Abstract
Klotho is an anti-aging protein with several therapeutic roles in the pathophysiology of different organs, such as the skeletal muscle and kidneys. Available evidence suggests that exercise increases Klotho levels, regardless of the condition or intervention, shedding some light on this anti-aging protein as an emergent and promising exerkine. Development of a systematic review and meta-analysis in order to verify the role of different exercise training protocols on the levels of circulating soluble Klotho (S-Klotho) protein. A systematic search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE through PubMed, EMBASE, CINAHL, CT.gov, and PEDro. Randomized and quasi-randomized controlled trials that investigated effects of exercise training on S-Klotho levels. We included 12 reports in the analysis, comprising 621 participants with age ranging from 30 to 65 years old. Klotho concentration increased significantly after chronic exercise training (minimum of 12 weeks) (Hedge’ g [95%CI] 1.3 [0.69–1.90]; P < 0.0001). Moreover, exercise training increases S-Klotho values regardless of the health condition of the individual or the exercise intervention, with the exception of combined aerobic + resistance training. Furthermore, protocol duration and volume seem to influence S-Klotho concentration, since the effect of the meta-analysis changes when subgrouping these variables. Altogether, circulating S-Klotho protein is altered after chronic exercise training and it might be considered an exerkine. However, this effect may be influenced by different training configurations, including protocol duration, volume, and intensity.
Similar content being viewed by others
Introduction
In Greek mythology, even the most powerful gods needed to bow to the three Moirai: Clotho, Lachesis, and Atropos. These three goddesses of fate personified the inescapable destiny of mortals by controlling the thread of life1. Clotho spun the thread of life, Lachesis measured and allotted it to each person, and Atropos was the thread cutter. In view of the Moirai story, when Kuro-o et al. discovered the gene that seems to control aging, they named it Klotho2.
Klotho is now considered a strong biomarker and therapy for several diseases3,4,5,6,7,8,9,10. In addition, it presents anti-inflammatory and antioxidant properties11,12,13. Klotho increases the affinity of fibroblast growth factors (FGFs) 19, 21, and 23 for their respective receptors14. The FGF23-Klotho axis acts by suppressing renal inorganic phosphate reabsorption and activating vitamin D biosynthesis14. This pathway prevents calciprotein particle formation and, consequently, reduces cardio-renal damage14,15. Furthermore, the inflammation and cardiac dysfunction provoked by aging are associated with partial Klotho deficiency16. Part of this mechanism is proposed to be due to Klotho’s capacity to regulate energy metabolism and induce the expression of antioxidant enzymes, including catalase and superoxide dismutase17,18.
Klotho supplementation improves blood pressure and renal parameters in a pre-clinical model of type 2 diabetes15,19. It also suppresses inflammation and improves cardiac function in aged, endotoxemic mice16. Zhang et al.20 demonstrated that the peroxisome-activated receptor-γ (PPAR-γ) increases Klotho expression, while the PPAR-γ antagonist inhibits Klotho in mouse kidney. Interestingly, exercise training promotes PPAR-γ activation in skeletal muscle and other tissues21,22,23, which might be linked to greater circulating levels of Klotho in different populations.
Exercise training induces Klotho secretion by skeletal muscle24, along with other molecules including irisin25, sestrin-226, and a myriad of microRNAs27. The molecules produced and secreted by tissues and organs as a direct or indirect consequence of exercise are called exerkines28,29. Due to their therapeutic potential as exercise mimetics, exerkines open a novel avenue for drug discovery28,29,30,31. Although Klotho has promising therapeutic potential in human pathology, it has never been considered an exerkine until now. Therefore, knowing what the effects of exercise are on S-Klotho expression and secretion may help scientists and coaches to prescribe the best protocols to increase the levels of this protein.
In the past few years, the interest of the scientific community and industry in the possibility of mimicking or potentializing the effects of exercise has increased considerably32,33. In this context, exerkines have been pursued as alternative treatments or strategies to improve health and/or performance28,29. Although it is an intriguing idea, there is still a long way to go before the creation of an exercise “pill or shot”. Therefore, it is worth investigating the possible bioactive compounds that should be targeted as novel therapies related to exercise mimetics. Indeed, some insights might appear in the study of Kurosu et al. who were able to attenuate aging-related senescence in mice with Klotho overexpression34. Thus, the increase in Klotho following exercise training may demonstrate a potential link between being physically active and delaying aging.
The aforementioned scenario may lead to a better training prescription aimed at anti-aging effects. Moreover, it could even generate a perspective for the use of Klotho measurement in the biological control of sports training and clinical rehabilitation programs. Here, we systematically described different exercise protocols capable of inducing Klotho levels. In that context, the purpose of the present study was to verify the role of different exercise training protocols on S-Klotho concentrations. The present review provides novel insights supporting exercise as a strong intervention to increase S-Klotho in humans. While this phenomenon appears to occur regardless of the condition (healthy or diseased) and training protocol (aerobic or resistance training), we outlined that the volume and duration of the intervention might play a significant role on S-Klotho changes.
Results
Participant characteristics
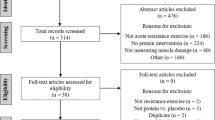
The PRISMA flow diagram of study selection is presented in Supplementary Fig. 1. From the 8 studies that met the inclusion criteria, 12 reports were included in the quantitative analysis. Amaro-Gahete et al.35 was split into 3 independent reports: (a) combined aerobic + resistance training; (b) only aerobic; (c) aerobic + electromyostimulation. Corrêa et al.36 was split into two independent reports: (a) conventional resistance training and (b) blood-flow restricted resistance training. Neves et al.37 was split into 2 independent reports: (a) conventional resistance training and (b) isometric resistance training.
There were five reports with healthy subjects and seven with diseased patients. The diseases analyzed were chronic obstructive pulmonary disease38, stage two of chronic kidney disease36, end-stage renal disease37,39, and coronary artery disease40. Moreover, five reports investigated the effect of resistance training36,37,38, five reports investigated aerobic training35,40,41,42, and only two studies examined the combined effect of aerobic and resistance training35,39. Publications ranged from 2014 to 2021. There was a total of 621 participants with age ranging from 30 to 65 years. According to the results of critical appraisal phase presented in supplementary Fig. 2, included studies are in low risk of biases.
Exercise training characteristics
The exercise training characteristics are presented in Table 1. The interventions lasted from 12 to 24 weeks (17 ± 6 weeks), performed between 2 and 5 days per week. Studies that applied a resistance training protocol used percentages of the maximum force or 1RM test36,38 and perceived exertion scale37 to control the intensity of the protocol. The increase in S-Klotho protein in these studies ranged from 8.39 to 88.51%. It was notable that the authors also observed associated benefits such as improvement in functional performance, bone mineral density, inflammatory biomarkers, and attenuation of chronic kidney disease progression (when applicable).
The studies that applied aerobic exercise used percentages of VO2max35 or HRmax40,41,42 to control the intensity of the protocol. The increase in Klotho protein ranged from 12 to 55.8%. A positive association was verified with higher muscle mass, improvement in arterial stiffness, pro-brain natriuretic peptide, and cardiac hypertrophic indices. Only two of the included studies assessed the combined effect of aerobic + resistance training35,39. Amaro-Gahete et al.35 prescribed the protocol based on the World Health Organization recommendation (aerobic: 60–65% of the reserve heart rate; resistance: 40–60% of 1RM). Klotho increased by 47.75% and seemed to be associated with higher muscle mass. Fakhrpour et al.39 prescribed aerobic training based on the Borg scale (45 min; 12–14 on Borg scale) and resistance training based on 1RM (40–65% of 1RM). The authors found an increase of 7.27% in Klotho levels, concomitant to an increase in muscle strength and functional performance.
Meta-analysis
Figure 1 illustrates the overall response of Klotho protein to exercise training. Chronic exercise training increased circulating Klotho, independent of the modality (Hedges’ g [95%CI] 1.3 [0.69–1.90]; P < 0.0001). Significant heterogeneity was found for Klotho (I2 = 90.69) responses. This is likely due to different conditions (healthy and diseased patients), protocols (aerobic, resistance, and aerobic + resistance training), and protocol durations in each study. Therefore, caution is required when interpreting the effect of exercise training on circulating Klotho values. We also performed the same meta-analysis with fixed-effect, and it did not deliver a different magnitude, effect, or significance compared with the random-effect meta-analysis.
Forest plot of the results from a random-effects meta-analysis shown as Hedges’ g with 95% CIs on Klotho concentrations. For each study, the square represents the Hedges’ g of the intervention effect with the horizontal line intersecting it as the lower and upper limits of the 95% CI. The rhombi represent the total effect.
Sub-group analysis
A sub-group meta-analysis was performed by stratifying the studies by population condition (healthy vs. presence of disease) and exercise training protocol (aerobic vs. resistance vs. combined training). The effects were maintained, independent of the condition (healthy: 0.82 [0.47–1.16], P < 0.0001); diseased: 1.55 [0.59–1.90], P < 0.0001), as described in Fig. 2. Klotho seems to increase significantly after resistance training (1.93 [0.73–3.12], P < 0.0001) and aerobic training (0.92 [0.60–1.25], P < 0.0001). However, there were no significant modifications in Klotho levels after combined training (0.39 [−0.05 to 0.83], P > 0.05), as described in Fig. 3. This response might be due to the different interventions, durations, and volumes, which could differentially influence the molecular pathways related to Klotho expression. Trying to address this issue, we performed a cumulative analysis of Klotho levels according to the protocol duration (12 to 24 weeks) and estimated training volume (60 to 210 min per week).
Forest plot of the results from a random-effects meta-analysis shown as Hedges’ g with 95% CIs of healthy and diseased patients on Klotho concentrations. For each study, the square represents the Hedges’ g of the intervention effect with the horizontal line intersecting it as the lower and upper limits of the 95% CI. The rhombi represent the weighted healthy, diseased, and total effect.
Forest plot of the results from a random-effects meta-analysis shown as Hedges’ g with 95% CIs of aerobic, resistance, and combined training on Klotho concentrations. For each study, the square represents the Hedges’ g of the intervention effect with the horizontal line intersecting it as the lower and upper limits of the 95% CI. The rhombi represent the weighted the aerobic, resistance, and combined training.
Figure 4 illustrates the cumulative analysis of Klotho response according to protocol duration. Interestingly, the protocol’s length seems to play a key role in Klotho responses. Moreover, Fig. 5 illustrates a possible inverted “U” shaped curve, pointing to the possibility of a dose–response related to Klotho changes and the protocol volume (minutes per week) whereas ~ 150 min per week appears to present the highest magnitude of Klotho change. Therefore, we pooled together S-Klotho concentrations of the studies around36,39,42, below35,37,41, and above35,38,40 150 min per week, and then analyzed them. The mean values were 1.65, 1.05, and 1.36, respectively, demonstrating a higher effect for the studies that performed a volume around 150 min per week.
Cumulative forest plot from a random-effect meta-analysis shown as Hedges’ g with 95% CIs of the protocol duration increase on Klotho concentrations. For each study, the square represents the Hedges’ g of the intervention effect with the horizontal line intersecting it as the lower and upper limits of the 95% CI.
Cumulative forest plot from a random-effect meta-analysis shown as Hedges’ g with 95% CIs of the increase of minutes per week on Klotho concentrations. For each study, the square represents the Hedges’ g of the intervention effect with the horizontal line intersecting it as the lower and upper limits of the 95% CI.
S-Klotho concentrations following exercise training in healthy and diseased subjects
Figure 3 of the Supplementary material shows that exercise training significantly increases Klotho in relation to the control group (S. Fig. 3A). This increase in mean values seems higher in healthy subjects (S. Fig. 3B,C).
Discussion
To date, this is the first meta-analysis to assess the pooled effect of different exercise training protocols on blood S-Klotho levels. Our main finding was that exercise training consistently increases S-Klotho levels (Fig. 1). This response might occur without a relationship to the healthy status (Fig. 2) and training protocol (Fig. 3). There is an exception for combined training, which is probably related to the different protocol duration, intensity, or volume used in the studies that assessed the combined training. It is noteworthy that the findings regarding combined training should be interpretated with caution, since we found only two eligible studies.
A previous systematic review43 in both human and animal models demonstrated that exercise may increase S-Klotho levels. The authors also demonstrated Klotho as an important protein for hampering senescence. Although our meta-analysis was comprehensive and included multiple studies, some other studies that did not meet the inclusion criteria are also in agreement with our conclusions (Supplementary Table 1). In summary, the excluded studies demonstrated that master athletes present higher levels of S-Klotho in comparison to non-athletes44,45. This fact could be associated with physical activity levels, higher muscle mass, increased fat oxidation, low cardiometabolic risk, and muscle strength46,47,48,49,50,51,52. Moreover, a bout of acute exercise seems to increase S-Klotho in women53, and also in healthy football players54. However, military operational stress reduces Klotho in service members55. Taken together, it seems that an excessively stressful condition could blunt S-Klotho production.
Exercise-induced S-Klotho: does oxidative stress play a role?
External and internal stress exposure plays a crucial role in exercise-induced molecules56,57. Strenuous exercise notably induces an increase in free radicals and reactive oxygen species, which is commonly known as oxidative stress58. This condition may lead to increase in muscle damage, toxins, and cell death56,58,59. In contrast, a transient increase in oxidative stress is necessary for aerobic-induced benefits, which normally trigger hormesis56,59. Hormesis is a common term used to describe an effect associated with toxic compounds that, in low doses, promote a beneficial effect on the exposed organism and, in high doses, present a toxic effect, leading to an inverse “U” shaped curve of optimal dose–response60. In this context, the relatively stable reactive oxygen species induced by muscle contraction, such as hydrogen peroxide and nitric oxide, may act as signaling molecules that improve cellular communication and function, aiming to reach stability60. Considering that S-Klotho may regulate and be regulated by reactive oxygen species, this protein increase should require a transient redox imbalance61. Another possible explanation on the exercise-induced Klotho levels might be related to the anti-inflammatory role of exercise. It known that inflammation decreases Klotho expression, leading to pre-mature aging and age-related issues62,63. Therefore, it is worthy to state that exercise-controlled inflammation may play a key role in increasing Klotho levels in human.
In our study, S-Klotho appears to be higher in trained people, regardless of the volume of training. Nevertheless, ~ 150 min per week seems to be the optimal volume to induce S-Klotho changes. This might be due to the transient increase in oxidative stress, leading to an upregulation of the antioxidant system and anti-inflammatory profile64,65,66,67,68. Considering that high exercise intensities might cause renal artery vasoconstriction69, the hypoxia induced by a limited blood-flow may promote a transient oxidative stress that would increase the antioxidant system, including S-Klotho. Taken together, there might be an optimal stress level for Klotho induction, and further studies should investigate the dose–response kinetics of this protein following different intensities and volumes. Finding the dose–response of exercise for this protein could lead to a better protocol prescription aimed at anti-aging effects, or assist in the assembly of training programs and periodization in high-performance sport. In summary, S-Klotho may be one of the main parts of a myriad of molecules that allow exercise training to be effective in the prevention and treatment of a plethora of diseases. Nevertheless, further studies should test this hypothesis.
Exercise training protocols in S-Klotho response
Exercise training seems to stimulate S-Klotho regardless of the protocol (resistance or aerobic training). However, even subgrouping the meta-analysis according to the type of intervention, we could observe some divergences in both resistance and aerobic training. From the studies with resistance training36,37,38, all of them applied full-body resistance training, using exercises that require several muscle groupings in the same movement. Boeselt et al.38 applied a total of four exercises, Neves et al.37 used a total of twelve exercises (for both dynamic and isometric training), and Corrêa et al.36 used eight exercises (for both training with and without blood-flow restriction).
As observed in Fig. 3, the study of Boeselt et al.38 did not present a significant effect that favors intervention. The protocol consisted of 15–20 sets of 2–4 repetitions. Although their final workload was similar to the other included studies, it is known that different training configurations, such as number of sets and repetitions, produce distinct metabolic responses in humans70. Moreover, time under tension is another variable that may influence the responses following resistance training71,72. Burd et al.71 provided evidence that greater muscle time under tension could increase mitochondrial and sarcoplasmic protein synthesis. Considering that Klotho is mainly influenced by metabolism11,14,20, it is possible that different resistance exercise prescriptions may lead to different S-Klotho responses.
Regarding the studies that applied aerobic training, two studies used a treadmill for the intervention35,40, one study used a cycle-ergometer41, and one study performed aerobic aquatic training42. There are plenty of physiological differences between these interventions73,74. However, all the included studies that applied an aerobic training protocol presented similar responses in S-Klotho production. This might be due to the impact of aerobic exercise stimulation on mitochondrial biogenesis75. As stated before, oxidative metabolism may influence Klotho production. Thus, aerobic training probably induces S-Klotho regardless of the protocol configuration, due to the overall mitochondrial stimulus.
Considering the aforementioned points, a combination of aerobic + resistance training should be the optimal protocol to increase S-Klotho in humans. To our surprise, there was no significant effect of this training intervention on Klotho. However, the present data should be interpreted with caution, because only two studies investigated the combined effect of both training models35,39. Moreover, we should consider all training configurations in the process to change molecular signatures in the organism (intensity and volume). In this regard, we hereby suggest a possible role of the training-volume-dependent manner to different concentrations of S-Klotho in humans.
Klotho: a potent target for exercise mimetics
We must highlight that an exercise mimetic is probably not the best intervention and will not solve physical inactivity, as already stated elsewhere32. Nonetheless, the potential role of an “exercise pill” is to promote benefits for populations that cannot perform physical activity. In the 30-year follow-up to the Dallas bedrest study76, 3 weeks of bedrest in 20-year-old men had a more profound impact on cardiovascular parameters than 30 years of aging. Considering that a person with spinal cord injury, coma, or post-surgical stage may spend up to 3 weeks in bedrest, a key question is raised here: can exercise mimetics mitigate the impacts caused by these conditions?
Here, we demonstrated Klotho as an emerging exerkine induced by different protocols of exercise training, providing clues to future investigations that might consider this anti-aging protein as a potent bioactive compound for the development of exercise mimetics.
Klotho: a potent target for sports sciences and medicine
The prevention and rehabilitation of various sports injuries has been widely studied77,78,79,80. Baseline characteristics such as muscle strength, body composition, and levels of athleticism may influence injury prediction78. Such properties seem to be constantly modulated by several molecule pathways, which include Klotho81,82,83. In rodents, a reduction in this protein led to the loss of muscle stem cell function81 and appeared to modulate myogenesis-accelerated muscle growth after injury83. In addition, a decrease in Klotho expression can contribute to cartilage damage in osteoarthritic mice82. Taken together, all this evidence points to a possible role of Klotho in the management of muscle and cartilage injuries.
Although most of the included studies demonstrated that chronic exercise training increases S-Klotho levels in humans, none of them presents a causal effect between Klotho and exercise-induced beneficial outcomes, such as functional performance and body composition. However, Phelps et al. showed that Klotho expression is required to enable exercise to have an effect on endurance capacity and skeletal muscle strength84. Sahu et al. verified that knocking down the Klotho gene in vivo appears to hamper the progression of muscle progenitor cell lineage, blunting muscle fiber regeneration85. This gene knockdown also provoked damage to mitochondrial DNA and impaired cellular bioenergetics. Therefore, the increase in S-Klotho identified in the present study might point towards a possible biomarker of the training status and it should be targeted by future studies, aiming to verify the causality between Klotho and the benefits outlined in this study induced by exercise training in humans.
Conclusion and outlook
In summary, our findings add new information about the effects of different exercise training protocols on S-Klotho levels. Another key message of the present study is that although the overall results support the claim that Klotho might be an exerkine associated with a myriad of health benefits, there is no consensus on the ideal exercise protocol to lead to a greater increase in Klotho concentrations. Furthermore, we speculate that Klotho may act as an exerkine, and its kinetics could be modified according to training volume, intensity, and duration. Klotho can possibly be used alone or in conjunction with other exerkines and baseline characteristics, through dynamic mathematical modeling, to assist physiologists and coaches in high-performance sports. Finally, Klotho is a candidate that should be targeted and explored by industries and researchers aiming to build and develop an exercise mimetic biotool. However, this conclusion should be interpretated with caution since the population and the protocol appear indicating possible sources of heterogeneity and the lower number of studies and samples.
Methods
Protocols and registration
We first performed a systematic review according to the Cochrane Handbook recommendations86 and reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines87. The protocol for this review was registered in the International Prospective Register of Systematic Reviews https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021243080 with registration number: CRD42021243080.
Criteria for considering studies for this review
We included randomized and quasi-randomized controlled trials (RCTs) that investigated the S-Klotho response after the following comparisons: (a) Resistance training versus no training; (b) aerobic training versus no training; (c) combined resistance + aerobic training versus no training. To be included in this review, studies needed to apply a chronic exercise protocol in humans.
No language restriction was applied in the search. There were no limitations on age, sex, or condition (diseased or healthy) since Klotho could be induced by exercise in any populations.
Search strategy and selection criteria
Two authors (TA and AR) independently reviewed published studies by searching the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE through PubMed, EMBASE, CINAHL, CT.gov, and PEDro from their inception through March 2021. All searches were adapted from the MEDLINE search strategy as reported: ("Exercise training” [MeSH]) AND ("klotho proteins" [MeSH). We reviewed the trials’ bibliographies, identifying and contacting some of the authors in the field to clarify trial eligibility or to identify additional published or unpublished data. Noteworthy, unpublished data was sought according to Young and Hopewell88.
Selection of the studies
Two review authors (TA and AR) independently checked the references identified by the search strategy. The full texts of all potentially relevant studies were obtained for independent assessment. Disagreements were solved through discussion, and a third review author (HLC) acted as arbitrator where necessary. All citations were downloaded into EndNote X9®, duplicates were removed, and an identification number was assigned to each article.
Data extraction
The same authors collected data in sufficient detail in order to better extract properties including studies based on PICO: Population: humans; Intervention: exercise training; Comparator, no exercise group; Outcome: Klotho response. We also extracted the associated benefits related to the Klotho response in each study. After extracting the data, two authors (TA and AR) graded the risk of bias in the included trials. Disagreements were resolved through discussion and a third reviewer (HLC) acted as moderator where necessary. Authors of primary studies did not extract data from their own studies. AR entered the data into the Software ReviewManager 5.4. (RevMan 5.4.) and HLC checked data entry. To plot results in graphs, WebPlotDigitizer v.4.1 (Austin, Texas, USA) software was used.
Assessment of risk of bias in the included studies
Two review authors independently assessed the risk of bias of all included studies. The assessment was according to the Cochrane Handbook for Systematic Reviews of Interventions86. The included studies were evaluated according to randomization sequence generation, allocation concealment, blinding (participants and outcomes), incomplete outcome data, selective outcome reporting, and other sources of bias. Disagreements were resolved by discussion between the two authors. The risk of bias was graded as “high risk of bias”, low risk of bias”, or “unclear risk of bias”.
Measures of exercise training effect and heterogeneity
Owing to the anticipated heterogeneity across studies due to different populations and conditions (health and diseases), we conducted a meta-analysis of random effects. The meta-analysis was performed with STATA 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). The same analyses were also performed in RevMan 5 to check the agreement between them and avoid errors. Due to the variety of kits used by the included studies to assess Klotho concentration, we used the standardized mean difference with corresponding 95% confidence intervals (CI) to calculate Hedges’ (adjusted) g. We took data from the post-training and post-control period. Studies with multiple treatment groups were split according to the type of intervention and analyzed as an independent study. Heterogeneity was assessed by visual inspection of the forest plots and by the I2 statistics86. As recommended, the I2 was interpreted as follows: 0–40% might not be important heterogeneity; 30–60% moderate heterogeneity; 50–90% substantial heterogeneity. P < 0.10 was adopted to point out statistically significant heterogeneity. The potential reasons for the heterogeneity were assessed through subgroup analysis.
Subgroup analysis
The subgroup analysis was performed with the variables that may influence the expression and production of molecular substances induced by exercise training. Therefore, one condition was the population: healthy vs. diseased patients. Another condition was the protocol of exercise training: aerobic vs. resistance vs. combined training. We also performed a cumulative analysis of the protocol duration (12 to 24 weeks) and the estimated volume (60 to 210 min per week) adopted in each study to verify the Klotho kinetics according to time.
Additional analysis
The mean values and deltas (post–pre) were plotted on GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California USA). A student-t test was performed comparing the deltas of Klotho concentrations in control group vs. exercise group. A three-way analysis of variance 2 × 2 × 2 (Intervention × Time × Condition) was performed to compare Klotho concentrations among healthy and diseased patients. A two-way mixed analysis of variance 2 × 2 (Intervention x Condition) was applied to compare the deltas of Klotho levels among groups. P < 0.05 was adopted for statistical significance. Furthermore, we constructed a table summarizing the main characteristics of the excluded studies (Supplementary Table 1).
References
Smyrnaeus, Q. The Fall of Troy Aeschylus, Prometheus Bound, 510–518: Theoi Project—Ananke "Theoi Project: Moirae and the Throne of Zeus". (1913).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. https://doi.org/10.1038/36285 (1997).
Forsberg, E. A., Olauson, H., Larsson, T. & Catrina, S. B. Effect of systemically increasing human full-length Klotho on glucose metabolism in db/db mice. Diabetes Res. Clin. Pract. 113, 208–210. https://doi.org/10.1016/j.diabres.2016.01.006 (2016).
Hu, M. C. & Moe, O. W. Klotho as a potential biomarker and therapy for acute kidney injury. Nat. Rev. Nephrol. 8, 423–429. https://doi.org/10.1038/nrneph.2012.92 (2012).
Hu, M. C. et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 78, 1240–1251. https://doi.org/10.1038/ki.2010.328 (2010).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123. https://doi.org/10.1074/jbc.C500457200 (2006).
Neyra, J. A. & Hu, M. C. Potential application of klotho in human chronic kidney disease. Bone 100, 41–49. https://doi.org/10.1016/j.bone.2017.01.017 (2017).
Tang, X. et al. Klotho: A tumor suppressor and modulator of the Wnt/β-catenin pathway in human hepatocellular carcinoma. Lab. Invest. J. Tech. Methods Pathol. 96, 197–205. https://doi.org/10.1038/labinvest.2015.86 (2016).
Yang, Y. L. et al. Long noncoding RNA NEAT1 is involved in the protective effect of Klotho on renal tubular epithelial cells in diabetic kidney disease through the ERK1/2 signaling pathway. Exp. Mol. Med. 52, 266–280. https://doi.org/10.1038/s12276-020-0381-5 (2020).
Lin, Y. & Sun, Z. In vivo pancreatic β-cell-specific expression of antiaging gene Klotho: A novel approach for preserving β-cells in type 2 diabetes. Diabetes 64, 1444–1458. https://doi.org/10.2337/db14-0632 (2015).
Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 389, 233–241. https://doi.org/10.1515/bc.2008.028 (2008).
Liu, F., Wu, S., Ren, H. & Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 13, 254–262. https://doi.org/10.1038/ncb2167 (2011).
Yamamoto, M. et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 280, 38029–38034. https://doi.org/10.1074/jbc.M509039200 (2005).
Kuro-o, M. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15, 27–44. https://doi.org/10.1038/s41581-018-0078-3 (2019).
Takenaka, T. et al. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. (Oxford, England) 225, 13190. https://doi.org/10.1111/apha.13190 (2019).
Hui, H. et al. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget 8, 15663–15676. https://doi.org/10.18632/oncotarget.14933 (2017).
Percy, C. J., Power, D. & Gobe, G. C. Renal ageing: Changes in the cellular mechanism of energy metabolism and oxidant handling. Nephrology (Carlton) 13, 147–152. https://doi.org/10.1111/j.1440-1797.2008.00924.x (2008).
Ravikumar, P. et al. α-Klotho protects against oxidative damage in pulmonary epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L566-575. https://doi.org/10.1152/ajplung.00306.2013 (2014).
Takenaka, T. et al. Klotho supplementation ameliorates blood pressure and renal function in DBA/2-pcy mice, a model of polycystic kidney disease. Am. J. Physiol. Renal Physiol. 318, F557-f564. https://doi.org/10.1152/ajprenal.00299.2019 (2020).
Zhang, H. et al. Klotho is a target gene of PPAR-gamma. Kidney Int. 74, 732–739. https://doi.org/10.1038/ki.2008.244 (2008).
Farzanegi, P., Dana, A., Ebrahimpoor, Z., Asadi, M. & Azarbayjani, M. A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 19, 994–1003. https://doi.org/10.1080/17461391.2019.1571114 (2019).
Spangenburg, E. E., Brown, D. A., Johnson, M. S. & Moore, R. L. Alterations in peroxisome proliferator-activated receptor mRNA expression in skeletal muscle after acute and repeated bouts of exercise. Mol. Cell. Biochem. 332, 225–231. https://doi.org/10.1007/s11010-009-0195-1 (2009).
Thomas, A. W. et al. Exercise-associated generation of PPARγ ligands activates PPARγ signaling events and upregulates genes related to lipid metabolism. J. Appl. Physiol. (Bethesda, Md.: 1985) 112, 806–815. https://doi.org/10.1152/japplphysiol.00864.2011 (2012).
Avin, K. G. et al. Skeletal muscle as a regulator of the longevity protein, Klotho. Front. Physiol. 5, 189. https://doi.org/10.3389/fphys.2014.00189 (2014).
Colaianni, G., Cinti, S., Colucci, S. & Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 1402, 5–9. https://doi.org/10.1111/nyas.13345 (2017).
Lenhare, L. et al. Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp. Gerontol. 97, 17–21. https://doi.org/10.1016/j.exger.2017.07.009 (2017).
Widmann, M., Nieß, A. M. & Munz, B. Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. (Auckland, NZ) 49, 509–523. https://doi.org/10.1007/s40279-019-01070-4 (2019).
Safdar, A., Saleem, A. & Tarnopolsky, M. A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 12, 504–517. https://doi.org/10.1038/nrendo.2016.76 (2016).
Safdar, A. & Tarnopolsky, M. A. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harbor Perspect. Med. https://doi.org/10.1101/cshperspect.a029827 (2018).
Lee, T. H. et al. Potential exerkines for physical exercise-elicited pro-cognitive effects: Insight from clinical and animal research. Int. Rev. Neurobiol. 147, 361–395. https://doi.org/10.1016/bs.irn.2019.06.002 (2019).
Yu, M., Tsai, S. F. & Kuo, Y. M. The therapeutic potential of anti-inflammatory exerkines in the treatment of atherosclerosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18061260 (2017).
Hawley, J. A., Joyner, M. J. & Green, D. J. Mimicking exercise: What matters most and where to next?. J. Physiol. 599, 791–802. https://doi.org/10.1113/jp278761 (2021).
Whitham, M. & Febbraio, M. A. The ever-expanding myokinome: Discovery challenges and therapeutic implications. Nat. Rev. Drug Discov. 15, 719–729. https://doi.org/10.1038/nrd.2016.153 (2016).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science (New York, NY) 309, 1829–1833. https://doi.org/10.1126/science.1112766 (2005).
Amaro-Gahete, F. J. et al. Exercise training increases the S-Klotho plasma levels in sedentary middle-aged adults: A randomised controlled trial. The FIT-AGEING study. J. Sports Sci. 37, 2175–2183. https://doi.org/10.1080/02640414.2019.1626048 (2019).
Corrêa, H. L. et al. Blood flow restriction training blunts chronic kidney disease progression in humans. Med. Sci. Sports Exerc. 53, 249–257. https://doi.org/10.1249/mss.0000000000002465 (2021).
Neves, R. V. P. et al. Dynamic not isometric training blunts osteo-renal disease and improves the sclerostin/FGF23/Klotho axis in maintenance hemodialysis patients: A randomized clinical trial. J. Appl. Physiol. (Bethesda, Md: 1985) 130, 508–516. https://doi.org/10.1152/japplphysiol.00416.2020 (2021).
Boeselt, T. et al. Benefits of high-intensity exercise training to patients with chronic obstructive pulmonary disease: A controlled study. Respir. Int. Rev. Thorac. Dis. 93, 301–310. https://doi.org/10.1159/000464139 (2017).
Fakhrpour, R. et al. Effect of sixteen weeks combined training on FGF-23, Klotho, and Fetuin-A levels in patients on maintenance hemodialysis. Iran. J. Kidney Dis. 14, 212–218 (2020).
Saghiv, M., Goldhammer, E. & Radzishevski, E. The impact of 12 weeks exercise training on circulating soluble-Klotho and pro-BNP in coronary artery disease patients. J. Cardiol. Vasc. Res. 1, 1–4 (2017).
Matsubara, T. et al. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 306, H348–H355. https://doi.org/10.1152/ajpheart.00429.2013 (2014).
Rahimi, S., Khademvatani, K. & Zolfaghari, M. R. Association of circular Klotho and insulin-like growth factor 1 with cardiac hypertrophy indexes in athlete and non-athlete women following acute and chronic exercise. Biochem. Biophys. Res. Commun. 505, 448–452. https://doi.org/10.1016/j.bbrc.2018.09.138 (2018).
Amaro-Gahete, F. J. et al. Role of exercise on S-Klotho protein regulation: A systematic review. Curr. Aging Sci. 11, 100–107. https://doi.org/10.2174/1874609811666180702101338 (2018).
Rosa, T. D. S. et al. Age-related decline in renal function is attenuated in master athletes. Int. J. Sports Med. https://doi.org/10.1055/a-1332-1594 (2021).
Rosa, T. S. et al. Sprint and endurance training in relation to redox balance, inflammatory status and biomarkers of aging in master athletes. Nitric Oxide Biol. Chem. 102, 42–51. https://doi.org/10.1016/j.niox.2020.05.004 (2020).
Amaro-Gahete, F. J. et al. Body composition and S-Klotho plasma levels in middle-aged adults: A cross-sectional study. Rejuvenation Res. 22, 478–483. https://doi.org/10.1089/rej.2018.2092 (2019).
Amaro-Gahete, F. J. et al. Association of physical activity and fitness with S-Klotho plasma levels in middle-aged sedentary adults: The FIT-AGEING study. Maturitas 123, 25–31. https://doi.org/10.1016/j.maturitas.2019.02.001 (2019).
Amaro-Gahete, F. J., De-la, O. A., Jurado-Fasoli, L., Ruiz, J. R. & Castillo, M. J. Association of basal metabolic rate and fuel oxidation in basal conditions and during exercise, with plasma S-klotho: The FIT-AGEING study. Aging 11, 5319–5333. https://doi.org/10.18632/aging.102100 (2019).
Amaro-Gahete, F. J. et al. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging 12, 2698–2710. https://doi.org/10.18632/aging.102771 (2020).
Crasto, C. L. et al. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res. 15, 295–301. https://doi.org/10.1089/rej.2011.1268 (2012).
Semba, R. D. et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: The InCHIANTI study. Eur. J. Appl. Physiol. 112, 1215–1220. https://doi.org/10.1007/s00421-011-2072-3 (2012).
Shardell, M. et al. Serum 25-hydroxyvitamin D, plasma Klotho, and lower-extremity physical performance among older adults: Findings from the InCHIANTI study. J. Gerontol. Ser. Biol. Sci. Med. Sci. 70, 1156–1162. https://doi.org/10.1093/gerona/glv017 (2015).
Santos-Dias, A. et al. Longevity protein klotho is induced by a single bout of exercise. Br. J. Sports Med. 51, 549–550. https://doi.org/10.1136/bjsports-2016-096139 (2017).
Mostafidi, E., Moeen, A., Nasri, H., GhorbaniHagjo, A. & Ardalan, M. Serum Klotho levels in trained athletes. Nephro-urology monthly 8, e30245. https://doi.org/10.5812/numonthly.30245 (2016).
Beckner, M. E. et al. Impact of simulated military operational stress on executive function relative to trait resilience, aerobic fitness, and neuroendocrine biomarkers. Physiol. Behav. 236, 113413. https://doi.org/10.1016/j.physbeh.2021.113413 (2021).
Ost, M., Coleman, V., Kasch, J. & Klaus, S. Regulation of myokine expression: Role of exercise and cellular stress. Free Radic. Biol. Med. 98, 78–89. https://doi.org/10.1016/j.freeradbiomed.2016.02.018 (2016).
Welc, S. S. & Clanton, T. L. The regulation of interleukin-6 implicates skeletal muscle as an integrative stress sensor and endocrine organ. Exp. Physiol. 98, 359–371. https://doi.org/10.1113/expphysiol.2012.068189 (2013).
Lushchak, V. I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 224, 164–175. https://doi.org/10.1016/j.cbi.2014.10.016 (2014).
Ji, L. L., Kang, C. & Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 98, 113–122. https://doi.org/10.1016/j.freeradbiomed.2016.02.025 (2016).
Calabrese, E. J., Iavicoli, I. & Calabrese, V. Hormesis: Its impact on medicine and health. Hum. Exp. Toxicol. 32, 120–152. https://doi.org/10.1177/0960327112455069 (2013).
Guo, Y. et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 238–251, 2018. https://doi.org/10.1016/j.bbadis.2017.09.029 (1864).
Ebert, T. et al. Inflammation and premature ageing in chronic kidney disease. Toxins. https://doi.org/10.3390/toxins12040227 (2020).
Izquierdo, M. C. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol. Dial. Transplant. 27(Suppl 4), 6–10. https://doi.org/10.1093/ndt/gfs426 (2012).
Agita, A. & Alsagaff, M. T. Inflammation, immunity, and hypertension. Acta Med. Indones. 49, 158–165 (2017).
Brunetta, H. S., Holwerda, A. M., van Loon, L. J. & Holloway, G. P. Exercise. Mitochondrial ROS and aging: Understanding exercise as a preventive tool. J. Sci. Sports Exerc. 2, 15–24 (2020).
El Assar, M., Angulo, J. & Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 65, 380–401. https://doi.org/10.1016/j.freeradbiomed.2013.07.003 (2013).
Lim, K. et al. α-Klotho expression in human tissues. J. Clin. Endocrinol. Metab. 100, E1308-1318. https://doi.org/10.1210/jc.2015-1800 (2015).
Papaconstantinou, J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells https://doi.org/10.3390/cells8111383 (2019).
Kotoku, K. et al. Effect of exercise intensity on renal blood flow in patients with chronic kidney disease stage 2. Clin. Exp. Nephrol. 23, 621–628. https://doi.org/10.1007/s10157-018-01685-3 (2019).
Lacerda, L. T. et al. Variations in repetition duration and repetition numbers influence muscular activation and blood lactate response in protocols equalized by time under tension. J. Strength Cond. Res. 30, 251–258. https://doi.org/10.1519/jsc.0000000000001044 (2016).
Burd, N. A. et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J. Physiol. 590, 351–362. https://doi.org/10.1113/jphysiol.2011.221200 (2012).
Morton, R. W. et al. Muscle fibre activation is unaffected by load and repetition duration when resistance exercise is performed to task failure. J. Physiol. 597, 4601–4613. https://doi.org/10.1113/jp278056 (2019).
Araneda, O. F., Contreras-Briceño, F., Cavada, G. & Viscor, G. Swimming versus running: Effects on exhaled breath condensate pro-oxidants and pH. Eur. J. Appl. Physiol. 118, 2319–2329. https://doi.org/10.1007/s00421-018-3958-0 (2018).
Millet, G. P., Vleck, V. E. & Bentley, D. J. Physiological differences between cycling and running: Lessons from triathletes. Sports Med. (Auckland, N.Z.) 39, 179–206. https://doi.org/10.2165/00007256-200939030-00002 (2009).
Vargas-Ortiz, K. et al. Aerobic training but no resistance training increases SIRT3 in skeletal muscle of sedentary obese male adolescents. Eur. J. Sport Sci. 18, 226–234. https://doi.org/10.1080/17461391.2017.1406007 (2018).
McGuire, D. K. et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 104, 1350–1357 (2001).
Bullock, G. S. et al. Methods matter: Clinical prediction models will benefit sports medicine practice, but only if they are properly developed and validated. Br. J. Sports Med. https://doi.org/10.1136/bjsports-2021-104329 (2021).
Cook, C. Predicting future physical injury in sports: It’s a complicated dynamic system. Br. J. Sports Med. 50, 1356–1357. https://doi.org/10.1136/bjsports-2016-096445 (2016).
Kox, L. S., Kuijer, P. P., Kerkhoffs, G. M., Maas, M. & Frings-Dresen, M. H. Prevalence, incidence and risk factors for overuse injuries of the wrist in young athletes: A systematic review. Br. J. Sports Med. 49, 1189–1196. https://doi.org/10.1136/bjsports-2014-094492 (2015).
Takeda, H., Nakagawa, T., Nakamura, K. & Engebretsen, L. Prevention and management of knee osteoarthritis and knee cartilage injury in sports. Br. J. Sports Med. 45, 304–309. https://doi.org/10.1136/bjsm.2010.082321 (2011).
Ahrens, H. E., Huettemeister, J., Schmidt, M., Kaether, C. & von Maltzahn, J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet. Muscle 8, 20. https://doi.org/10.1186/s13395-018-0166-x (2018).
Gu, Y., Ren, K., Wang, L. & Yao, Q. Loss of Klotho contributes to cartilage damage by derepression of canonical Wnt/β-catenin signaling in osteoarthritis mice. Aging 11, 12793–12809. https://doi.org/10.18632/aging.102603 (2019).
Welc, S. S., Wehling-Henricks, M., Kuro, O. M., Thomas, K. A. & Tidball, J. G. Modulation of Klotho expression in injured muscle perturbs Wnt signalling and influences the rate of muscle growth. Exp. Physiol. 105, 132–147. https://doi.org/10.1113/ep088142 (2020).
Phelps, M., Pettan-Brewer, C., Ladiges, W. & Yablonka-Reuveni, Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 14, 729–739. https://doi.org/10.1007/s10522-013-9447-2 (2013).
Sahu, A. et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 9, 4859. https://doi.org/10.1038/s41467-018-07253-3 (2018).
Higgins, J. P. et al. Cochrane Handbook for Systematic Reviews of Interventions (Wiley, 2019).
Shamseer, L. P. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ (Clin. Res. Ed.) 350(g7647), 2015. https://doi.org/10.1136/bmj.g7647 (2015).
Young, T. & Hopewell, S. Methods for obtaining unpublished data. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.MR000027.pub2 (2011).
Acknowledgements
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001. To the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq).
Author information
Authors and Affiliations
Contributions
H.L.C., H.S.B., R.V.P.N., P.L.R.S. and T.S.R. wrote the manuscript; A.T.O.R., T.M.A., A.L.R., F.S.H., M.A.S.M., O.L.F. and L.A.D. contributes in data collection, data interpretation and English review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corrêa, H.d., Raab, A.T.O., Araújo, T.M. et al. A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine. Sci Rep 12, 17587 (2022). https://doi.org/10.1038/s41598-022-22123-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22123-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.