Abstract
Guidelines have shifted to now recommend endoscopic eradication therapy for Barrett’s esophagus (BE) with low and high-grade dysplasia. Previously, esophagectomy was the standard therapy for high-grade dysplasia. However, it is unclear to what degree ablation therapy has affected utilization of esophagectomy. In this retrospective observational cohort study of BE patients without cancer from the Premier Healthcare Database, the prevalence of utilization of endoscopic ablation therapy and of esophagectomy in BE were calculated and temporal trends were evaluated. A total of 938, 333 BE cases were included in the study. There was a significantly increasing trend of ablation over the period 2006 to 2010 (Annual Percentage Change (APC); 95% CI 0.56% [0.51%, 0.61%]), a significantly decreasing trend for the period 2011 to 2015 (APC; 95% CI − 0.15% [− 0.20%, − 0.11%]), and a shallow increasing trend for the period 2016 to 2019 (APC; 95% CI 0.09% [0.06%, 0.11%]). For esophagectomy, there was a significantly decreasing trend for the period 2006 to 2009 (APC; 95% CI − 0.03% [− 0.04%, − 0.02%]; P < 0.001) that corresponded to the uptrend in utilization of endoscopic ablation. There was a stable trend of esophagectomy over the period 2010 to 2019 (APC; 95% CI − 0.0006% [− 0.0002%, 0.0005%]; P = 0.1947). Adoption and increased utilization of endoscopic ablation therapy for BE has coincided with a decrease in esophagectomy, and is the predominate method of therapy for BE with dysplasia.
Similar content being viewed by others
Introduction
Barrett’s esophagus (BE) is a precancerous condition in which normal squamous esophageal mucosa is replaced by specialized intestinal metaplasia1. It is a well-known risk factor for esophageal adenocarcinoma (EAC). BE is prevalent in 1–2% of the population. BE is thought to develop into cancer in a step-wise fashion from low-grade dysplasia to high-grade dysplasia, and eventually to EAC2. Endoscopic eradication therapy (ERT) is indicated for patients with dysplasia and superficial cancer (T1a)1,3. ERT consists of endoscopic resection and ablation. Endoscopic resection is indicated for raised superficial lesions followed by ablation of the remaining Barrett’s segment. Currently available ablation platforms are radiofrequency ablation (RFA) and cryotherapy3,4,5. Currently, esophagectomy is reserved for patients with cancer staged T1b (sm2 and 3) and higher, dysplastic BE not amenable to endoscopic therapy (i.e. bulky raised disease not amenable to endoscopic resection), or persistent dysplastic BE: ractory to endoscopic therapy. However, prior to the development of ERT, patients with high-grade dysplasia were also treated with esophagectomy6.
Although esophagectomy is a definitive way of curing BE and associated dysplasia, it can bring considerable morbidity and mortality. The mortality rate is 2% and the morbidity rate is 10%7,8,9. Operative complications can include anastomotic leaks, respiratory complications, and swallowing problems. Thus, avoiding esophagectomy is an advantage of ERT. It should be noted that outcomes of esophagectomy have never been compared to outcomes to ERT in a prospective randomized fashion. Given the comorbidities associated with esophagectomy, such a trial is not expected7.
The goal of endoscopic ablation is for complete eradication of intestinal metaplasia to prevent the formation of cancer1. Prospective studies have shown that ablation therapy after removal of raised lesions by endoscopic resection can prevent the progression to cancer in dysplastic BE3,4,5. This should correlate to a decrease in esophagectomy rates. However, there is limited real-world data examining esophagectomy rates for therapy in BE and it’s comparison during pre-ERT to post ERT eras. It is unclear if the esophagectomy rate for BE is truly declining. The aim of this study is to compare pre-ERT to post-ERT esophagectomy utilization in comparison to ablation therapy utilization in a large healthcare database.
Methods
Study design and data source selection
We conducted a retrospective observational cohort study using the Premier Healthcare Database (PHD, Charlotte, North Carolina, USA). Informed consent from each patient in the database is not possible due to lack of no identifying information available. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
The PHD is a large, U.S. hospital-based, service-level, all-payer database. Inpatient admission data include over 127 million visits with more than 11 million per year since 2012, representing approximately 25 percent of annual U.S. inpatient admissions. Outpatient encounters include over 947 million outpatient visits with more than 102 million visits per year since 2012. Outpatient visits to emergency departments, ambulatory surgery centers and alternate sites of care are included. The PHD contains data from over 244 million unique patients. Patients can be tracked in the same hospital across the inpatient and hospital-based outpatient settings. There are more than 700 hospitals providing data yearly since 201210. In terms of data quality, for most data elements, less than 1% of patient records have missing information, and for key elements, such as demographics and diagnostic information, less than 0.01% have missing data11.
This database was chosen for its scope, as it includes both inpatients and outpatients. This allows for estimation of the prevalence of (1) all BE patients in a single year within the database; (2) endoscopic ablation procedures (usually outpatient procedures); and (3) surgical esophagectomies (which require an inpatient recovery). The underlying diagnosis of BE in the database population was integral to determine the utilization rate of endoscopic ablation and esophagectomy in BE.
Based on data availability, 14 years (2005–2019) of PHD data were used. The PHD is considered exempt from Institutional Review Board oversight as dictated by Title 45 Code of Federal Regulations, Part 46 of the United States, specifically 45 CFR 46.101(b)(4). In accordance with the HIPAA Privacy Rule, disclosed data from Premier are considered de-identified per 45 CFR 164.506(d)(2)(ii)(B) through the “Expert Determination” method. The study was exempt from the Zucker School of Medicine at Hofstra/Northwell institutional board review due to the database containing no patient identifiable information. The need for informed consent was waived by the Zucker School of Medicine at Hofstra/Northwell institutional board review due to the retrospective nature of the study and lack of patient identifying information in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Cohort selection
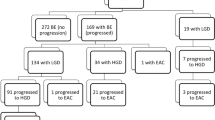
To calculate the prevalence of utilization of ERT and esophagectomy among patients with BE, we included all adult patients who were diagnosed with BE each year of the study period. A patient with BE was identified by using International Statistical Classification of Diseases the 9th revision (ICD-9-CM) or the 10th revision (ICD-10-CM) diagnosis codes of BE (Appendix 1). ERT was identified by using the Current Procedural Terminology (CPT®) codes (Appendix 1). Esophagectomy was identified by using International Statistical Classification of Diseases the 9th revision (ICD-9-CM) or the 10th revision (ICD-10-PCS) surgical codes. We only included patients with a primary diagnosis of BE for these procedures. Billing codes for radiofrequency ablation and cryotherapy were not used as they are the same and thus cannot be differentiated within a dataset.
A patient with BE was counted once during a one-year period. The same patient could be counted again in subsequent years if this patient had at least one visit with a BE diagnosis during that year and met selection criteria. To this end, we could capture all BE patients who could potentially receive ERT or esophagectomy during the study period. When a patient had multiple hospital visits during a one-year period, the first visit that had a BE diagnosis was considered the index diagnosis. CY2006 was the first year in which an index BE diagnosis was made. We used the first year’s available data (CY2005) as a buffer to apply to our cohort selection criteria. Patients were excluded if they (1) had a diagnosis of esophageal or stomach cancer 6 months prior to and during the year of index diagnosis; (2) had an esophageal ablation procedure within 12 months prior to index diagnosis; (3) had an endoscopic resection on the same day as an esophageal ablation procedure. The rationale for these exclusion criteria are as follows: (1) early versus advanced esophageal or gastric cancer could not be differentiated in this database, and as curative ablation therapy is inappropriate for advanced cancer, only patients with BE and low or high grade dysplasia without cancer were included; (2) ERT for BE should be performed only after the index diagnosis is made; (3) endoscopic resection and ablation for BE do not usually occur on the same day.
Outcome measures
The primary outcome was to determine the utilization of esophageal ablation and esophagectomy among patients with BE without cancer in the United States. The prevalence of ERT and of esophagectomy were evaluated on a yearly basis during the study period. The secondary outcome was to identify differences in patient demographics and hospital characteristics among BE patients who underwent ERT versus those who underwent esophagectomy.
Statistical analysis
Univariate analysis was used to compare patient demographics and hospital characteristics. Chi-square test was used for categorical variables. The prevalence and associated 95% CIs in utilization of ablation and esophagectomy in each year was calculated. To evaluate temporal trends in utilization of ablation, both the unadjusted relative risk (RR) and adjusted relative risk (aRR) for annual change were calculated, as linear trends in utilization of ablation and esophagectomy across the years of our study period were not observed. These analyses were performed using generalized estimating equation (GEE) models with a robust covariance estimator to adjust for provider (hospital) clustering effect. The index BE diagnosis year was treated as a categorical variable and the year with the highest prevalence of utilization of the procedure was selected as a reference year (for ERT - CY2010, for esophagectomy - CY2006).
To calculate the aRR of ablation, a priori–specified covariates were used, including age, sex, race, marital status, health insurance type, hospital bed size, regions, teaching hospital and urban hospital. To assess temporal trends in utilization of esophagectomy, the adjusted relative risk (aRR) was calculated by adjusting for provider (hospital) clustering effect only. Due to the extremely low number of esophagectomy cases in the study cohort, a best-fit model with the priori–specified covariates was not able to be found. Statistical significance was two-tailed and was set at P < 0.05. Data preparation and analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary NC, USA.
Results
A total of 938, 333 patients with BE were included in the study. The overall prevalence of ERT and esophagectomy for BE was 2018 per 100,000 and 20 per 100,000 BE patients, respectively (Table 1). The use of ERT increased from 626 (95% CI 530–735) per 100,000 BE patients in 2006 to 2019 (95% CI 1983–2158) per 100,000 BE patients in 2019. Overall, there was a significantly increasing trend for ERT over the period 2006 to 2010 (Annual Percentage Change (APC); 95% CI 0.56% [0.51%, 0.61%]), a significantly decreasing trend for the period 2011 to 2015 (APC; 95% CI − 0. 15% [− 0.20%, − 0.11%]), and a shallow increasing trend for the period 2016 to 2019 (APC; 95% CI 0.09% [0.06%, 0.11%]) (Fig. 1).
During the same time period of increased utilization of ERT, the utilization of esophagectomy decreased from 104 (95% CI 68–154) per 100,000 BE patients in 2006 to 7 (95% CI 3–14) per 100,000 BE patients in 2019.
Overall, there was a significantly decreasing trend for esophagectomy for the period 2006 to 2009 (APC; 95% CI − 0.03% [− 0.04%, − 0.02%]; P < 0.001), which corresponded to the uptrend in utilization of ERT. There was a stable trend for esophagectomy over the period 2010 to 2019 (APC; 95% CI − 0.0006% [− 0.0002%, 0.0005%]; P = 0.1947) (Table 2 and Fig. 1).
Patient and hospital characteristics of each group are summarized in Table 3. Patients with BE who underwent ERT were more likely to be between 55 and 64 years old (25.4% vs. 27.9%, P < 0.0001), male (57.5% vs. 71.9%, P < 0.0001), Hispanic (3.5% vs. 4.1%, P < 0.0001), married (54.3% vs. 61.8%, P < 0.0001), and have commercial health insurance (33.3% vs. 40.4%, P < 0.0001) than patients with BE that did not undergo ERT. Furthermore, patients treated in hospitals with 500 or more beds (25.8% vs. 42.4, P < 0.0001), who lived in the Southern region (38.5% vs. 49.2%, P < 0.0001) and were treated at a teaching hospital (43.1% vs. 48.3%, P < 0.0001) were also more likely to undergo esophagus ablation than no ERT.
Patients with BE who underwent esophagectomy were more likely to be between 55 and 64 years old (P = 0.0036), male (57.7% vs. 74.0%, P < 0.0001), and have commercial health insurance (33.4% vs. 44.2%, P = 0.0095) than patients with BE that did not undergo esophagectomy. Patients who were treated in hospitals with 500 or more beds (26.1% vs. 58.9, P < 0.0001), who lived in the Southern region (38.7% vs. 46.4%, P = 0.0077) and were treated at a teaching hospital (43.2% vs. 68.2%, P < 0.0001) were also more likely to have an esophagectomy than no esophagectomy (Table 3).
Finally, patients with BE who were between 35 and 54 years old (P = 0.0022), had Medicaid health insurance (4.4% vs. 9.9%, P = 0.0022), were treated in hospitals with 500 or more beds (42.4% vs. 58.9%, P < 0.0001), who lived in the Northeast region (10.8% vs. 20.3%, P = 0.0003) and were treated at a teaching hospital (48.3% vs. 68.2%, P < 0.0001) were more likely to have esophagectomy than to undergo ERT.
Discussion
In this study we examined the utilization trends in endoscopic ablation and esophagectomy for management of BE without cancer. We found that increased utilization of ERT correlated with a decrease in the utilization of surgical esophagectomy. Based on these trends, it can be inferred that endoscopic ablation therapy has helped decrease utilization of surgical esophagectomy for management of BE without cancer. To our knowledge this is the first study that has shown these trends. Although it was previously assumed that ablation therapy decreased esophagectomy rates, it was unclear if this truly was the case as real world practices in the management of BE were not previously assessed.
We found that the increased use of ERT occurred primarily between 2007 and 2010. While the pivotal trial that demonstrated the success of RFA in the treatment of BE was formally published in 20093, the interim results were initially presented in 2008. In addition, there was a multicenter US registry on the use of RFA that was published in 200812. Use of RFA for the treatment of BE began prior to these publications and the trials began in 2007, which corresponds to the observed increased uptick of ERT in this study period.
This study also aimed to examine if there were any patient or hospital factors that predicted the use of ERT versus esophagectomy for management of BE without cancer. We found similar patient characteristics for both patients undergoing ERT and esophagectomy, likely reflecting the typical patient population that requires therapy for BE (male, middle aged, from the southern region, treated in teaching hospitals, and those with commercial insurance). However, we did find some risk factors that did predispose patients to esophagectomy over endoscopic ablative therapy. These included younger patients (age 35–54), patients with Medicaid insurance, those treated in larger hospitals (> 500 beds), and treatment in the Northeast region. Younger patients are generally better surgical candidates, and thus this may be the reason for this risk factor. It is unclear why the other risk factors predispose to esophagectomy. Without individual patient details, it is difficult to examine why these may be risk factors.
It should be noted that although ERT decreases the need for upfront surgery, esophagectomy is still part of the armamentarium in the management of BE. Esophagectomy still should be utilized for patients with refractory strictures either intrinsic to BE or iatrogenic due to endoscopic therapy; for dysplastic BE refractory to an array of ablation modalities, or for bulky raised BE not amenable to resection. Our data shows that surgery is still being utilized, although at a low rate (Fig. 1, Table 1). Furthermore, although ERT is safe, there are still small risks associated with it including bleeding, perforation, and stricture formation. ERT also requires multiple sessions to eradicate the BE and requires ongoing endoscopic surveillance after complete eradication, and carries the risk of interval cancers. Although this study shows the positive effects of endoscopic ablation therapy of BE in the United States, the rate of esophageal cancer continues to rise in the United States13,14. In fact, of about 10,000 esophageal adenocarcinomas diagnosed each year in the USA, only about 7% are identified through current screening approaches13. Most esophageal adenocarcinoma is being diagnosed at a late stage in which ERT or esophagectomy cannot be offered to the patient. Thus, improved screening tools and non-invasive devices are needed to better screen for BE with dysplasia15,16,17,18,19,20,21. Endoscopic ablation therapy will not be fully utilized to its potential without enhancing our approach to better screen for Barrett’s with dysplasia.
Our study has several strengths. It uses a large database of BE patients that capture both inpatient and outpatient billing procedure codes and diagnoses; in addition, patients can be tracked between procedures. Thus we are able to estimate utilization of both ERT and esophagectomy for BE. We use the same database to determine the number of patients diagnosed with BE each year to estimate esophagectomy and ERT rates rather than report the crude number of procedures performed for each modality. This allows for a more accurate estimation of endoscopic or surgical therapy utilization for management of BE. In addition, we exclude cancer cases to truly gauge how BE with low-grade and high-grade dysplasia is being managed. Including cancer cases in the dataset would create a heterogeneous group, as the dataset cannot differentiate between superficial (T1a) and invasive (T2 and above) esophageal cancer. In addition, the dataset cannot differentiate between squamous cell cancer and adenocarcinoma (the cancer for which BE is the major risk factor); and is thus another reason to exclude BE patients with cancer.
Our study does have limitations. This study estimates the utilization trends of ERT and esophagectomy using one large database. It does not capture every procedure in the USA and thus does not report on the incidence of these procedures. In addition, like any study of this nature, it relies on accurate billing and diagnosis codes. Finally, the database cannot distinguish between BE with dysplasia and nondysplastic BE. It is assumed that most BE without cancer undergoing therapy is dysplastic BE per the national guidelines1,22. In addition, the database cannot distinguish between radiofrequency ablation and other forms of ablation therapy (e.g., cryotherapy and photodynamic therapy) as they are all billed under the same code. Although RFA is the most utilized ablation therapy and is currently the only recommended therapy in the guidelines for eradication of dysplastic BE, photodynamic therapy was the first ablation modality1,22,23.
In conclusion, BE ablative therapy has been shown to be an effective tool to eradicate dysplasia3,5. This study shows that with increasing ablation therapy utilization, the rates of esophagectomy for BE decreased. As discussed, further efforts need to be aimed at better screening for BE with dysplasia to utilize the full potential of ablation therapy in BE.
Data availability
The dataset used for this study will be provided upon request as allowed per Premier Database user guidelines. Requests can be directed to the corresponding author.
Abbreviations
- BE:
-
Barrett’s esophagus
- APC:
-
Annual percentage change
- EAC:
-
Esophageal adenocarcinoma
- RFA:
-
Radiofrequency ablation
- PHD:
-
Premier Health Database
References
Shaheen, N. J. et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 111(1), 30–50 (2016) (quiz 51).
Qumseya, B. J. et al. Disease progression in Barrett’s low-grade dysplasia with radiofrequency ablation compared with surveillance: systematic review and meta-analysis. Am. J. Gastroenterol. 112(6), 849–865 (2017).
Shaheen, N. J. et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 360(22), 2277–2288 (2009).
Phoa, K. N. et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 311(12), 1209–1217 (2014).
Canto, M. I. et al. Multifocal cryoballoon ablation for eradication of barrett’s esophagus-related neoplasia: a prospective multicenter clinical trial. Am. J. Gastroenterol. 115(11), 1879–1890 (2020).
Wani, S. & Sharma, P. Another strike against esophagectomy for high-grade dysplasia in Barrett’s esophagus?. Clin. Gastroenterol. Hepatol. 6(2), 128–129 (2008).
Wani, S. & Sharma, P. Challenges with endoscopic therapy for Barrett’s esophagus. Gastroenterol. Clin. N. Am. 44(2), 355–372 (2015).
Wani, S. et al. Management of high-grade dysplasia and intramucosal adenocarcinoma in Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 10(7), 704–711 (2012).
Bennett, C. et al. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 143(2), 336–346 (2012).
Incorporated, P. Premier healthcare database white paper: data that informs and performs. Prem. Appl. Sci. 2, 1–15 (2020).
Fisher, B. T., Lindenauer, P. K., Feudtner, C. In-hospital databases, in Pharmacoepidemiology 244–258 (Wiley-Blackwell, 2012). https://doi.org/10.1002/9781119959946.ch16.
Ganz, R. A. et al. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. multicenter registry. Gastrointest. Endosc. 68(1), 35–40 (2008).
Vaughan, T. L. & Fitzgerald, R. C. Precision prevention of oesophageal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 12(4), 243–248 (2015).
Peters, Y. et al. Barrett oesophagus. Nat. Rev. Dis. Prim. 5(1), 35 (2019).
Rubenstein, J. H. et al. Prediction of Barrett’s esophagus among men. Am. J. Gastroenterol. 108(3), 353–362 (2013).
Rubenstein, J. H. et al. Validation and comparison of tools for selecting individuals to screen for Barrett’s esophagus and early neoplasia. Gastroenterology 158(8), 2082–2092 (2020).
Rubenstein, J. H. & Thrift, A. P. Risk factors and populations at risk: selection of patients for screening for Barrett’s oesophagus. Best Pract. Res. Clin. Gastroenterol. 29(1), 41–50 (2015).
Fitzgerald, R. C. et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet (London, England). 396(10247), 333–344 (2020).
Moinova, H. R. et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 10(424), eaao5848 (2018).
Iyer, P. G. et al. Validation of a methylated DNA marker panel for the nonendoscopic detection of Barrett’s esophagus in a multisite case-control study. Gastrointest. Endosc. 94, 498–505 (2021).
Sami, S. S. et al. Comparative cost effectiveness of reflux-based and reflux-independent strategies for Barrett’s esophagus screening. Am. J. Gastroenterol. 116, 1620 (2021).
Sharma, P. et al. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: expert review. Gastroenterology 158(3), 760–769 (2020).
Weusten, B. et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 49(2), 191–198 (2017).
Author information
Authors and Affiliations
Contributions
The conception and design was by A.J.T.; The analysis and interpretation of the data was by A.J.T., J.Z., J.Q., and J.H.; Drafting of the article was by A.J.T., J.Z., K.R.; Critical revision of the article for important intellectual content was by A.J.T., J.Z., K.R., J.Q., J.H., and P.C.B.; Final approval of the article was by A.J.T., J.Z., K.R., J.Q., J.H., and P.C.B.; All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
AJT is a consultant for Pentax Medical. JZ is an employee of Medtronic. JQ is an employee of Medtronic. JH is an employee of Medtronic. PCB is a consultant for Medtronic, Olympus America, Apollo Overstitch, Creo Medical. KR has no conflicts of interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trindade, A.J., Zhang, J., Raphael, K.L. et al. Utilization trends for endoscopic ablation therapy and esophagectomy in Barrett’s esophagus from 2005 to 2019. Sci Rep 12, 17619 (2022). https://doi.org/10.1038/s41598-022-21838-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21838-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.