Abstract
“Blue carbon” wetland vegetation has a limited freshwater requirement. One type, mangroves, utilizes less freshwater during transpiration than adjacent terrestrial ecoregions, equating to only 43% (average) to 57% (potential) of evapotranspiration (\(ET\)). Here, we demonstrate that comparative consumptive water use by mangrove vegetation is as much as 2905 kL H2O ha−1 year−1 less than adjacent ecoregions with \({E}_{c}\)-to-\(ET\) ratios of 47–70%. Lower porewater salinity would, however, increase mangrove \({E}_{c}\)-to-\(ET\) ratios by affecting leaf-, tree-, and stand-level eco-physiological controls on transpiration. Restricted water use is also additive to other ecosystem services provided by mangroves, such as high carbon sequestration, coastal protection and support of biodiversity within estuarine and marine environments. Low freshwater demand enables mangroves to sustain ecological values of connected estuarine ecosystems with future reductions in freshwater while not competing with the freshwater needs of humans. Conservative water use may also be a characteristic of other emergent blue carbon wetlands.
Similar content being viewed by others
Introduction
Water will be among the most limiting natural resource of the future, influencing ecological flows among coastal environments and soon affecting at least 80% of the world’s population directly1,2. Competition for water between humans and natural ecosystems is critical in driving outcomes for natural ecosystem health, and water supply is projected to increasingly shift toward human use3. Selection of “nature-based solutions” to sequester carbon or enhance climate resilience could simultaneously consider the water economy of the ecosystem doing the work. To that end, “blue carbon” wetlands may have emergent but yet unrecognized ecological value.
Blue carbon wetlands comprise a variety of coastal ecosystems – most notably mangroves, saltmarshes and seagrasses4 – but also include upper estuarine tidal wetlands, and adjacent ecosystems such as salt flats and macroalgal communities5. As a group, they provide tremendous value to human societies6,7. Among these, blue carbon wetlands store large amounts of carbon in aboveground biomass and in soils as their roots trap sediments and expand soil volumes8,9. As a result, blue carbon wetlands have increasingly become important for environmental management of coasts and for use as self-sustaining nature-based solutions in adapting coastlines to rising seas10.
Mangrove vegetation is located within the intertidal zone11 and tolerates but does not always require saline water12 (but see ref.13). Mangroves extract freshwater from ultrafiltration of seawater through root tissue14,15 and consume freshwater differentially when it is readily available (groundwater, rainfall at low tide)16,17. Once water is absorbed by roots and the metabolic costs of excluding salt are incurred, bulk water transport from roots-to-leaves is driven by pressure gradients created as water is transpired18. Especially under saline soil conditions, mangroves have been recognized as having high plasticity in adjusting their rates of leaf-level water use efficiency to accommodate osmotic gradients19.
Of 214 evaluations (number of species × number of studies) of leaf-level water-use efficiency published over the last three decades (source: Scopus/Web of Science), most confirm what was originally presented from a 1989 field study20, that intrinsic leaf-level photosynthetic water use efficiency (\({WUE}_{int}\): ratio of leaf-level net photosynthetic rate to stomatal conductance to water vapor) becomes higher as the salinity of water within mangrove roots increases (Fig. S1). This relationship is seemingly amplified by high atmospheric vapor pressure deficit; however, leaf-level marginal water costs [i.e., the ratio of change in actual water used (water cost) vs. change in photosynthetic rate (carbon gained)] are not strongly influenced by atmospheric moisture or temperature changes over diel cycles for mangroves21. Nevertheless, high rates of water use efficiency at the leaf-level have promoted the idea that mangroves are able to use less water as stress gradients increase. Indeed, salinity is one of those stressors that affect both \({WUE}_{int}\) and marginal water costs in ways that may also influence water use by whole trees and canopies.
In this study, we assess whether one type of blue carbon wetland – mangroves – may be particularly efficient in the amount of water they use at leaf, tree, and ecosystem scales during the process of atmospheric carbon capture15. Mangroves can, in many locations, grow with limited freshwater availability17, their roots extracting freshwater from seawater14, and they can obtain some freshwater directly through foliage22,23,24,25. We explore three questions. First, are leaf-level and tree-level water use strategies in mangroves similarly conservative? Second, how much water does mangrove vegetation use? Third, is mangrove vegetation water use low compared to water use from non-wetland ecosystems (e.g., terrestrial forests) or from vegetation that arises from the conversion of mangroves to alternative land uses (e.g., oil palm plantations)? We answer these questions by exploring a pulse of recent studies and model development that allow insight across scales.
Results
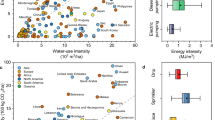
Mangrove water use efficiency is reported over a range of scales; as long-term, instantaneous, or intrinsic, and at the leaf, plant, or ecosystem level, each necessitating a range of assumptions. This 3 × 3 matrix of scale is dominated in the mangrove literature by leaf-level \({WUE}_{int}\), with fewer assessments of water use efficiency at other spatial or temporal scales. In our analysis, median \({WUE}_{int}\) of terrestrial woody plant species ranged from 57 μmol CO2 (mol H2O)−1 from dry sub-humid environments to 88–95 μmol CO2/(mol H2O) in arid and semi-arid environments (Fig. 1a). Median \({WUE}_{int}\) for mangrove leaves was 67 μmol CO2/(mol H2O)−1 (range, 10 – 212) which is only slightly higher than shrubs and trees from dry sub-humid environments (p < 0.05, Dunn’s ranked sums).
Leaf-intrinsic water use efficiency ( \({WUE}_{int}\) ) and individual tree water use for mangroves versus terrestrial woody vegetation. (a) \({WUE}_{int}\) for a mix of seedlings, saplings, and trees of upland species from arid, semi-arid, dry semi-arid, and humid environments60 versus mangrove, depicting median (center line and values), upper and lower quartiles (box limits), 1.5 × interquartile ranges (whiskers), and outliers (points) as box-and-whisker plots, and sample sizes depicted within parentheses. (b) Individual tree water use (median) from sap flow studies conducted on mangrove trees relative to diameter at breast height (dbh), with comparison to upland trees (from ref.61).
However, lack of differentiation among comparative water use efficiencies at the leaf-level did not scale. Instead, upland forest trees used, on average, 3.5 L H2O day−1 cm−1 of dbh (diameter at breast height, ~ 1.3 m above ground) while mangrove trees used only 1.4 L H2O day−1 cm−1 of dbh (Fig. 1b) (statistical Q = 6.06, p < 0.001, Dunn’s ranked sums). Therefore, from sap flow-derived individual tree water flux data, we can begin to understand organizational scales at which transpirational water loss is most limited from mangrove vegetation.
Scaling further to the canopy, we discovered that slopes of relationships relating canopy transpiration to net primary productivity (\({E}_{c}\)-to-NPP) for mangroves were similar between locations in China and the US (Fig. S2), despite being developed on sites separated by half the globe. Furthermore, slopes of both relationships differed significantly from zero (p < 0.001; r2 ≥ 0.955) suggesting the potential to use NPP of focal mangrove forests to estimate \({E}_{c}\) where data on \({E}_{c}\) were unavailable. We developed the following equation:
where \({E}_{c}\) is in units of mm H2O year−1 and NPP is in units of kg C m-2 year−1. Note that 1 mm H2O is equivalent to 1 L H2O per square meter of ground area.
Using data from 26 published records reporting on 71 mangrove study sites located in the Florida-Caribbean Region (N = 24) and the Asia–Pacific Region (N = 47) that had sufficient data to derive approximate NPP values, we used Eq. (1) to estimate what \({E}_{c}\) might be in order to support estimated NPP for those 71 locations (Fig. 2). Average \({E}_{c}\)-to-\(ET\) ratios for mangroves was 43.4% (± 3.0%, S.E.) when we assume \({E}_{c}\) from sites under study-reported environmental conditions, but as high as 57.4% (± 4.0%, S.E.) when we consider the potential for higher \({E}_{c}\) at sites with lower porewater salinity.
Location of 71 mangrove study sites from the Florida-Caribbean and Indo-Pacific regions from which net primary productivity (NPP, kg C m-2 year−1) data were used to determine canopy transpiration (\({E}_{c}\)). Polygons (pink) over the oceans represent 20 °C summer and winter isotherms influencing mangrove distributions (updated from Duke et al. 1998, Glob. Ecol. Biogeogr. Letts., v. 7, p. 27–47). Insets represent comparative average \({E}_{c}\), \(ET\), and NPP values for litter, wood, and roots assuming a continuous mangrove coverage versus \(ET\) at a scale of 1 km2 for each of the two regions. Box plot depictions are the same as in Fig. 1. Base image created using ArcMAP 10 (Esri, Inc., Redlands, California, USA), https://desktop.arcgis.com/en/arcmap/ .
Mangrove \({E}_{c}\)-to-\(ET\) ratios were approximately 27% less than that of tropical rainforests and about 4% less than that of Mediterranean shrublands (Table 1). Thus, average water use differences between mangroves and other ecoregions ranged from 121 to 2905 kL ha−1 year−1, or from − 302 to 1399 kL ha−1 year−1 versus potential water use from mangroves assuming low salinity. Under low salinity scenarios, higher water use by mangroves would occur when compared with temperate coniferous forest, desert, and Mediterranean shrubland. However, the majority of differences in consumptive water use reductions by mangroves are associated with the tropics where 93% of all mangroves occur (Table 1).
Discussion
Surface or groundwater seeping through mangroves and out to the ocean is not necessarily available for direct human consumption, but unconsumed freshwater that flows to marine and estuarine environments has inherently high value because of its importance in supporting wetland and coastal ocean productivity, and thus, sustained ecological processes within adjacent estuaries26. Given that over 80% of freshwater currently consumed globally by humans is associated with food production27, food and water security are intricately linked. For this reason, consumption of marine fish in lieu of crops, for example, has been shown to save as much as 50% of a region’s freshwater resource28. Mangroves contribute to this efficiency by supporting marine and estuarine food webs at low freshwater costs. Additionally, unlike many terrestrial ecosystems, mangrove survival can be potentially sustained for short periods of time (albeit at lower productivity) during periodic (or even sustained) reductions in freshwater to the coast29, at least to a point30.
Mangroves also have lower water use than alternative land uses to which they have been converted. For example, oil palm (Elaeis guineensis Jacq.) plantations, have replaced 18,467 ha of mangroves in Indonesia, Myanmar, Malaysia, and Thailand between the years 2000–201231. These plantations use more water through \({E}_{c}\) than the mangroves they have replaced. \({E}_{c}\) from mature oil palm plantations (> 12 years old) range from 5332 to 70%33 of \(ET\) in areas with adequate rainfall or irrigation. By scaling this ratio to the converted land areas from those four countries, converting mangrove to oil palm has potentially led to an additional 21.6 to 58.4 GL H2O year−1 toward transpirational water loss that would simultaneously reduce water flows toward estuaries. As oil palm plantations often need irrigation in dry years, the additional amount of freshwater needed to sustain oil palm \({E}_{c}\) becomes unavailable for human use and to support ecological processes during irrigation years, when societal demands are likely to be highest34. Furthermore, freshwater use by mangroves would not compete directly with humans under most land use or climate change scenarios.
If there are large carbon costs of water uptake and transport for mangroves growing in saline intertidal environments, then maintaining plant growth must be balanced by low water costs of carbon gain at some organizational scale19. Initial water use studies that measured sap flow in mangroves actually found appreciable rates of water flux in the outer sapwood of trees. This result suggested that the velocity of xylem water ascent in mangroves may not be distinctively low, despite the environmental conditions under which mangroves develop. For example, median sap velocities of mangroves from outer sapwood locations averaged ~ 0.13 mm s−1 in Borneo and Hawaii, USA35,36, which did not differentiate strongly from median velocities of ~ 0.13–0.16 mm s−1 in adjacent upland tropical dipterocarp or heath forest trees35. However, volume of water flux in mangroves is low versus many terrestrial forest trees (Fig. 1b) because sap flow with depth into sapwood (from cambium beyond depths of 2 cm) attenuates quickly37,38, which reduces the total amount of water ascending the tree stem to become available to foliage. Canopy leaf-area is adjusted in sync with stem capacity for water transport; with canopies dying back when water is less available or expanding as water supply increases39,40. This suggests that limited water use in mangroves may be a function of stem adjustments in water transport with changing environmental conditions, which may be imposed in concert with leaf-scale response.
To expand on this idea mechanistically, we applied the BETTINA Model41, which was previously parameterized for a widely adapted and globally distributed mangrove genus, Avicennia42. Simulations provide theoretical estimations of individual tree water use versus tree size and salinity given adequate water and light resources (Fig. 3). Depending on the salinity, trees exhibit differential biomass allocation patterns and biomass structural differences that influence water use. In fact, modelled trees become shorter but have a proportionally larger stem diameter with increasing salinity (Fig. 3a), which is in good agreement with field data43. The structural adjustments under saline conditions result in hydraulic architecture (e.g., modified canopy area) that ensure optimal water potentials and sap flow with increasing environmental stress. Thus, in addition to inherently lower whole-tree water use in mangroves compared to upland trees, saline conditions exert additional constraints to whole-tree mangrove water use. For example, water use can become theoretically high (> 150 L H2O day −1), and far greater than the literature has documented to date (Fig. 3b), when mangrove trees are large and without major constraints on water use imposed by high salinity; also affecting actual-to-potential transpiration (Fig. S3). For modelled trees, the water use of a tree growing at 70 psu (double the salt concentration of seawater) is 21% the water use of a tree growing with zero salinity (Fig. 3b).
BETTINA model simulations applied to tree size and salinity vs. water use. (a) Individual tree water use from BETTINA modelling studies relative to dbh for different salinities within mangroves. Each solid line represents one tree over the time of simulation with trees adapting their allometry to variation in soil salinity (from blue for 0 psu to red for 80 psu). For comparison, dashed lines mark the water uptake at different salinities with no allometric adaptation (average tree allometry as growing at 40 psu); solid black line represents empirical individual tree water use estimated for mangroves in this study. (b) Simulated individual tree water use of each mangrove tree at an age of 200 years.
Plasticity in water use along environmental gradients has also been studied from anatomical and physiological perspectives in mangroves44. However, this topic remains little explored at the whole-tree architectural level. Past studies reveal two important considerations. First, mangrove tree species growing under increasingly saline environments can have greater xylem vessel grouping and vessel density, as well as reduced vessel diameters and lengths than their low-salinity conspecifics, which is proposed to reduce the risk of embolisms associated with highly negative water potentials45. Second, some mangroves – those that secrete salt from their leaf and bark surfaces – can dynamically control xylem ion composition46 to increase hydraulic conductivity47 as water potential becomes more negative, whilst maintaining relatively stable stomatal conductance (and transpiration). This dynamic xylem sap osmolality control increases the water available to the transpiration stream without actively increasing transpiration, further reducing the risk of embolisms under salt stress46.
For our analysis focused at the broader canopy scale, we report average \({E}_{c}\) alongside of location-specific \(ET\) at the scale of 1-km2 from the MODIS satellite product (MOD16-A3), which is an analytical approach used by Schlesinger & Jasechko48 to permit direct comparisons of sap flow-derived transpiration rates and \(ET\) among a suite of different ecoregional vegetation types. It is important to recognize that this approach provides an approximation of \({E}_{c}\) from theoretically continuous vegetation coverage versus MODIS-derived \(ET\) over the same ground area; i.e., MODIS would also include areas without mangroves (Fig. 4). MODIS-derived \(ET\) includes several non-\({E}_{c}\) mechanisms of water loss as well as sub-areas not continuously occupied by vegetation, so we considered water use for mangroves compared to non-mangrove ecoregions using \({E}_{c}\)-to-\(ET\) ratios to better control for different, but inherent, \(ET\) water fluxes from non-transpirational sources.
Water flows in mangrove forests associated with either evapotranspiration (ET) or canopy transpiration (Ec), and how areas from MODIS-derived ET data and Ec data would relate for mangroves, as well as generally from the literature for other ecosystems. (a) Inset represents a comparison of \({E}_{c}\)-to-\(ET\) ratio for mangroves versus tropical rain forests, where most of the world’s mangroves associate. (b) Inset represents known water fluxes in mangrove root zones that contribute to Ec, and ultimately to ET.
At least four assumptions apply to this \({E}_{c}\)-to-\(ET\) ratio analysis. First, NPP data from each mangrove wetland location, and thus \({E}_{c}\) approximations, are from the study plots used in the cited studies, and therefore, may not represent the average regional structure of the entire mangrove forest over 1 km2. We assume that plots are representative of the larger stand. Second, our approximations of \({E}_{c}\) focus just on the dominant vegetation and do not include evaporation from flooded soil, saturated soil, tidal creeks, and leaf-intercepted water, or the tide-energy influences on water losses, which would be part of what \(ET\) would include49. Third, our sap flow generated determinations of \({E}_{c}\) may exclude some components of the understory. However, the degree to which source-study NPP measurements included saplings and small trees of the understory would influence our estimates of \({E}_{c}\); the more inclusive of those understory NPP components from the original data sources, the better our \({E}_{c}\) approximations. Finally, for the salinity component specific to mangroves, while freshening of water has the potential to increase \({E}_{c}\)-to-\(ET\) ratios, we assume that \(ET\) across larger spatial scales is altered only modestly with salinity reduction. To our knowledge, salinity-to-\(ET\) relationships have not been assessed at relevant scales to consider further.
Empirical assessment of \({E}_{c}\)-to-\(ET\) ratios is limited to only a few mangrove studies. On sites in Southwest Florida, USA with moderate stand basal areas (23–28 m2 ha−1) and average canopy heights (10–11 m), regional \(ET\) was 1029–1048 mm H2O year−1 while dominant canopy \({E}_{c}\) was 350–511 mm H2O year−1, or 34–50% of \(ET\) 50. Short mangrove forests (< 5 m tall) on Hainan Island, China, used only 269–357 mm H2O year−1 versus an \(ET\) of 1134–1166 mm H2O year−1, or 23–31% of \(ET\) 51. Mangrove stands did use as much as 872 mm H2O year−1 versus an \(ET\) rate of 1378 mm year−1 (or 63% of \(ET\)) in an Everglades mangrove stand, also in south Florida, but in a location were the stand had moderate salinity concentrations (~ 25 psu), greater basal area (41 m2 ha−1), and taller trees (19 m)50. \({E}_{c}\)-to-\(ET\) ratios ranged from 30 to 66% among three additional mangrove sites in China, with these ratios linked to seasonal leaf area index and salinity change52. Unlike the direct linear increases in \({WUE}_{int}\) described at the leaf-level as salinity increased (Fig. S1)20, mangrove \({E}_{c}\) remained fairly constant over salinities of ~ 10 to 28 psu and decreased beyond52. Thus, empirical estimations of \({E}_{c}\)-to-\(ET\) ratios fall within the range of our derived values. In fact, any discrepancies trended toward more water-use conservative \({E}_{c}\)-to-\(ET\) ratios for mangrove vegetation.
By aligning global mangrove area with the distribution of adjacent ecoregions to mangroves, water use differences from our analysis scale to 34.7 Teraliters (TL) H2O year−1 if comparable areas to mangrove are assumed (Table 1, Table S1). We add this perspective to demonstrate that having mangroves along a coast can indeed be consequential to water cycling locally as well as globally. Even under an assumption of low soil porewater salinity, consumptive water use by mangroves is still comparatively low (by 16.2 TL H2O year−1). Likewise, our predictive variable (NPP) for approximating \({E}_{c}\) among the world’s mangroves is linked strongly to rainfall, explaining up to 86% of global carbon stocks among mangroves when compared with other climatic variables, such as atmospheric temperature53. Thus, mangroves receiving higher rainfall benefit from reduced salinities and increased nutrients, which in turn illicit productivity gains requiring more freshwater use by trees.
Finally, there is a growing concern for the ability to feed the Planet’s increasing population with limited renewable freshwater resources27,54, due to changes in diets, increased demand for freshwater resources to support fossil fuel extraction55, and competition between water used for agricultural production of biofuels and food56. Placing potential water use reductions by mangroves within this food-energy-water nexus requires understanding of the multiple ecosystem services that mangrove wetlands provide, which encompass food resources, building materials and energy utilization. The quantification and comparison of freshwater consumption in the production of 268 goods and services can be determined from estimates of \({E}_{c}\) by attributing water use per hectare to consumable weights (Mg) of resultant products in marine and blue carbon ecosystems. Water used in the production of a consumable product accompanies that product as a legacy requirement during local or global trade as the product realizes its destination and ultimate use; this water is defined as “virtual water content”57.
When we evaluate virtual water content from mangroves versus relevant ecoregions based on global estimates of the economic value of ecosystem services for each58, we estimate that the value of food production per hectare for a tropical rainforest (e.g.), in USD, is approximately $ 0.27 mm−1 of H2O but for mangroves it is $ 2.20 mm−1 of H2O (Table S2). Similarly, the raw material value is $ 0.11 mm−1 of H2O for tropical rainforests compared to six-fold higher value for mangroves. Over time, such analysis may reveal previously unrealized returns on investments in protection and restoration of mangroves, and possibly of other emergent blue carbon wetlands.
Conclusions
Efficient and conservative use of freshwater at the individual tree and stand levels by mangroves equate to global water use differentials in the trillions of liters annually compared to adjacent ecoregions and alternate land use area. We can gain additional information on levels of water use from other blue carbon wetlands through expanding eco-hydrologic studies, especially as these wetlands may also be efficient in maintaining their functions with reduced freshwater availability. Low freshwater use by mangroves could significantly augment the global blue carbon wealth of nations, recently estimated to be $190.67 ± 30 billion year−1 (USD)59. High productivity of mangroves for their low usage of freshwater compared to land uses that have replaced them provides evidence of additional benefits to the ecology of associated estuaries that are dependent on freshwater flows. The water economy of ecosystems may require greater worldwide consideration as humans look for options to ameliorate, limit, and adapt to future water shortages while sustaining coastal productivity and ecological connections. The conservative water use of blue carbon ecosystems may add to their value and function as nature-based solutions, and to coastal resilience as freshwater availability is reduced.
Materials and methods
At least 11 coastal ecosystems have been considered based on a minimum of actionably defined criteria to be “blue carbon ecosystems”. These include mangrove wetlands, tidal marshes (salt, brackish, fresh), seagrasses, salt flats, freshwater (upper estuarine) tidal forests, macroalgae, phytoplankton, coral reef, marine fauna (fish), oyster reefs, and mud flats5; all but three of these would be considered wetlands, with salt flats and mud flats being examples of non-emergent (plant) blue carbon wetlands. Herein, we focus on mangroves.
Adjusting intrinsic leaf-level photosynthetic water use efficiency (\({WUE}_{int}\)) in response to environmental gradients (Introduction)
We used data provided by B.F. Clough & R.G. Sims20, which presented leaf-scale net photosynthesis (\({P}_{n}\) [sic]; μmol CO2 m−2 s−1), stomatal conductance (\({g}_{w}\): mol m−2 s−1), leaf-intercellular CO2 concentrations (\({c}_{i}\): μl l−1), and intrinsic photosynthetic water use efficiency (\({WUE}_{int}\): \(\frac{{P}_{n}}{{g}_{w}}\)) for 19 mangrove species occupying 9 different sites in Papua New Guinea and northern Australia. These field data were collected using an infrared gas analyzer (model Li-6000, Li-Cor Biosciences, Inc., Lincoln, NE, USA) attached to leaves at saturation light levels (reported as > 800 μmol PPFD m−2 s−1). Soil salinity at the time of data collection ranged from 10 to 49 psu, and median long-term atmospheric temperature and relative humidity among sites ranged from 19.9 to 27.4 °C and 35.1 to 92.2%, respectively (Fig. S1)20. These data were among the first to offer insight from field study into the plasticity of mangroves across a range of natural salinity and aridity gradients to adjust leaf-level \({WUE}_{int}\) as needed for local environmental condition. While it is not new for trees to adjust their \({WUE}_{int}\) when they develop in arid, semi-arid, or even some humid and tropical environments60, what is distinctive is that mangroves may be further driven to water savings by salinity gradients as a condition of development.
\({{\varvec{W}}{\varvec{U}}{\varvec{E}}}_{{\varvec{i}}{\varvec{n}}{\varvec{t}}}\) and individual tree water use of mangrove wetlands versus terrestrial ecosystems
For Fig. 1a, we compare leaf-level \({WUE}_{int}\) data collected from 17 published papers (using maximum and minimum values), providing 67 independent measurements of \({WUE}_{int}\) for mangroves (Table S3). While we mention in the main text that as many as 214 independent measurements of water use efficiency are available, not all of these present raw \({P}_{n}\) or \({g}_{w}\) data, with some reporting leaf transpiration (\({T}_{r}\)) which do not enable reporting of intrinsic water use efficiencies. Also, we strategically included studies from reproducible experimental designs and readily available papers. Mangrove species included in this review represented a global distribution of greenhouse and field observations, and encompassed species in the following mangrove genera: Rhizophora, Avicennia, Laguncularia, Bruguiera, Aegialitis, Aegiceras, Ceriops, Sonneratia, Kandelia, Excoecaria, Heritiera, Xylocarpus, and Conocarpus.
We then accessed an existing database (n = 11,328 observations) that reported raw \({P}_{n}\) and \({g}_{w}\) data from 210 upland deciduous and evergreen shrubs and trees of savannah, boreal, temperate, and tropical habitats60. From these data, we evaluated a range of upland tree and shrub species that occurred and developed naturally in environments along a global gradient of vapor pressure deficit (i.e., atmospheric moisture and temperature), including arid, semi-arid, dry semi-humid, and humid locations.
For Fig. 1b, we started with a review by Wullschleger et al.61 that provides maximum individual tree water use data (L H2O day−1) from 52 published studies representing 67 species of upland trees from around the world. Of those studies, dbh values (8 to 134 cm) were provided alongside 47 individual tree water use values. Maximum individual tree water use and dbh (4.1 to 45.3 cm) were available from the original source for 8 mangrove studies representing 7 species from French Guiana, Mayotte Island (Indian Ocean), China, Florida (USA), and Louisiana (USA) (Table S4). These represent the extent of published sap flow data that provided both individual tree water use and dbh from mangroves (numerically); e.g., we could not extract specific individual tree water use versus dbh from a Moreton Bay (Australia) study site62, south Florida study site63, or from five additional study sites in China51,52. However, regressions for two of the Chinese study sites provided over two years51 indicated that mangrove trees from a suite of species ranging in dbh from 8 to 24 cm used approximately 0.76 and 9.31 L H2O day−1, or 0.53 L H2O day−1 cm−1 of dbh. These apparent rates were even lower than what was reported as average for mangroves in Fig. 1b of 1.4 L H2O day−1 cm−1. The mangrove species included in this analysis were Avicennia germinans (L.) L., Laguncularia racemosa (L.) C.F. Gaertn., Rhizophora mangle L., Ceriops tagal (Perr.) C.B. Rob., Rhizophora mucronata Lam., Sonneratia apetala Buch.-Ham, and Sonneratia caseolaris (L.) Engl.. Additional comparative mangrove species reported by B. Leng & K.-F. Cao51 included Bruguiera sexangula (Lour.) Poir., Bruguiera sexangula var. rhynchopetala W.C. Ko, Excoecaria agallocha L., Rhizophora apiculata Blume, Sonneratia alba Sm., and Xylocarpus granatum J. Koenig.
Estimation of canopy transpiration (\({{\varvec{E}}}_{{\varvec{c}}}\)) from net primary productivity data
Estimates of carbon uptake from CO2 can provide insight into the water use requirement for that uptake of carbon64. We used leaf-level instantaneous water use efficiency (\({WUE}_{ins}\): \(\frac{{P}_{N}}{{T}_{r}})\), which relates to net CO2 uptake from leaves of the dominant mangrove forest canopy relative to the specific amount of water used, and developed a predictive relationship (predicted) for determining mangrove net primary productivity (NPP) values from \({E}_{c}\) using \({WUE}_{ins}\). For A. germinans, L. racemosa, and R. mangle forest components, we used light-saturated, leaf-level \({WUE}_{ins}\) values of 3.82 ± 0.3, 4.57 ± 0.3, and 5.15 ± 0.4 mmol CO2 (mol H2O)−1 [± 1 SE], respectively, from mangrove saplings and small trees of south Florida65. \({WUE}_{ins}\) values were stratified by species relative to basal area distributions on each south Florida study plot, converted from molar fractions of H20 (from \({E}_{c}\) determination) and CO2 to molecular weights, and multiplied by \({WUE}_{ins}\) with applicable unit conversions to attain kg CO2 m−2 year−1. This value was multiplied by 0.273 to yield kg C m−2 year−1.
This predictive relationship was validated in two independent ways. First, for one of the calibration sites (lower Shark River, Everglades National Park, Florida, USA), we modeled \({E}_{c}\) from sap flow data50, determined NPP from \({WUE}_{ins}\) calculations relative to the amount of water the stand used, and had independent measurements of net ecosystem exchange (NEE) of CO2 between the mangrove ecosystem and atmosphere from an eddy flux tower66. For this site, NPP estimation and NEE were closely aligned once soil CO2 effluxes were accounted; respiratory CO2 effluxes from soil and pneumatophores were determined to be 1.2 kg C m−2 year−1 from previous study67. Using our NPP estimations from \({WUE}_{ins}\) calculations and subtracting soil and pneumatophore CO2 effluxes of 1.2 kg C m−2 for 2004 and 0.8 kg C m−2 for 2005 (partial year), NPP becomes 0.96 kg C m−2 for 2004 and 0.85 kg C m−2 for January to August of 2005 (see Observed 1, Florida on Fig. S2). Our approach underestimated NPP from \({E}_{c}\) relative to measurements from eddy covariance by 0.21 kg C m-2 for 2004 (within 17.5% of predicted) and was nearly identical for 2005 (within 0.02 kg C m−2, or 2% of predicted).
Second, we wanted to determine whether \({E}_{c}\)-to-NPP predictions developed on a few sites in south Florida, USA, represented other global sites, so we included an analysis from several mangrove sites in Guangdong Province, China, to represent an entirely different location. Similar to south Florida analyses, we combined data for NPP from co-located sites of \({E}_{c}\) determination using sap flow techniques. NPP of the mangrove forests were measured using multiple procedures (including eddy flux) for improved accuracy68,69. The relationships of predicted NPP versus \({E}_{c}\) and observed NPP versus \({E}_{c}\) did not differ between Florida and China (t = 0.48, p = 0.643).
Projecting mangrove \({{\varvec{E}}}_{{\varvec{c}}}\) to other locations
We reviewed data from 26 published records that report mangrove NPP, or enough data to estimate NPP, from 71 study sites located in the Florida-Caribbean Region (25 sites) and Asia–Pacific Region (46 sites) (Table S5). Table S1 reveals mangrove literature sources used, as well as how NPP was estimated from values provided in the original source; itemizes assumptions for determinations of aboveground NPP, wood production, litter production, and root production from various ratios70; and reveals unit conversions.
We then convert NPP to \({E}_{c}\) for all 71 sites using the predicted curve in Fig. S2 (Eq. 1, main text), and provide summary results by location in Table S1. Regional \(ET\) data were extracted from the MODIS Global Evapotranspiration Project (MOD16-A3), which are provided at a resolution of 1-km. The locations of mangrove NPP study sites were identified, assigned to a single 1-km2 grid in MOD16, and \(ET\) was extracted from that grid and used for \({E}_{c}\)-to-\(ET\) comparison. Average \(ET\) from single cells (1 km2) was combined with the average of up to 8 additional neighboring cells to provide comparative \(ET\) projections over up to 9 km2 for each location from 2000 to 2013 to compare sensitivity among suites of the specific MODIS16-A3 cells selected over land. When neighboring cells were completely over water, they were excluded since component mangrove forest \({E}_{c}\) estimation was not possible from the cells. Estimates of \(ET\) by individual cells used to compare with mangrove \({E}_{c}\) versus up to 9 cells differed by an average of only 43 mm H2O year−1 (± 16 mm H2O year−1, S.E.). Therefore, we use \(ET\) from individual, overlapping \({E}_{c}\) cells in Table S1.
The average \({E}_{c}\)-to-\(ET\) ratio from mangroves was subtracted from \({E}_{c}\)-to-\(ET\) ratio for specific ecoregions48, and this ratio difference was assumed to represent net water use strategy affecting differences by the dominant vegetation between ecosystem types. We were also mindful that salinity reductions can affect \({E}_{c}\). We used scaled (0–1) mean and standard deviations from \({WUE}_{int}\) data previously reported for mangroves (Fig. S1)20. Standard deviation was 32% of mean \({WUE}_{int}\) related to salinity gradients, and if we re-scale this deviation to \({E}_{c}\) data and add it to the mean \({E}_{c}\) to assume low salinity, average \({E}_{c}\)-to-\(ET\) ratio becomes 57.4%. This is theoretical and assumes a relatively linear relationship between \({WUE}_{int}\) and \({E}_{c}\).
Comparative water use scaling among ecoregions
Table 1 presents the projected reduction in water used through \({E}_{c}\) if a mangrove \({E}_{c}\)-to-\(ET\) ratio was applied to tropical rainforest (290.52 mm H2O year−1), temperate deciduous forest (131.76 mm H2O year−1), tropical grassland (110.77 mm H2O year−1), temperate grassland (46.48 mm H2O year−1), temperate coniferous forest (54.96 mm H2O year−1), desert (22.99 mm H2O year−1), and Mediterranean shrubland (12.08 mm H2O year−1). To convert potential water use differences to kL H2O ha−1 year−1 (as presented in the abstract), the following calculation is used (using the example of tropical rainforest):
For comparisons made to mature (> 12 years) oil palm (Elaeis guineensis Jacq.) plantations, \({E}_{c}\)-to-\(ET\) ratio was assumed to range from 5332 to 70%33, for a water use difference of 1170 and 3160 kL H2O ha−1 year−1, respectively, relative to annual global mangrove \(ET\) (of 1172 mm). We multiply these values by the 18,467 ha of land area that was converted from mangroves to oil palm31 to attain potential water use differences of 21.6 to 58.4 GL H2O year−1 from avoided conversion of mangrove to oil palm in this region.
Global water use scaling
In order to determine how much global mangrove area is adjacent to each ecoregion, we conducted a cross-walk between terrestrial ecoregions71 and those used by Global Mangrove Watch in the 2010 classification of global mangrove area72. Terrestrial ecoregions used by Schlesinger & Jasechko48 were then able to be associated with specific mangrove areas (Table S6). In other words, given a specific ecoregion, we determined how much mangrove area would be occurring within that same ecoregional geography. Global mangrove area assignment to those ecoregions mapped within 0.1% of the total mangrove area of 13,760,000 ha reported in Bunting et al.72. To convert kL H2O ha−1 year−1 to GL H2O year−1 among ecoregions, the following calculation was used (continuing with the example of tropical rainforest, which has an area of adjacent mangroves of 112,331.9 km2):
Agent-based modelling of individual tree water use (Discussion)
The BETTINA model simulates the growth of mangrove trees as a response to above- and below-ground resources, i.e. light and water41. In the model, an individual tree is described by four geometric measures, including stem radius, stem height, crown radius and root radius; attributing functional relevance in terms of resource uptake. Aiming to maximize resource uptake, new biomass is allocated to increase these measures in an optimal but not constant proportion. Water uptake of the tree is driven by the water potential gradient between the soil and the leaves. Thus, porewater salinity is part of what determines the water availability for plants.
With the BETTINA model, we simulated the growth of nine individual mangrove trees under different salinity conditions, ranging between 0 and 80 psu, while all other environmental and tree-specific conditions were kept constant. Simulation time was 200 years so that trees could achieve very close to their maximum possible size, and the hydrological parameters were similar to that reported previously42. We can show that the ratio of the actual transpiration to the potential transpiration decreases with increasing salinity; plants use less water. Potential transpiration was the transpiration of a given tree without a simulated reduction in water availability due to porewater salinity. These parameter details are presented graphically for mangroves (Fig. S3), comparing porewater salinity along a gradient against the ratio of actual-to-potential individual tree transpiration.
Further, BETTINA simulation results include morphological plasticity adjustments to allometry. To highlight this, we also displayed results assuming a constant allometry as for 40 psu. Naturally, for this arbitrary benchmark the solid and the dashed line coincide (Fig. 3a). Adaptation to higher salinities improves water uptake (primarily girth and root growth), thus the adapted trees (solid lines) have a higher water uptake than the average allometry (dashed lines) for salinities below 40 psu. Lower salinities promote increase of height and crown radius to improve light availability. That is why the adapted trees have a lower water uptake than an average tree would for salinities above 40 psu. Tree water use decreases with increasing salinity (Fig. 3b), as \({WUE}_{int}\) coincidently increases (Fig. S1).
Virtual water use explained (Discussion)
Water is required to produce products or acquire services from natural ecosystems; e.g., forest products, fisheries biomass, nutrient processing (nitrification, denitrification), food production. If a net kilogram of a food is grown on a hectare of land where water is abundant and that kilogram of food requires 400 mm of water to be produced, the export of that food to an area of low water availability provides an ecosystem service in the amount of 1 kg of food, plus 400 mm of “virtual” water not actually needed at the destination but used at the source. This water is defined as the product’s “virtual water content”56. There is a rich body of literature exploring the concept of virtual water73,74, but we expand on this concept here as a comparison among 7 ecoregions48 and mangroves. Raw data used for calculations are presented in Table S2.
Statistical analysis
Data for leaf-level \({WUE}_{int}\) comparisons between terrestrial woody plants and mangroves, as well as individual tree water use by dbh for both terrestrial and mangrove trees, were not normally distributed. We used a Kruskal–Wallis ANOVA based on ranks, and the Dunn’s Method for difference tests. Individual tree water use by dbh for both terrestrial and mangrove trees were determined using linear regression, mostly applied to mean values. For a couple of mangrove studies, only median values were extractable from minimum and maximum values. Likewise, all other data relationships were best fit with linear models, including the calibration curves between \({E}_{c}\) and NPP. All data were analyzed using SigmaPlot (v. 14.0, Systat, Inc., Palo Alto, California, USA).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files], or from https://bitbucket.org/gsglobal/leafgasexchange for data reported in Medlyn et al.60. Model code for the BETTINA model (a sub-routine of the model, MANGA) is available at, https://github.com/mcwimm/bc_wetlands.
References
Vörösmatry, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561. https://doi.org/10.1038/nature09440 (2010).
Bhaduri, A. et al. Achieving sustainable development goals from a water perspective. Front. Environ. Sci. 4, 64. https://doi.org/10.3389/fenvs.2016.00064 (2016).
Dynesius, M. & Nilsson, C. Fragmentation and flow regulation of river systems in the northern third of the world. Science 266, 753–762. https://doi.org/10.1126/science.266.5186.753 (1994).
Mcleod, E. et al. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. https://doi.org/10.1890/110004 (2011).
Lovelock, C. E. & Duarte, C. M. Dimensions of blue carbon and emerging perspectives. Biol. Lett. 15, 20180781. https://doi.org/10.1098/rsbl.2018.0781 (2019).
Duke, N. C. et al. A world without mangroves. Science 317, 41–42. https://doi.org/10.1126/science.317.5834.41b (2007).
Ewel, K. C., Twilley, R. R. & Ong, J. Different kinds of mangrove forests provide different goods and services. Glob. Ecol. Biogeogr. Lett. 7, 83–94. https://doi.org/10.1111/j.1466-8238.1998.00275.x (1998).
Alongi, D. M. Blue Carbon: Coastal Sequestration for Climate Mitigation (Springer, Switzerland, 2018).
Rogers, K. et al. Wetland carbon storage controlled by millennial-scale variation in relative sea-level rise. Nature 567, 91–95. https://doi.org/10.1038/s41586-019-0951-7 (2019).
Taillardat, P., Friess, D. A. & Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 14, 20180251. https://doi.org/10.1098/rsbl.2018.0251 (2018).
Lewis, R. R. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24, 403–418. https://doi.org/10.1016/j.ecoleng.2004.10.003 (2005).
Krauss, K. W. & Ball, M. C. On the halophytic nature of mangroves. Trees Struct. Funct. 27, 7–11. https://doi.org/10.1007/s00468-012-0767-7 (2013).
Nguyen, H. T., Stanton, D. E., Schmitz, N., Farquhar, G. D. & Ball, M. C. Growth responses of the mangrove Avicennia marina to salinity: Development and function of shoot hydraulic systems require saline conditions. Ann. Bot. 115, 397–407. https://doi.org/10.1093/aob/mcu257 (2015).
Scholander, P. F. How mangroves desalinate seawater. Physiol. Plant. 21, 251–261. https://doi.org/10.1111/j.1399-3054.1968.tb07248.x (1968).
Reef, R. & Lovelock, C. E. Regulation of water balance in mangroves. Annals Bot. 115, 385–395. https://doi.org/10.1093/aob/mcu174 (2015).
Ewe, S. M. L., Sternberg, L. d. S. L. & Childers, D. L. Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia 152, 607–616. https://doi.org/10.1007/s00442-007-0699-x (2007).
Santini, N. S., Reef, R., Lockington, D. A. & Lovelock, C. E. The use of fresh and saline water sources by the mangrove Avicennia marina. Hydrobiol. 745, 59–68. https://doi.org/10.1007/s10750-014-2091-2 (2015).
Tyree, M. T. The cohesion-tension theory of sap ascent: Current controversies. J. Exp. Bot. 48, 1753–1765. https://doi.org/10.1093/jxb/48.10.1753 (1997).
Ball, M. C. Salinity tolerance in the mangroves, Aegiceras corniculatum and Avicennia marina I Water use in relation to growth, carbon partitioning and salt balance. Funct. Plant Biol. 15, 447–464. https://doi.org/10.1071/PP9880447 (1988).
Clough, B. F. & Sim, R. G. Changes in gas exchange characteristics and water use efficiency of mangroves in response to salinity and vapour pressure deficit. Oecologia 79, 38–44. https://doi.org/10.1007/BF00378237 (1989).
Farquhar, G. D., Schulze, E. D. & Kuppers, M. Responses to humidity by stomata of Nicotiana glauca L and Corylus avellana L are consistent with the optimization of carbon dioxide uptake with respect to water loss. Funct. Plant Biol. 7, 315–327. https://doi.org/10.1071/PP9800315 (1980).
Nguyen, H. T. et al. Leaf water storage increases with salinity and aridity in the mangrove Avicennia marina: Integration of leaf structure, osmotic adjustment and access to multiple water sources. Plant Cell Environ. 40, 1576–1591. https://doi.org/10.1111/pce.12962 (2017).
Coopman, R. E. et al. Harvesting water from unsaturated atmospheres: Deliquescence of salt secreted onto leaf surfaces drives reverse sap flow in a dominant arid climate mangrove, Avicennia marina. New Phytol. 231, 1401–1414. https://doi.org/10.1111/nph.17461 (2021).
Steppe, K. et al. Direct uptake of canopy rainwater causes turgor-driven growth sports in the mangrove Avicennia marina. Tree Physiol. 38, 979–991. https://doi.org/10.1093/treephys/tpy024 (2018).
Hayes, M. A. et al. Foliar water uptake by coastal wetland plants: A novel acquisition mechanism in arid and humid subtropical mangroves. J. Ecol. 108, 2625–2637. https://doi.org/10.1111/1365-2745.13398 (2020).
Gillson, J. Freshwater flow and fisheries production in estuarine and coastal systems: Where a drop of rain is not lost. Rev. Fisheries Sci. 19, 168–186. https://doi.org/10.1080/10641262.2011.560690 (2011).
Carr, J. A., D’Odorico, P., Laio, F. & Ridolfi, L. Recent history and geography of virtual water trade. PLoS ONE 8, e55825. https://doi.org/10.1371/journal.pone.0055825 (2013).
Gephart, J. A., Pace, M. L. & D’Odorico, P. Freshwater savings from marine protein consumption. Environ. Res. Lett. 9, 014005. https://doi.org/10.1088/1748-9326/9/1/014005 (2014).
Galeano, A., Urrego, L. E., Botero, V. & Bernal, G. Mangrove resilience to climate extreme events in a Colombian Caribbean Island. Wetland Ecol. Manag. 25, 743–760. https://doi.org/10.1007/s11273-017-9548-9 (2017).
Duke, N. C. et al. Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: a severe ecosystem response, coincidental with an unusually extreme weather event. Mar. Freshw. Res. 68, 1816–1829. https://doi.org/10.1071/MF16322 (2017).
Richards, D. R. & Friess, D. A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Nat. Acad. Sci. 113, 344–349. https://doi.org/10.1073/pnas.1510272113 (2016).
Röll, A. et al. Transpiration in an oil palm landscape: Effects of palm age. Biogeosciences 12, 5619–5633. https://doi.org/10.5194/bg-12-5619-2015 (2015).
Dufrêne, E., Dubos, B., Rey, H., Quencez, P. & Saugier, B. Changes in evapotranspiration from an oil palm stand (Elasis guineensis Jacq) exposed to seasonal soil water deficits. Oléagineux 48, 105–120 (1993).
Merten, J. et al. Water scarcity and oil palm expansion: Social views and environmental processes. Ecol. Soc. 21, 5. https://doi.org/10.5751/ES-08214-210205 (2016).
Becker, P., Asmat, A., Mohamad, J., Moksin, M. & Tyree, M. T. Sap flow rates of mangrove trees are not unusually low. Trees Struc. Funct. 11, 432–435. https://doi.org/10.1007/s004680050104 (1997).
Zimmermann, U. et al. High molecular weight organic compounds in the xylem sap of mangroves: Implications for long-distance water transport. Botanica Acta 107, 218–217. https://doi.org/10.1111/j.1438-8677.1994.tb00789.x (1994).
Krauss, K. W., Young, P. J., Chambers, J. L., Doyle, T. W. & Twilley, R. R. Sap flow characteristics of neotropical mangroves in flooded and drained soils. Tree Physiol. 27, 775–783. https://doi.org/10.1093/treephys/27.5.775 (2007).
Zhao, H. et al. Anatomical explanations for acute depressions in radial pattern of axial sap flow in two diffuse-porous mangrove species: Implications for water use. Tree Physiol. 38, 277–287. https://doi.org/10.1093/treephys/tpx172 (2018).
McDowell, N. G. et al. Processes and mechanisms of coastal woody-plant mortality. Glob. Change Biol. https://doi.org/10.1111/gcb.16297 (in press).
Shinozaki, K., Yoda, K., Hozumi, K. & Kira, T. A quantitative analysis of plant form-the pipe model theory I Basic analyses. Jpn. J. Ecol. 14, 97–105. https://doi.org/10.18960/seitai.14.3_97 (1964).
Peters, R., Vovides, A. G., Luna, S., Grüters, U. & Berger, U. Changes in allometric relations of mangrove trees due to resource availability – a new mechanistic modelling approach. Ecol. Model. 283, 53–61. https://doi.org/10.1016/j.ecolmodel.2014.04.001 (2014).
Peters, R. et al. Partial canopy loss of mangrove trees : Mitigating water scarcity by physical adaptation and feedback on porewater salinity. Estuar. Coast. Shelf Sci. 248, 106797. https://doi.org/10.1016/j.ecss.2020.106797 (2021).
Vovides, A. G. et al. Morphological plasticity in mangrove trees: Salinity-related changes in the allometry of Avicennia germinans. Trees Struc. Funct. 28, 1413–1425. https://doi.org/10.1007/s00468-014-1044-8 (2014).
Lovelock, C. E., Krauss, K. W., Osland, M. J., Reef, R. & Ball, M. C. The physiology of mangrove trees with changing climate. In Tropical Tree Physiology (eds Goldstein, G. & Santiago, L. S.) (Springer, New York, 2016).
Robert, E. M. R., Koedam, N., Beeckman, H. & Schmitz, N. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. Funct. Ecol. 23, 649–657. https://doi.org/10.1111/j.1365-2435.2009.01551.x (2009).
López-Portillo, J. et al. Dynamic control of osmolality and ionic composition of the xylem sap in two mangrove species. Am. J. Bot. 101, 1013–1022. https://doi.org/10.3732/ajb.1300435 (2014).
López-Portillo, J., Ewers, F. W. & Angeles, G. Sap salinity effects on xylem conductivity in two mangrove species. Plant Cell Environ. 28, 1285–1292. https://doi.org/10.1111/j.1365-3040.2005.01366.x (2005).
Schlesinger, W. H. & Jesechko, S. Transpiration in the global water cycle. Agri. For. Meteorol. 189–190, 115–117. https://doi.org/10.1016/j.agrformet.2014.01.011 (2014).
Barr, J. G. et al. Summertime influences of tidal energy advection on the surface energy balance in a mangrove forest. Biogeosciences 10, 501–511. https://doi.org/10.5194/bg-10-501-2013 (2013).
Krauss, K. W., Barr, J. G., Engel, V., Fuentes, J. D. & Wang, H. Approximations of stand water use versus evapotranspiration from three mangrove forests in southwest Florida, USA. Agri. For. Meteorol. 213, 291–303. https://doi.org/10.1016/j.agrformet.2014.11.014 (2015).
Leng, B. & Cao, K.-F. The sap flow of six tree species and stand water use of a mangrove forest in Hainan, China. Glob. Ecol. Conserv. 24, e10233. https://doi.org/10.1016/j.gecco.2020.e01233 (2020).
Liang, J. et al. Evapotranspiration characteristics distinct to mangrove ecosystems are revealed by multiple-site observations and a modified two-source model. Water Resour. Res. 55, 11250–11273. https://doi.org/10.1029/2019WR024729 (2019).
Sanders, C. J. et al. Are global mangrove carbon stocks driven by rainfall?. J. Geophys. Res. Biogeosci. 121, 2600–2609. https://doi.org/10.1002/2016JG003510 (2016).
Varis, O., Keskinen, M. & Kummu, M. Four dimensions of water security with a case of the indirect role of water in global food security. Water Sec. 1, 36–45. https://doi.org/10.1016/j.wasec.2017.06.002 (2017).
Rosa, L., Davis, K. F., Rulli, M. C. & D’Odorico, P. Environmental consequences of oil production from oil sands. Earth’s Future 5, 158–170. https://doi.org/10.1002/2016EF000484 (2017).
Rulli, M. C., Bellomi, D., Cazzoli, A., De Carolis, G. & D’Odorico, P. The water-land-food nexus of first-generation biofuels. Sci. Rep. 6, 22521. https://doi.org/10.1038/srep22521 (2016).
Allan, J. A. Virtual water: a strategic resource. Groundwater 36, 545–547. https://doi.org/10.1111/j.1745-6584.1998.tb02825.x (1998).
Costanza, R. et al. Changes in the global value of ecosystem services. Glob. Environ. Change 26, 152–158. https://doi.org/10.1016/j.gloenvcha.2014.04.002 (2014).
Bertram, C. et al. The blue carbon wealth of nations. Nat. Clim. Change 11, 704–709. https://doi.org/10.1038/s41558-021-01089-4 (2021).
Medlyn, B. E. et al. How do leaf and ecosystem measures of water-use efficiency compare?. New Phytol. 216, 758–770. https://doi.org/10.1111/nph.14626 (2017).
Wullschleger, S. D., Meinzer, F. C. & Vertessy, R. A. A review of whole-plant water use studies in trees. Tree Physiol. 18, 499–512. https://doi.org/10.1093/treephys/18.8-9.499 (1998).
Vandegehuchte, M. W. et al. Long-term versus daily stem diameter variation in co-occurring mangrove species: Environmental versus ecophysiological drivers. Agri. For. Meteorol. 192–193, 51–58. https://doi.org/10.1016/j.agrformet.2014.03.002 (2014).
Hao, G.-Y. et al. Hydraulic redistribution in dwarf Rhizophora mangle trees driven by interstitial soil water salinity gradients: Impacts on hydraulic architecture and gas exchange. Tree Physiol. 29, 697–705. https://doi.org/10.1093/treephys/tpp005 (2009).
Beer, C., Reichstein, M., Ciais, P., Farquhar, G. D. & Papale, D. Mean annual GPP of Europe derived from its water balance. Geophys. Res. Lett. 34, L05401. https://doi.org/10.1029/2006GL029006 (2007).
Krauss, K. W. Growth, Photosynthetic, and Water Use Characteristics of South Florida Mangrove Vegetation to in Response to Varying Hydroperiod (Ph.D. thesis, University of Louisiana at Lafayette, 2004).
Barr, J. G. et al. Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J. Geophys. Res. 115, G02020. https://doi.org/10.1029/2009JG001186 (2010).
Troxler, T. G. et al. Component-specific dynamics of riverine mangrove CO2 efflux in the Florida coastal Everglades. Agri. For. Meteorol. 213, 273–282. https://doi.org/10.1016/j.agrformet.2014.12.012 (2015).
Peng, C. J. et al. Vegetation carbon stocks and net primary productivity of the mangrove forests in Shenzhen China. Chin. J. Appl. Ecol. 27, 2059–2065. https://doi.org/10.13287/j.1001-9332.201607.029 (2016).
Gu, X. et al. The mangrove blue carbon sink potential: Evidence from three net primary production assessment methods. For. Ecol. Manag. 504, 119848. https://doi.org/10.1016/j.foreco.2021.119848 (2022).
Bouillon, S. et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles. https://doi.org/10.1029/2007GB003052 (2008).
Olson, D. M. et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 51, 933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 (2001).
Bunting, P. et al. The global mangrove watch – a new 2010 global baseline of mangrove extent. Remote Sens. 10, 1699. https://doi.org/10.3390/rs10101669 (2018).
D’Odorico, P. et al. The global food-energy-water nexus. Rev. Geophys. 56, 456–531. https://doi.org/10.1029/2017RG000591 (2018).
D’Odorico, P. et al. Global virtual water trade and the hydrological cycle: patterns, drivers, and socio-environmental impacts. Environ. Res. Lett. 14, 053001. https://doi.org/10.1088/1748-9326/ab05f4 (2019).
Acknowledgements
We thank the U.S. Geological Survey Climate Research and Development Program, Environments Program, Southeast Climate Adaptation Science Center, and LandCarbon Program for providing funds to support KWK, EJW, and JC. We thank the Volkswagen Foundation (VolkswagenStiftung, grant No. 94844) for providing research support to UB, RP, HB, AV, and MCW; and UK’s Natural Environment Research Council (NERC, grant No. NE/P014127/1) for providing research support to PB and AV. This review was also in part supported by Australian Research Council awards FL20010013 (to CEL), DP180102969 (to MCB), DP150104437 (to MCB and CEL), DP1096749 (to MCB and CEL), and DP180103444 (to RER), as well as the National Natural Science Foundation of China award 42076176 (to LC). We thank Michael J. Baldwin (USGS) for assistance with WUEint literature reviews and compiling MODIS satellite \(\mathrm{ET}\) data. Technical Contribution No. 7090 of the Clemson University Experiment Station. This material is based in part upon work supported by the NIFA/USDA, under project number SC-1700590. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
K.W.K., C.E.L., and L.C. conceived the idea presented herein and co-wrote the first draft. K.W.K., C.E.L., L.C., J.A.D., and J.C. conducted literature searches and data extraction from published accounts. U.B., R.P., A.V., M.C.W., and M.C.B contributed the modeling and hydraulic constraint perspective. J.C. contributed the virtual water perspective. E.J.W. contributed to methodology and editing. P.B. contributed ecoregional-specific global mangrove areas. All authors added perspective from their on-going research focusing on various aspects of water use by mangroves and contributed significantly to the writing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krauss, K.W., Lovelock, C.E., Chen, L. et al. Mangroves provide blue carbon ecological value at a low freshwater cost. Sci Rep 12, 17636 (2022). https://doi.org/10.1038/s41598-022-21514-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21514-8
This article is cited by
-
Stronger increases but greater variability in global mangrove productivity compared to that of adjacent terrestrial forests
Nature Ecology & Evolution (2024)
-
Practical Guide to Measuring Wetland Carbon Pools and Fluxes
Wetlands (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.