011).
Giles, J. T. et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann. Rheum Dis. 73(8), 1487–1494 (2014).
Zhu, J., Zhou, Y., Chen, X. & Li, J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J. Rheumatol. 41(7), 1282–1289 (2014).
Kelly, C. A. et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatology (Oxford) 53(9), 1676–1682 (2014).
Ingegnoli, F. et al. Use of antibodies recognizing cyclic citrullinated peptide in the differential diagnosis of joint involvement in systemic sclerosis. Clin. Rheumatol. 26(4), 510–514 (2007).
Wu, R. et al. Increased prevalence of anti-third generation cyclic citrullinated peptide antibodies in patients with rheumatoid arthritis and CREST syndrome. Clin. Rev. Allergy Immunol. 32(1), 47–55 (2007).
Santiago, M. et al. A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti-CCP assay (CCP3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin. Rheumatol. 27(1), 77–83 (2008).
Ueda-Hayakawa, I. et al. Usefulness of anti-cyclic citrullinated peptide antibody and rheumatoid factor to detect rheumatoid arthritis in patients with systemic sclerosis. Rheumatology 49(11), 2135–2139 (2010).
Polimeni, M., Feniman, D., Skare, T. & Nisihara, R. M. Anti-cyclic citrullinated peptide antibodies in scleroderma patients. Clin. Rheumatol. 31(5), 877–880 (2012).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9), 1725 (1997).
Petri, M. et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64(8), 2677–2686 (2012).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292(7), 344–347 (1975).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292(8), 403–407 (1975).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: An American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 62(9), 2569–2581 (2010).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198(5), e44–e68 (2018).
Bastos, A. L., Corrêa, R. A. & Ferreira, G. A. Tomography patterns of lung disease in systemic sclerosis. Radiol. Bras. 49(5), 316–321 (2016).
Macintyre, N. et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 26(4), 720–735 (2005).
Bogliolo, L. et al. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J. Rheumatol. 32(3), 511–515 (2005).
Kakumanu, P. et al. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies, but their sera also react with unmodified arginine-containing peptide. Arthritis Rheum 58(6), 1576–1581 (2008).
Koga, T. et al. Determination of anti-cyclic citrullinated peptide antibodies in the sera of patients with liver diseases. Clin. Exp. Rheumatol. 26(1), 121–124 (2008).
Kakumanu, P. et al. Citrulline dependence of anti-cyclic citrullinated peptide antibodies in systemic lupus erythematosus as a marker of deforming/erosive arthritis. J. Rheumatol. 36(12), 2682–2690 (2009).
Kang, B. H. et al. Clinical significance of serum autoantibodies in idiopathic interstitial pneumonia. J. Korean Med. Sci. 28(5), 731–737 (2013).
Payet, J. et al. Anticyclic citrullinated peptide antibodies in rheumatoid and nonrheumatoid rheumatic disorders: Experience with 1162 patients. J. Rheumatol. 41(12), 2395–2402 (2014).
Laustriat, G. et al. Anti-citrullinated peptides antibodies in systemic sclerosis: Meta-analysis of frequency and meaning. Joint Bone Spine 85(2), 147–153 (2018).
Zamora-Legoff, J. A., Krause, M. L., Crowson, C. S., Ryu, J. H. & Matteson, E. L. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 69(3), 542–549 (2017).
Assayag, D. et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 19(4), 493–500 (2014).
Yamakawa, H. et al. Interstitial lung disease associated with anti-citrullinated peptide/protein antibody-positive anti-synthetase syndrome. J. Thorac. Dis. 10(10), 5924–5931 (2018).
van der Helm-van Mil, A. H., Verpoort, K. N., Breedveld, F. C., Toes, R. E. & Huizinga, T. W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 7(5), R949-958 (2005).
Demoruelle, M. K. et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol. 69(6), 1165–1175 (2017).
Reynisdottir, G. et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 66(1), 31–39 (2014).
Sparks, J. A. & Karlson, E. W. The roles of cigarette smoking and the lung in the transitions between phases of preclinical rheumatoid arthritis. Curr. Rheumatol. Rep. 18(3), 15 (2016).
Fischer, A. et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir. Med. 106(7), 1040–1047 (2012).
Lucchino, B. et al. Identification of subclinical lung involvement in ACPA-positive subjects through functional assessment and serum biomarkers. Int. J. Mol. Sci. 21(14), 5162 (2020).
Kamalaksha, S., White, D. H. N. & Solanki, K. K. Significance of combined anti-CCP antibodies and rheumatoid factor in a New Zealand cohort of patients with systemic sclerosis. Int. J. Rheum Dis. 21(7), 1430–1435 (2018).
Funding
This work was supported by the Basic Science Research Program (2021R1F1A1059528 to J.J.S.) through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology.
Author information
Authors and Affiliations
Contributions
J.W.H. designed the report and wrote the paper; Y.J.H., C.H.P., and H.J.C. participated in data acquisition and interpretation; J.D.M., J.Y.P., S.W.L., and Y.B.P. drafted and revised the manuscript; C.H.P. and J.J.S. designed the concept and approved the final paper. All authors have taken care to ensure the integrity of this work, and the final manuscript has been seen and approved by all authors.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ha, J.W., Hong, Y.J., Cha, H.J. et al. A retrospective analysis of the relationship between anti-cyclic citrullinated peptide antibody and interstitial lung disease in systemic sclerosis. Sci Rep 12, 19253 (2022). https://doi.org/10.1038/s41598-022-23180-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23180-2
This article is cited by
-
The interplay between rheumatic diseases and pulmonary health
Rheumatology International (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.