Abstract
The African coconut beetle Oryctes monoceros and Asiatic rhinoceros beetle O. rhinoceros have been associated with economic losses to plantations worldwide. Despite the amount of effort put in determining the potential geographic extent of these pests, their environmental suitability maps have not yet been well established. Using MaxEnt model, the potential distribution of the pests has been defined on a global scale. The results show that large areas of the globe, important for production of palms, are suitable for and potentially susceptible to these pests. The main determinants for O. monoceros distribution were; temperature annual range, followed by land cover, and precipitation seasonality. The major determinants for O. rhinoceros were; temperature annual range, followed by precipitation of wettest month, and elevation. The area under the curve values of 0.976 and 0.975, and True skill statistic values of 0.90 and 0.88, were obtained for O. monoceros and O. rhinoceros, respectively. The global simulated areas for O. rhinoceros (1279.00 × 104 km2) were more than that of O. monoceros (610.72 × 104 km2). Our findings inform decision-making and the development of quarantine measures against the two most important pests of palms.
Similar content being viewed by others
Introduction
The African coconut beetle Oryctes monoceros (Olivier) and the Asiatic rhinoceros beetle O. rhinoceros (L.) are sap-sucking coleopteran pests of palms. The rhinoceros beetles are, economically, the greatest threat to the palm industry1. The two species have similar biology and ecology2. Adult beetles bore into the apical section of the palm through the basal parts of the leaves and enter the heart of the unfolded leaves, inflicting physical damage at the growing point of the infested palm. The damage may lead to the subsequent death of the palm3. In tropical Africa, O. monoceros can cause up to 40% damage to coconut2. The estimated losses of O. rhinoceros to coconuts in India and Indonesia are approximately 10%, while losses to oil palm have been estimated to be as high as 25% in Malaysia and the South Pacific regions1,4,5,6.
Oryctes monoceros and O. rhinoceros attack over 30 palm species, among which the most economically important ones are oil palm, coconut, and date palms7. Apart from palms, the rhinoceros beetles also attack sugarcane8. Management strategies for the two pests include chemical pesticides, old fishing nets for trapping adults, removal of adults with the metal hook, and destruction of breeding sites which include dead logs, cow dung, and organic manure. Biological control using the entomopathogenic fungus Metarhizium anisopliae has been proved useful in reducing the population of O. rhinoceros in the Philippines and Indonesia9,10. The entomopathogenic Oryctes rhinoceros nudivirus (previously known as Baculovirus oryctes) has provided control of invasive populations of O. rhinoceros in the Pacific islands6 and has also been tested in Tanzania with less success11.
Oryctes monoceros undergoes a complete metamorphosis. Its breeding sites are found in rotten logs, compost, and decaying vegetation. Adults lay eggs in these organic materials and subsequently hatch into 1st instars, the latter taking about 10–13 days. The 1st larval stage may take 9–20 days to develop into the 2nd instar12, and about 34–54 days thereafter to move into the 3rd instar. The final stage where pupae develop into adults requires about 20–30 days, depending on food availability and prevailing environmental conditions. O. monoceros may live for up to six months12,13. Similarly, O. rhinoceros, undergoes a complete metamorphosis. Its eggs hatch in 8–12 days, and after that, the larvae spend their whole larval stage inside the breeding medium. The larva requires 80–200 days to develop: the first instar takes 10–21 days, the second instar 12–21 days, and the third instar 60–125 days. The beetle then goes through an 8–13-day prepupal stage before pupating in a pupal chamber built out of the feeding substrate. Pupae last for 17–30 days, after which they emerge as adults that can live for up to 6 months or longer14,15,16.
Oryctes monoceros is distributed throughout the tropical regions of Africa and has been recently reported in Yemen17. In contrast, O. rhinoceros is indigenous only to South and Southeast Asia6 which may be due to lack of a contiguous land mass. Its major pathways of transmission have been the transportation of host materials by humans, floating logs carried with ocean currents, and the shipment of wartime equipment18. For instance, the spread of O. rhinoceros has been associated with the transport of the pest on commercial soil products19. At various possible entry points in the United States of America, live adults of O. rhinoceros have been intercepted five times, originating once from China, Malaysia, Sri Lanka, and twice from Indonesia7, highlighting the potential for dispersion of the beetle outside its native range.
Advancements in geographic information systems (GIS) and remote sensing technology, as well as quick advances in relative statistical modeling and analysis, have offered new indicators for biogeographical studies on pests20. Ecological niche models are commonly employed in forecasts of climate change to quantify its potential impacts on regional ecology and biogeography21. The models are a group of approaches that combine species occurrence records with environmental data to create a correlative model of the environmental variables that can meet a species’ ecological requirements and predict its potential habitat22. The models have been used to obtain the following outputs: (a) to assess the relative suitability of habitats known to be occupied by the species, (b) to evaluate the relative suitability of habitats in geographic areas not known to be occupied by the species, (c) to predict changes in the suitability of habitats over time given a specific scenario for environmental change, and (d) to estimate the species occurrence23,24. Bioclimatic Prediction and Modeling System (BIOCLIM), Genetic Algorithm for Rule-set Prediction (GARP), CLIMEX model, Random Forest (RF), Booted Regression Trees (BRT) and the maximum entropy (MaxEnt) are applied as the main ecological niche models. The latter is a machine learning algorithm that employs the theory of maximum entropy25,26,27. This model relies on known occurrence records of a species and corresponding environmental variables to analyze and predict the geographical distribution of the species when the entropy reaches the highest point under limited conditions27. There is a risk of over-fitting and bias in the present locations for MaxEnt modeling, and this can limit the model's performance28,29. However, the model has several advantages including the use of presence only records because species absence records are rarely available or reliable, the model results are continuous, which allows classification of levels of suitability in different areas, and the model uses a generative approach by incorporating environmental data from the study area thereby avoiding the need for absence data25,27,30. MaxEnt has a high simulation accuracy, and has been widely used in the prediction of favorable locations for insects such as Dalbulus maidis (DeLong) (Hemiptera: Cicadellidae)31, (Coleoptera: Cerambycidae)32,33, Lycorma delicatula White (Hemiptera: Fulgoridae)34, Daktulosphaira vitifoliae Fitch (Homoptera: Phylloxeridae)35, and Planococcus ficus Signoret (Hemiptera: Pseudococcidae)36.
Climate change and global warming will have a significant impact on species distribution and abundance, as well as the extent of pest losses, affecting crop output and food security37. Research has shown that increased temperatures, rising CO2 levels in the atmosphere, and changing precipitation patterns all have a substantial impact on agricultural production and agricultural insect pests38. Given that climate change will lead to a rise in pest outbreaks and changes in pest behavior and risk of invasion37,39, it is essential to understand how climate change influences the geographical distribution of agricultural pests such as O. monoceros and O. rhinoceros, which is a prerequisite for developing ecological friendly integrated pest management (IPM) strategies40. Previous studies on O. monoceros and O. rhinoceros have mainly focused on management strategies, biological characteristics, and environmental factors that influence the population dynamics and abundance10,41. However, risk maps for surveillance and monitoring are quite insufficient at this moment7. Therefore, in this study, for the first time, we have used the MaxEnt model and ArcGIS software to quantify and map the geographical distribution of O. monoceros and O. rhinoceros.
Methods
We divided the analysis process into four steps: (1) acquisition of occurrence records; (2) preparation of environment variables; (3) MaxEnt modeling; and (4) production of the potential distribution maps for O. monoceros and O. rhinoceros. The technical flow chart of our study is depicted in Fig. 1.
Occurrence records of O. monoceros and O. rhinoceros
A 3-year nationwide survey to collect field data was conducted in palm plantations in Ghana for O. monoceros. The locations where O. monoceros was found were geolocated using a handheld GPS device. The presence of any of the developmental stages (i.e., eggs, larvae, pupae, and adults) and damaged symptoms were considered the existence of the pest. Since it is critical to have enough data points for accurate modelling, the field data were enriched by an extensive scientific literature search utilizing online databases such as Web of Science, Science Direct, Google, Google Scholar, PubMed, and MEDLINE (Supplemental information Table S1). The locations of occurrence O. rhinoceros were obtained from scientific literature by an extensive article search utilizing online databases such as Web of Science, Science Direct, Google, Google Scholar, PubMed, and MEDLINE (Supplemental information Table S1) by searching online using keywords, i.e., Oryctes rhinoceros and O. rhinoceros. East longitudes and north latitudes were transformed to positive values, while west longitudes and south latitudes were converted to negative values27,33,42. The latitudes and longitudes were proofread for accuracy using Google Earth. Duplicate records, fuzzy records, and neighboring records were all eliminated based on MaxEnt's requirements43. Overall, 322 and 304 occurrence records for O. monoceros and O. rhinoceros, respectively, were used for mapping the global geographical distribution of the two pests (Fig. 2).
Occurrence records of O. monoceros and O. rhinoceros. ESRI ArcMap 10.2.2 (https://support.esri.com/en/Products/Desktop/arcgis-desktop/arcmap/10-2-2#downloads).
Environmental variables
Environmental variables affect the habitat and ecological niche of species27. These variables, together with the occurrence records, were used to model and map the spatial distribution of the two pests. The environmental variables consisted of climatic variables, as well as human, and geographical factors (Table 1).
The bioclimatic variables used for the mapping were obtained from the Global Climate Data website (Version 2.0, http://www.worldclim.org/), spanning 30 years from 1970 to 2000. The NASA Surface Meteorology and Solar Energy (https://eosweb.larc.nasa.gov/) global mean annual relative humidity datasets were transformed from a shape file to a raster layer as one of our input data, and 2.5 arc minutes with a spatial resolution of 5 km. As for the geographical factors, previous work showed that vegetation affected infestations of O. rhinoceros in young oil palms replanting in Malaysia44. It was also observed that some insects are limited to a small range of altitudes, whereas others are found in a large range of elevations45. Thus, we included vegetation and elevation as the geographical factors in the mapping of the two pests. In a series of four studies, researchers observed that cover crops reduced the abundance of the beetles44. Moreover, Normalized Difference Vegetation Index (NDVI) are commonly used to represent the development of plant canopy21,46,47,48,49. Therefore, NDVI dataset with a 5 × 5 km spatial resolution and a 15-day interval temporal resolution, obtained from Mapping Studies (GIMMS) group (http://glcf.umd.edu/), were used as a substitute of vegetation for this work. We computed the mean yearly NDVI using these datasets from 1982 to 2015 and used it as one of the input layers for machine learning models21. Land cover also affects the diversity and distribution of pests50. Because thermal accumulation drives development in many ectothermic species, elevated urban temperatures have the most pronounced consequences on ectotherms51,52. In this study, we used Pearson correlation to remove collinearity among predictor variables and those with correlation coefficients |r|≥ 0.7 were excluded from the final model (Table 2). As a result, we were able to select 10 environmental variables for the final simulation (Table 3).
Modeling analysis
MaxEnt is a machine learning algorithm that employs the theory of maximum entropy25,26,27. This model relies on known occurrence records of an organism and corresponding environmental variables to analyze and predict the geographical distribution of the organism when the entropy reaches the highest point under limited conditions27. The entropy formula can be defined as:
ln this natural logarithm, π as the unknown probability distribution over a finite set of pixels X within the study area is approximated by \(\widehat{\pi }\). For each x, \(\widehat{\pi }\) must be assigned a non-negative probability, when the integration of all the probabilities must equal one.
In this study, MaxEnt software version 3.4.1 was used to quantify the potential global distribution of O. monoceros and O. rhinoceros. The model has the advantage of being user-friendly for only requiring a small sample size to make accurate predictions53,54. In this study, 100 repetitions of the ten-fold cross-validation were performed for O. monoceros and O. rhinoceros in MaxEnt, respectively, and every model was run for 500 iterations to reduce model uncertainty. The area under the curve (AUC) and true skill statistic (TSS) were used to quantify the accuracy of the approach55,56. The AUC value lies from 0 to 1, with a value closer to 1 indicating higher model prediction accuracy, whereas an AUC value greater than 0.7 corresponds to better performance55. The TSS value ranges from − 1 to 1, and the closer the value is to 1, the better the prediction is, with ranges between 0.6 and 1 indicating a higher model accuracy56. The model estimates the contribution of the different environmental variables to the geographical distribution of the pests, and the mechanism by which the variables specifically affect the distribution is presented through the marginal response curve. To quantify the areas suitable for both O. monoceros and O. rhinoceros, we employed ArcGIS software’s inbuilt Tabulate Area tool (version 10.1)57,58.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Prediction accuracy of the MaxEnt model
The projected spatial distribution results and the actual distribution of O. monoceros and O. rhinoceros have a high degree of overlap, indicating that the results can be applied to the appropriate regionalization of these species (Fig. 2a and b). The MaxEnt model performance was determined using the distribution points of O. monoceros and O. rhinoceros, and 10 environmental variables. The test data of AUC for O. monoceros and O. rhinoceros were 0.976 and 0.975, respectively (Fig. 3a and b). The TSS value for O. monoceros was 0.90, whereas 0.88 was obtained for O. rhinoceros.
Global potential distribution of O. monoceros and O. rhinoceros
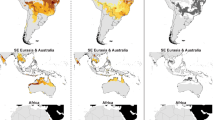
The simulated suitable areas for O. monoceros and O. rhinoceros covered the present-day known occurrence records of the pests (Fig. 4a and b). The model predicts overlaps of highly suitable areas for O. monoceros and O. rhinoceros, especially in parts of the north and east coasts of South America; west, south, and east coasts of Africa; south and east coasts of Asia; and a few suitable areas scattered in the coastal regions of Northern Oceania. The mapping shows that parts of Ghana, Nigeria, Tanzania, Mozambique and Côte d'Ivoire in Africa; Indonesia, India, Malaysia, Thailand, and the Philippines in Asia; Brazil, Venezuela and Colombia in the Americas; and Papua New Guinea in Oceania, that produce large quantities of palms are also suitable for the two pests. However, the results show that suitable global areas for O. rhinoceros (1279.00 × 104 km2) are greater than that of O. monoceros (610.72 × 104 km2) (Table 4).
Global potential distribution maps generated by MaxEnt for: (a) O. monoceros; (b) O. rhinoceros. MaxEnt 3.4.1 (https://biodiversityinformatics.amnh.org/open_source/maxent/) and ESRI ArcMap 10.2.2 (https://support.esri.com/en/Products/Desktop/arcgis-desktop/arcmap/10-2-2#downloads).
The response curves of the three most important environmental variables with regard to their suitability for the prediction of O. monoceros and O. rhinoceros global distribution are shown in Fig. 5. The response curve for temperature annual range shows that there is less chance of O. monoceros colonisation when temperature annual range increases (Fig. 5a). The predictive probability of the O. monoceros to the land cover variable demonstrates that the closed shrublands is the most critical class determining the geographic suitability for O. monoceros occurrence (Fig. 5b). Moreover, the predictive probability of the presence of O. monoceros colonisation was high when precipitation seasonality ranged between 40 and 80 mm (Fig. 5c). With O. rhinoceros, the predictive probability of its geographic suitability decreased when temperature annual range increases (Fig. 5d), while probability of colonisation increases with an increase of precipitation in wettest month (Fig. 5e). The response curve obtained for elevation showed that the predictive probability of O. rhinoceros colonisation was high at elevations ranging between 0 and 2000 m (Fig. 5f).
Response curves of the most significant environmental variables in mapping suitable areas of O. monoceros (a–c) and O. rhinoceros (d–f). Note: In part b, 1–16 means evergreen needleleaf forests, evergreen broadleaf forests, deciduous needleleaf forests, deciduous broadleaf forests, mixed forests, closed shrublands, open shrublands, woody savannas, savannas, grasslands, permanent wetlands, croplands, urban and built-up lands, cropland/natural vegetation mo-saics, permanent snow and ice, and barren, respectively.
Contribution of environmental variables
Our results showed that the temperature annual range (Bio7, 47.2%) contributed most to the O. monoceros model, followed by land cover (31.2%), precipitation seasonality (Bio13, 6.1%), and precipitation of wettest month (Bio11, 5.5%) (Table 5). The cumulative contribution of the four variables was 90%. For O. rhinoceros, temperature annual range (Bio7, 33.6%) contributed most to the model, followed by precipitation of wettest month (Bio11, 28.6%), elevation (14.2%), and land cover (8.8%). The four factors contributed 85.2% in total to the O. rhinoceros model (Table 5).
Discussion
Ecological niche modeling has provided insight into identifying areas suitable for species and estimating the potential impact of environmental variables on their geographical distribution55. It has been quite helpful for mapping the niche shifts of plant diseases59,60, pests61, and plants62,63. In the present study, we conducted ecological niche modelling by combining MaxEnt with elevation of O. monoceros and O. rhinoceros, the two most harmful pests to worldwide palms.
We obtained the global potential distribution maps of O. monoceros and O. rhinoceros from the MaxEnt modeling. The prediction accuracy for the two pests based on AUC and TSS was good61,64, suggesting that the quantification of the geographical distribution was reliable and could provide critical guidance for policy formulation and mitigation measures, especially in regions where the pests have not emerged yet. We also generated the predicted global distribution map of the two pests using aeronautical reconnaissance coverage geographical information system. The tool has been applied in mapping the geographical distribution of pests, such as Spodoptera frugiperda (J.E.Smith) (Lepidoptera: Noctuidae) in central Asia65, Bactrocera dorsalis Hendel (Diptera: Tephrididae) in China66, and Episimus utilis Zimmerman (Lepidoptera: Tortricidae) in Brazil67.
Climate change, playing a significant role in the dispersal and outbreaks of agricultural pests, has been associated with the rise in temperature68. Recent climate models predict a 1 °C increase in global mean annual temperatures by 2025 and a potential 3 °C rise by the end of the century69. As a result, the anthropogenically induced climatic change caused by increased quantities of the earth’s atmospheric greenhouse gases is projected to impact agricultural pests considerably70. Our study analyzed the important environmental variables that influence the distribution of O. monoceros and O. rhinoceros based on the MaxEnt model. Our models’ predictions identified temperature annual range, followed by land cover, and then precipitation seasonality as the most important environmental variables that determined the distribution of O. monoceros. In contrast, Aidoo et al.58 reported that annual temperature variation, followed by seasonality of temperature, then isothermality, were the main environmental variables determining the distribution of O. monoceros based on Boosted regression tree (BRT) model.
According to the response curve, closed shrublands were the most important land cover variable affecting the distribution of O. monoceros. The presence of dead woods and dead palm trunks serve as a breeding site for the pest, whereas cover crops serve as a barrier preventing the beetles from identifying breeding sites10. For O. rhinoceros, temperature annual range contributed most to the prediction, followed by annual precipitation, suggesting that the temperature condition is more important than rainfall condition in defining the distribution of the pest. In contrast, minimum temperature of coldest month, followed by precipitation of wettest month were reported by Hao et al.57 as the main drivers of the global distribution of O. rhinoceros based on BRT model. Xu et al.71 reported that the precipitation of the wettest month was the most important driver of the potential distribution of O. rhinoceros. The variation in the modeling results could be associated with the modeling technique, environmental variables and the number of O. rhinoceros distribution points. Nevertheless, previous studies have shown that temperature affects the biology of O. rhinoceros, and the preferred temperature for the development and survival of O. rhinoceros ranges from 27 to 29 °C and relative humidity ranging from 85 to 95%72,73.
Temperature is one of the most important abiotic factors affecting the growth, development, reproduction, and survival of insects74. In this study, we found that temperature annual range was the most critical environmental variable for both beetles. The impact of temperature on insect growth varies with the species, but it is quite certain that lower temperatures usually result in a slower rate of development75. More than that, temperature affects a variety of biological properties of insects, including the sex ratio76, adult lifespan, survival, fecundity, and fertility77, leading to a significant impact on insect colonization, distribution, abundance, behavior, life history, and fitness78,79.
The risk map of O. monoceros shows a potential expansion to suitable areas outside its current known distributed areas, notably in Latin America and Asia. Similarly, the simulation of potential areas suitable O. rhinoceros also covers areas outside the current distribution of the pest. These areas include parts of West and East Africa, Oceania, and Latin America. The globalization of the international horticulture plant trade has increased the risk of inadvertent spread of leaf beetles from their original geographic locations to uninfected areas80. The unintentional transport of plant materials on agricultural equipment such as farm machinery and tools may be the most likely mode of spread to areas that the pest has not yet arrived. The record of O. monoceros in Yemen17 suggests that the pest can invade new regions, including Asia and other regions as same as we predicted in our study. An earlier study associated with the spread of O. rhinoceros from South and Southeast Asia to Guam to the transport of commercial soil products19. It is therefore imperative for stakeholders and plant regulatory services and NGOs to take an interest in safeguarding the oil palm, coconut, and date palm industry to stay alert for the pests as well as devising countermeasures for the control and prevention of O. monoceros and O. rhinoceros, especially in uninvaded areas.
Our findings showed that the total areas suitable for O. monoceros was 610.72 × 104 km2. Of this area, the percentage of suitable area in the native range was slightly lower than that of the predicted habitat suitability. Specifically, our simulation results show that about 55.8% of the predicted suitable areas were found outside the native range of O. monoceros. In general, the global suitable areas for O. monoceros, as simulated in this study, was slightly lower than that of Aidoo et al.58. The observed variation may be that the present study included human, climatic and geographic factors in the present simulation, while Aidoo et al.58 considered only climatic and geographic factors. Notwithstanding, there also some similarities in the areas predicted to be suitable for O. monoceros in both studies. The simulation for O. rhinoceros using the BRT model show that there are suitability in the major palm producing countries57 which is consistent with MaxEnt modeling, as suitable areas are found in Indonesia, Malaysia, Tanzania, India, Philippines and Brazil. In the present study, suitable areas for O. rhinoceros was found to be 1279.00 × 104 km2 with about 52.3% habitat suitability outside its native range. These predicted areas are less than that of Hao et al.57, and the variations could be associated with the input data, such as land cover, urban accessibility and vegetation. It could also be due to the modeling approach used for the different studies. Previous studies showed that different modeling methods affected ecological niche predictions of species differently81,82.
In this study, urban accessibility was the least (0.1%) important variable influencing the distribution of O. monoceros, while the same variable ranked seventh (2.8%) among the most important variables determining the distribution of O. rhinoceros. The results showed that the relative contribution of elevation to O. monoceros and O. rhinoceros models were 0.5 and 14.2%, respectively. While the relative contribution of vegetation to O. moncoeros model was 3.8, 1.6% was obtained for O. rhinoceros. Moreover, the relative contribution of urban accessibility for O. monoceros differed from that of O. rhinoceros, with the latter contributing to 2.8% of its model. This, however, suggests that the two species responded differently to these human and geographical factors and had a significant influence on the extent of the geographical distribution of the two species, as illustrated in the risk maps. The effects of land cover, urban accessibility, elevation, and NDVI could also influence host distribution through their effects on host food and habitats. For example, species' habitat requirements are likely to be influenced by the presence of nearby bushes83. Moreover, vegetation on the ground, the effect of cover crops like natural, legume, or grass, and the presence of bare ground all have an impact on the number of beetles in their breeding sites84.
Conclusions
For the first time, we have combined MaxEnt with elevation, vegetation, urban accessibility, land cover, and bioclimatic variables to determine the potential geographical distribution of O. monoceros and O. rhinoceros. Our study has created risk maps for the two major pests of palms to facilitate decision-making and the timely launching of preventive measures. The risk maps identify suitable areas outside the currently distributed regions of the pests. In this study, we found that thermal conditions were the most important factors governing the spread of pests.
Data availability
All data generated or analysed during this study are included in this published article and the supplementary information files.
Code Availability
Please contact the corresponding author for code of decent request.
References
Manjeri, G., Muhamad, R. & Tan, S. G. Oryctes rhinoceros beetles, an oil palm pest in Malaysia. Annu. Res. Rev. Biol. 4, 3429–3439 (2014).
Allou, K., Morin, J. P., Kouassi, P., Nklo, F. H. & Rochat, D. Oryctes monoceros trapping with synthetic pheromone and palm material in Ivory Coast. J. Chem. Ecol. 32, 1743–1754 (2006).
Alibert, H. Study on the insect pests of oil palm in Dahomey. Rev. Botan. Appl. 18, 745–773 (1936).
Catley, A. The coconut rhinoceros beetle Oryctes rhinoceros (L) [Coleoptera: Scarabaeidae: Dynastinae]. PANS Pest Articles News Summar. 15, 18–30 (1969).
Fauzana, H., Sutikno, A. & Salbiah, D. Population fluctuations Oryctes rhinoceros L. beetle in plant oil palm (Elaeis guineensis Jacq.) given mulching oil palm empty bunch. Cropsaver Int. J. Trop. Insect Sci. 1, 42–47 (2018).
Paudel, S., Mansfield, S., Villamizar, L. F., Jackson, T. A. & Marshall, S. D. Can biological control overcome the threat from newly invasive coconut rhinoceros beetle populations (Coleoptera: Scarabaeidae)? A review. Ann. Entomol. Soc. Am. 114, 247–256 (2021).
Molet, T. In CPHST Pest Datasheet for Oryctes rhinoceros. USDA-APHIS-PPQCPHST. Revised July 2014 (2013).
Hinckley, A. D. Ecology of the coconut rhinoceros beetle, Oryctes rhinoceros (L.) (Coleoptera: Dynastidae). Biotropica 1973, 111–116 (1973).
Sitepu, D., Kharie, S., Waroka, JS & Motulo, HFJ. Methods for the production and use of Marhizium anisopliae against Oryctes rhinoceros. In Integrated Coconut Pest Control Project—Annual report of Coconut Research Institute—Manado, North Sulawesi, Indonesia 104–111 (1988).
Philippe, R. & Dery, S. K. Coconut research and development. CORD 20, 43–51 (2004).
Purrini, K. Baculovirus oryctes release into Oryctes monoceros population in Tanzania, with special reference to the interaction of virus isolates used in our laboratory infection experiments. J. Invertebr. Pathol. 53, 285–300 (1989).
Ukeh, D. A., Usua, E. J. & Umoetok, S. B. A. Notes on the biology of Oryctes monoceros (OLIV.) A pest of palms in Nigeria. World J. Agric. Res. 2, 33–36 (2003).
Dry, F. W. Notes on the coconut beetle (Oryctes monoceros, Ol.) in Kenya Colony. Bull. Entomol. Res. 13, 103–107 (1922).
Bedford, G. O. Biology, ecology, and control of palm rhinoceros beetles. Annu. Rev. Entomol. 25, 309–339 (1980).
Khoo, K. C., Yusoff, M. N. M. & Lee, T. W. Pulp and paper of oil palm trunk. In Research Pamphlet No.107: Oil Palm Stem Utilisation, Kuala Lumpur, Malaysia, FRIM 51–65 (1991).
Giblin-Davis, R. M. Borers of palms. In Insects on Palms (eds Moore, D. et al.) (CABI Publishing, Wallingford, 2001).
Drumoni, A. & Ponchel, Y. Première capture au Yémen d’ Oryctes (Rykanoryctes) monoceros (Olivier, 1789) et confirmation de la présence de cette espèce africaine dans la Péninsule Arabique (Coleoptera, Dynastidae). Entomol. Afr. 15, 25–29 (2010).
Lever, R. J. A. W. Pests of the Coconut Palm (Food and Agriculture Organization of the United Nations, Rome, 1969).
Moore, A. Rhinoceros beetle pest found in Guam and Saipan. In Pest Alert, Suva, Fiji: Plant Protection Service, Secretariat of the Pacific Community (2007).
Zhang, K., Yao, L., Meng, J. & Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. Sci. 634, 1326–1334 (2018).
Ding, F., Fu, J., Jiang, D., Hao, M. & Lin, G. Mapping the spatial distribution of Aedes aegypti and Aedes albopictus. Acta Trop. 178, 155–162 (2018).
Valencia-Rodríguez, D., Jiménez-Segura, L., Rogéliz, C. A. & Parra, J. L. Ecological niche modeling as an effective tool to predict the distribution of freshwater organisms: The case of the Sabaleta Brycon henni (Eigenmann, 1913). PLoS ONE 16, e0247876 (2021).
Escobar, L. E., Qiao, H., Cabello, J. & Peterson, A. T. Ecological niche modeling re-examined: A case study with the Darwin’s fox. Ecol. Evol. 8, 4757–4770 (2018).
Warren, D. L. & Seifert, S. N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 21, 335–342 (2011).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 (2006).
Phillips, S. J. Transferability, sample selection bias and background data in presence-only modelling: A response to Peterson et al. (2007). Ecography 31, 272–278 (2008).
Elith, J. et al. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57 (2011).
Phillips, S. J. & Dudík, M. Modeling of species distributions with MaxEnt: New extensions and a comprehensive evaluation. Ecography 31, 161–175 (2008).
Arnold, J. D., Brewer, S. C. & Dennison, P. E. Modeling climate-fire connections within the Great basin and Upper Colorado River Basin. Fire Ecol. 10, 64–75 (2014).
Phillips, J. S. & Elith, J. On estimating probability of presence from use-availability or presence-background data. Ecology 94, 1409–1419 (2013).
Santana, P. A. Jr., Kumar, L., Da Silva, R. S., Pereira, J. L. & Picanço, M. C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest. Manag. Sci. 75, 2706–2715 (2019).
Li, et al. Predicting the current and future distributions of Brontispa longissima (Coleoptera: Chrysomelidae) under climate change in China. Glob. Ecol. Conserv. 25, e01444 (2021).
Li, T. et al. Direct and indirect effects of environmental factors, spatial constraints, and functional traits on shaping the plant diversity of montane forests. Ecol. Evol. 10, 557–568 (2020).
Namgung, H., Kim, M. J., Baek, S., Lee, J. H. & Kim, H. Predicting potential current distribution of Lycorma delicatula (Hemiptera: Fulgoridae) using MaxEnt model in South Korea. J. Asia Pac. Entomol. 23, 291–297 (2020).
Ji, W., Gao, G. & Wei, J. Potential global distribution of Daktulosphaira vitifoliae under climate change based on MaxEnt. Insects. 12, 347 (2021).
Ji, W., Han, K., Lu, Y. & Wei, J. Predicting the potential distribution of the vine mealybug, Planococcus ficus under climate change by MaxEnt. J. Crop. Prot. 137, 105268 (2020).
Sharma, HC & Prabhakar, CS. Impact of climate change on pest management and food security. In Integrated Pest Management 23–36 (Academic Press, Cambridge, 2014).
Skendžić, S., Zovko, M., Živković, I. P., Lešic, V. & Lemić, D. The impact of climate change on agricultural insect pests. Insects. 12, 440 (2021).
Ward, N. L. & Masters, G. J. Linking climate change and species invasion: An illustration using insect herbivores. Glob. Change Biol. 13, 1605–1615 (2007).
De Queiroz, D. L., Burckhardt, D. & Majer, J. Integrated pest management of eucalypt psyllids (Insecta, Hemiptera, Psylloidea). In Integrated pest management and pest control-current and future tactics. INTECH 2012, 385–412 (2012).
Hochberg, M. E. & Waage, J. K. A model for the biological control of Oryctes rhinoceros (Coleoptera: Scarabaeidae) by means of pathogens. J. Appl. Ecol. 28, 514–531 (1991).
Liu, Y. et al. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth). Ecol. Eng. 141, 105612 (2019).
Wang, R. et al. Predictions of potential geographical distribution of Diaphorina citri (Kuwayama) in China under climate change scenarios. Sci. Rep. 10, 1–9 (2020).
Wood, B. J. Studies on the effect of ground vegetation on infestations of Oryctes rhinoceros (L.) (Col., Dynastidae) in young oil palm replantings in Malaysia. Bull Entomol. Res. 59, 85–96 (1969).
Mittal, I. C. Survey of scarabaeid (Coleoptera) fauna of Himachal Pradesh (India). J. Entomol. Res. 24, 259–269 (2000).
Zheng, C., Jiang, D., Ding, F., Fu, J. & Hao, M. Spatiotemporal patterns and risk factors for scrub typhus from 2007 to 2017 in southern China. Clin. Infect. Dis. 69, 1205–1211 (2019).
Chen, S., Ding, F., Hao, M. & Jiang, D. Mapping the potential global distribution of red imported fire ant (Solenopsis invicta Buren) based on a machine learning method. Sustainability. 12, 10182 (2020).
Ding, F. et al. Infection and risk factors of human and avian influenza in pigs in south China. Prev. Vet. Med. 190, 105317 (2021).
Jiang, D. et al. Spatiotemporal patterns and spatial risk factors for Visceral leishmaniasis from 2007 to 2017 in Western and Central China: A modelling analysis. Sci. Total Environ Sci. 764, 144275 (2021).
Méndez-Rojas, D. M., Cultid-Medina, C. & Escobar, F. Influence of land use change on rove beetle diversity: A systematic review and global meta-analysis of a mega-diverse insect group. Ecol. Indic. 122, 107239 (2021).
Oke, T. R. City size and the urban heat island. Atmos. Environ. 7, 769–779 (1973).
Briere, J. F., Pracros, P., Le Roux, A. Y. & Pierre, J. S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 28, 22–29 (1999).
Zeng, Y., Low, B. W. & Yeo, D. C. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Eco. Model. 341, 5–13 (2016).
Fand, B. B. et al. Invasion risk of the South American tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in India: Predictions based on MaxEnt ecological niche modelling. Int. J. Trop. Insect Sci. 40, 1–11 (2020).
Li, W. J. et al. Potential distribution prediction of natural Pseudotsuga sinensis forest in Guizhou based on Maxent model. J. For. Res. 48, 47–52 (2019).
McIntyre, S., Rangel, E. F., Ready, P. D. & Carvalho, B. M. Species-specific ecological niche modelling predicts different range contractions for Lutzomyia intermedia and a related vector of Leishmania braziliensis following climate change in South America. Parasit. Vectors 10, 1–15 (2017).
Hao, M. et al. Global potential distribution of Oryctes rhinoceros, as predicted by boosted regression tree model. Glob. Ecol. Conserv. 37, e02175 (2022).
Aidoo, O. F. et al. The impact of climate change on potential invasion risk of Oryctes monoceros worldwide. Front. Ecol. Evol. 10, 633 (2022).
Aidoo, O. F. et al. Lethal yellowing disease: Insights from predicting potential distribution under different climate change scenarios. J. Plant Dis. Prot. 2021, 1–13 (2021).
Ruheili, A. M. A., Boluwade, A. & Subhi, A. M. A. Assessing the Impact of Climate Change on the Distribution of Lime (16srii-B) and Alfalfa (16srii-D) Phytoplasma Disease Using MaxEnt. Plants. 10, 460 (2021).
Wang, R. et al. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri (Kuwayama), in China using the MaxEnt model. PeerJ 7, e7323 (2019).
He, S. T. & Jing, P. F. Prediction of potential distribution areas of Salvia bowleyana Dunn. in China based on MaxEnt and suitability analysis. J Anhui Agri. Sci. 8, 2311–2314 (2014).
Chahouki, M. A. Z. & Sahragard, H. P. Maxent modelling for distribution of plant species habitats of rangelands (Iran). Pol. J. Ecol. 64, 453–467 (2016).
Shabani, F., Kumar, L. & Ahmadi, M. Assessing accuracy methods of species distribution models: AUC, specificity, sensitivity and the true skill statistic. Glob. Int. J. Hum. Soc. Sci. 18, 6–18 (2018).
Baloch, M. N., Fan, J., Haseeb, M. & Zhang, R. Mapping potential distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in central Asia. Insects. 11, 172 (2020).
Wang, N., Li, Z., Wu, J., Rajotte, E. G., Wan, F & Wang, Z. The potential geographical distribution of Bactrocera dorsalis (Diptera: Tephrididae) in China based on emergence rate model and ArcGIS. In International Conference on Computer and Computing Technologies in Agriculture 399–411. (Springer, Boston, 2008).
Manrique, V., Cuda, J. P., Overholt, W. A. & Diaz, R. Temperature-dependent development and potential distribution of Episimus utilis (Lepidoptera: Tortricidae), a candidate biological control agent of Brazilian peppertree (Sapindales: Anacardiaceae) in Florida. Environ. Entomol. 37, 862–870 (2008).
Das, D. K., Singh, J. & Vennila, S. Emerging crop pest scenario under the impact of climate change–a brief review. AgroPhysics. 11, 13–20 (2011).
Porter, J. H., Parry, M. L. & Carter, T. R. The potential effects of climatic change on agricultural insect pests. Agric. For. Meteorol. 57, 221–240 (1991).
Trenberth, K. E. Climate change caused by human activities is happening and it already has major consequences. J. Energy Nat. Resour. Law. 36, 463–481 (2018).
Xu, D., Zhuo, Z., Li, X. & Wang, R. Distribution and invasion risk assessment of Oryctes rhinoceros (L.) in China under changing climate. J. Appl. Entomol. 146, 385–395 (2022).
Sushil, K. & Mukhtar, A. Effect of temperature and humidity on biology of rhinoceros beetle, Oryctes rhinoceros Linn. on oil palm. J. Appl. Anim. Res. 18, 108–112 (2007).
Sabidin, N. N. E. The effect of climate change to the population of rhinoceros beetle (Oryctes rhinoceros) at selected oil palm plantation. In Bachelor of Science Thesis Dissertation. Universiti Teknologi MARA. https://ir.uitm.edu.my/id/eprint/22754. (2018).
Yadav, R. & Chang, N. T. Effects of temperature on the development and population growth of the melon thrips, Thrips palmi, on eggplant, Solanum melongena. J. Insect Sci. 14, 78 (2014).
Ju, R. T., Wang, F. & Li, B. Effects of temperature on the development and population growth of the sycamore lace bug, Corythucha ciliata. J. Insect Sci. 11, 1–12 (2011).
Zheng, F. S., Du, Y. Z., Wang, Z. J. & Xu, J. J. Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae). Insect Sci. 15, 375–380 (2008).
Azrag, A. G. et al. Modelling the effect of temperature on the biology and demographic parameters of the African coffee white stem borer, Monochamus leuconotus (Pascoe) (Coleoptera: Cerambycidae). J. Therm. Biol. 89, 102534 (2020).
Aidoo, O. F. et al. The African citrus triozid Trioza erytreae Del Guercio (Hemiptera: Triozidae): Temporal dynamics and susceptibility to entomopathogenic fungi in East Africa. Int. J. Trop. Insect Sci. 41, 563–573 (2021).
Leonard, A. et al. Predicting the current and future distribution of the edible long-horned grasshopper Ruspolia differens (Serville) using temperature-dependent phenology models. J. Therm. Biol. 95, 102786 (2021).
Roy, B. A. et al. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 12, 457–465 (2014).
Shabani, F., Kumar, L. & Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 6, 5973–5986 (2016).
Cianci, D., Hartemink, N. & Ibáñez-Justicia, A. Modelling the potential spatial distribution of mosquito species using three different techniques. Int. J. Health Geogr. 14, 10 (2015).
Zelazny, B. & Alfiler, A. Oryctes rhinoceros (Coleoptera: Scarabaeidae) larva abundance and mortality factors in the Philippines. Environ. Entomol. 15, 84–87 (1986).
Wood, B.J. Studies on the effect of ground vegetation on infestations of Oryctes rhinoceros (L.)(Col., Dynastidae) in young oil palm replantings in Malaysia. Bull. Entomol. Res. 59, 85–96 (1969).
Acknowledgements
We thank the University of Environment and Sustainable Development and Council for Scientific and Industrial Research, both in Ghana, and the State Key Laboratory of Resources and Environmental Information Systems, Institute of Geographical Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, 100101, China for supporting this study.
Funding
This research is supported and funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20010203).
Author information
Authors and Affiliations
Contributions
O.F.A. provided the data. O.F.A., S.A.M., C.B., E.T. and K.D.N. conceived, designed research and wrote the manuscript and F.D., D.J., T.M., D.W., M.H. analyzed the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aidoo, O.F., Ding, F., Ma, T. et al. Determining the potential distribution of Oryctes monoceros and Oryctes rhinoceros by combining machine-learning with high-dimensional multidisciplinary environmental variables. Sci Rep 12, 17439 (2022). https://doi.org/10.1038/s41598-022-21367-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21367-1
This article is cited by
-
Morphometric characterization and comparison of the African rhinoceros beetle (AfRB) Oryctes monoceros Olivier (Coleoptera: Dynastidae) populations from different agro-ecological zones in Ghana
International Journal of Tropical Insect Science (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.