Abstract
Endometriosis is a complex and chronic disease, whose multifactorial nature has encouraged a deep investigation on the role of lifestyle factors. A strong association between alcohol intake and endometriosis risk has already been shown. We aimed to confirm this association, considering the updated literature. 23 eligible studies were identified through comprehensive literature search of PubMed and EMBASE (May 2012–October 2021). A borderline statistical significance was found comparing any alcohol consumption with no consumption (unadjusted OR 1.14; 95% CI: 0.99–1.31, p = 0.06), in contrast with a previous meta-analysis. However, we confirmed the significant association between moderate alcohol intake and endometriosis (unadjusted OR 1.22, 95% CI: 1.03–1.45, p = 0.02), also performing a sensitivity analysis (unadjusted OR 1.27, 95% CI: 1.04–1.54). Our partly divergent evidence reflects the tough challenge of isolating the impact of specific factors on the natural history of multifactorial diseases. Indeed, on one hand alcohol could be adopted by patients as a self-management therapy and on the other, it could favor the disease, promoting positive feedback with inflammatory mediators and oxidative stress. Our study encourages further investigation on the role of modifiable lifestyle factors and highlights the opportunity to adopt them to prevent or at least limit endometriosis progression.
Similar content being viewed by others
Introduction
Endometriosis is a progressive and hormone-related disease that strongly impacts on woman’s physical, mental, and social well-being1. Due to its debilitating nature and difficult clinical management, research has deeply investigated its possible pathogenesis. Despite decades of research efforts, the comprehension of endometriosis pathophysiology is still elusive and inconclusive2,3,4,5, but a unique cause seems not plausible. As for other chronic illnesses6,7, genetics and inflammation could be a common denominator of different pathogenetic mechanisms8. The pathway pain-stress-inflammation could play a key role in the development, progression, and exacerbations of endometriosis. Indeed, women with endometriosis seem to be caught in a “vicious circle of high stress perception, inflammation and disease progression”9. Besides, while intrauterine and neonatal exposure to prolonged physical stress stimuli could be linked to the future development10, in adults chronic stress might directly enhance the progression11. Inline, increased inflammatory markers have been documented not only in endometriosis lesions but also in peritoneal fluid and even in the peripheral blood of patients affected12,13,14.
A consequent important issue is to establish whether and how promoters of inflammation could influence endometriosis risk15,16. It is not surprising that modifiable lifestyle factors, such as diet, caffeine, environment, and smoking, all factors possibly associated with inflammation, have been explored in this regard17,18,19,20,21,22,23,24. Alcohol has already gained a certain attention. A meta-analysis from our group proved a significant correlation between alcohol intake and the occurrence of endometriosis20, based on the published papers until 201225,26,27,28,29,30,31,32,33,34,35,36,37,38,39. However, given the preponderance of retrospective studies on the topic, at the time we claimed the need to confirm these findings. Therefore, the objective of this study was to verify the correlation between alcohol consumption and endometriosis risk, through a state-of-the-art systematic review and a meta-analysis.
Methods
Information sources
This systematic review was designed to meet the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines40. Systematic research was conducted to search for relevant articles in which the impact of alcohol on endometriosis risk was discussed. The search terms “endometriosis” and “diet”, “nutrition”, “alcohol”, “vitamin”, “fat”, “vegetable,” were used as a combination of free text and as Medical Subject Heading (MeSH) terms (Pubmed) or Emtree terms (Embase) and temporally limited “from 2012/05/31 to 2021/10/11” (See search strategy in Supplementary file S1).
Eligibility criteria
Inclusion criteria were:—case–control, cohort or cross-sectional study reporting original data from May 2012 to October 2021;—clinical or histological diagnosis of endometriosis;—presence of number or percentage of subjects with and without endometriosis according to alcohol intake;—full-length articles, published in English.
Search strategy and data collection
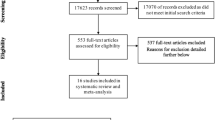
Data collection for our study followed the methodology of the previous one published in 201319. Our research was registered in PROSPERO (ID: CRD42021282108). Figure 1 shows the selection procedure, according to PRISMA 202040. First, two reviewers (LLP and FC) screened PUBMED and EMBASE to identify potential eligible studies. After excluding duplicated reports, they separately assessed all articles on title and abstract and selected relevant articles potentially meeting the inclusion criteria. They both read the full text of potentially eligible papers to assess whether they could be included. Full-text articles were reviewed, and discrepancies were discussed until consensus was reached among the authors. Exclusion reasons for potentially eligible studies were evaluation of intrauterine exposure to maternal alcohol intake or qualitative analysis of alcohol intake, in studies aimed to verify other associations.
Finally, data were extracted into Table 1, where we also considered the previous reports (n = 15). In the table, the following items were described: authors and publication date; country of origin; study design; number and age of participants; confounding factors; key findings.
Statistical analysis
Statistical analyses were performed using Revman (Review Manager [Computer program], version 5.3; The Cochrane Collaboration, 2014) and STATA (STATA, version 10.0; StataCorp LP, College Station, TX, 2012). We pooled the unadjusted odds ratios (OR) by computing the random-effect model weighed for the inverse variance. To assess the heterogeneity across studies, we conducted a test based on the chi-square distribution. The funnel plot and Egger’s test were used to detect publication bias41,42. Two sensitivity analyses were also performed. In one, the data by Parazzini et al.35 were excluded because the reference category included women who consumed less than 0.5 drinks per week and not only the non-consumers. Furthermore, the category of moderate drinkers included women consuming relatively low amount (i.e. 0.5–8 drinks/week) in comparison to other studies. In another sensitivity analysis, the results by Bérubé25 were excluded because heavy drinkers were identified using a cut-off lower than the other studies (i.e., ≥ 9 drinks/month) and because they reported the prevalence OR (POR). We performed a further analysis excluding both studies (Bérubé et al. and Parazzini et al.) in order to evaluate the joint impact of these studies on the overall ORs.
Quality assessment
The quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS)43. Studies were evaluated according to three broad categories: selection of study groups, comparability of study groups, and assessment of outcome (cohort studies) or ascertainment of exposure (case–control or cross-sectional studies). The maximum score was 9.
Results
Systematic review
Selected articles are shown in Fig. 1. We identified 8 papers from May 2012 up to September 2021 to be assessed for the systematic review. Considering those selected for our 2013 study (n = 15)20, we counted a total number of 23 studies. In Table 1, we reported the main methodological characteristics of both the previous and the current selected articles, for a more complete information.
USA was the country for three papers44,45,46, one was conducted in Brazil49, and the other four were set in Europe47,48,50,51. The diagnosis of endometriosis was obtained by a surgical or clinical approach. Only Schink et al. 50 did not specify the diagnostic method to detect endometriosis. In Table 2, we detailed the cutoffs of alcohol drinking of the selected papers, according to the classification levels used in the previous meta-analysis20. Few articles reported the specific thresholds used47,48,51. In one study the category of “no alcohol intake” also included infrequent consumption (< 1 glass/week); for this reason, we excluded this study in the meta-analysis48. Moreover, in two papers, the cut-offs did not allow a precise classification between infrequent and moderate, while in another one between moderate and heavy consumption48,51,52.
Cross-sectional studies
Only one of the newly selected articles had a cross-sectional design51. Saha et al., aimed to investigate the relationship between modifiable life-style factors and endometriosis in a cohort of 28,882 women: while a positive association between smoking or coffee intake with endometriosis was observed, they could not find a similar result considering alcohol consumption. Even taking into consideration the amount of alcohol per week, the association was not significant.
Cohort studies
Three cohort studies were identified after the previous review20. Prescott et al.45 designed a prospective cohort study to evaluate a possible link between endometriosis and infertility. Alcohol, expressed in terms of grams/day, was only mentioned as a covariate for their analysis. Hemmert et al.46 work stands out from the others for its nature: it was a multicenter prospective cohort design aimed to evaluate lifestyle exposure prior to endometriosis diagnosis. They observed null findings between endometriosis and alcohol intake, considering 473 women. In contrast, Ek et al.48 observed an inverse association between this habit and endometriosis, based on 172 women’s reported questionnaires.
Case–control studies
Most newly selected papers were case–control studies44,47,49,50. Both results from Schink et al.50 and Da Silva et al.49 agreed to deny an association between alcohol and endometriosis; indeed, they both observed that alcohol intake tended to be higher in unaffected patients. In contrast, Ricci et al. found an increased endometriosis risk among alcohol users47.
Meta-analysis
A total of 22 papers were included in the meta-analysis. Figure 2 depicted the study-specific and pooled ORs for any versus no alcohol intake. We were not able to find an overall statistically significant association between any alcohol consumption and endometriosis risk (unadjusted OR 1.14; 95% CI: 0.99–1.31) although a borderline statistical significance was observed (p = 0.06).
We also evaluated the effect of alcohol intake according to the number of drinks (Figs. 3, 4). None of the new papers reported a consumption attributable to the “infrequent” category, as adopted in the meta-analysis of Parazzini et al.20. For this reason, we did not report the forest plot. Thus, considering “infrequent” vs no alcohol intake, the OR remained 1.14 (95%CI 0.86–1.52)20. We found out a statistically significant association only when comparing moderate versus no alcohol consumers (p = 0.02) with a summary OR of 1.22 (95% CI, 1.03–1.45). In contrast, in case of heavy alcohol intake, the result was not significant, with an OR of 1.07 (95%CI, 0.90–1.27).
Moderate/regular versus no alcohol consumption. It presented the results of the analyses of moderate intake vs no alcohol intake. As in Fig. 2, endometriosis risk due to regular alcohol consumption is expressed in terms of unadjusted odds ratio (OR).
Heavy versus no alcohol consumption. It presented the results of the analyses of heavy intake vs no alcohol intake. As in Fig. 2, endometriosis risk due to heavy alcohol consumption is expressed in terms of unadjusted odds ratio (OR).
We performed two sensitivity analyses. In one, we excluded the data by Parazzini et al.35: the OR for moderate versus no alcohol consumption was 1.27 (95% CI, 1.04–1.54). In the second sensitivity analysis, the OR estimate for heavy versus no alcohol consumption was 1.01 (95% CI, 0.85–1.19) when we excluded the study of Bérubé et al.25. To assess the joint impact of the data by Bérubé et al. and Parazzini et al., we performed further analyses by excluding both the studies: the overall ORs were 1.14 (95% CI, 0.97–1.33), 1.22 (95% CI, 1.01–1.49) and 1.03 (95% CI, 0.83–1.28) for any, moderate and heavy versus no alcohol consumption, respectively.
Data from Schink et al.50 and Prescott et al.45 could not be included in the meta-analysis as they reported the value of alcohol intake as means of grams per day. Intriguingly, in both papers, unaffected women were more likely to drink alcohol than affected ones, even if without statistically significance. Data were then analyzed according to the time of alcohol intake (current, former or both). Compared to the previous meta-analysis, only one additional study provided information on this aspect44 (Table 3). However, compared to our previous analysis20, the added data did not significantly change the summary ORs. The updated estimates are reported in Table 3.
Finally, only Ricci et al.47 exploited the effect of different types of beverages, observing a positive, although not significant, association between alcohol intake and endometriosis risk, regardless of wine or beer or spirit.
Supplementary Figure S1 showed the funnel plot for any versus no alcohol consumption. There was no asymmetry in the funnel plot, thus suggesting the absence of publication bias; the Egger test was not significant.
Evaluation of the study quality according to the Newcastle–Ottawa Scale43, was reported in Supplementary Table S3. Using the NOS tool, high study quality (scale = 7–9) was detected in 16 out of 19 case–control studies and in all the cohort and cross-sectional studies.
Discussion
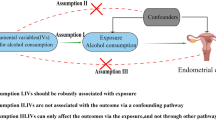
Alcohol consumption in endometriosis has deserved particular attention for many reasons. As summed up by Parazzini et al., alcohol may interfere with estrogen production, that is critically linked to endometriosis20,25,52,53. Moreover, alcohol could be implied in the vicious circle “pain-stress-inflammation”9,54. In 2021, one of the early effects of COVID-19 pandemic was an increase of alcohol consumption for 29.2% endometriosis patients55. Notably, psychiatric disorders (bipolar, depressive, anxiety, and stress-related syndromes) and endometriosis may be intertwined56,57,58 so that endometriosis patients may more likely suffer of these conditions59. However, it is not clear whether psychiatric comorbidities burden on endometriosis or whether they are the consequence of painful symptoms. Nevertheless, it is not difficult to figure out that chronic pain as well as impaired psychological well-being may encourage alcohol misuse60,61. Alcohol consumption could be framed as a possible wrong self-medication to cope with either stressful or painful events. Indeed, Gao et al., observed a higher risk of developing alcohol/drug dependence disorders (HR 1.93; 95%CI, 1.71–2.18) rather than other psychiatric conditions in endometriosis population. On the other hand, they also observed the opposite: alcohol/drug dependence disorders were at higher risk for a subsequent endometriosis diagnosis (HR 1.94; 95%CI, 1.84–2.04)56.
To note, alcohol metabolism influences pro-inflammatory pathways and oxidative stress62,63,64. Collecting all these premises, alcohol could be involved in endometriosis in two different ways: on one hand, it could be an effect of the disease, adopted by patients as a self-management therapy for pain and stressful events or as an expression of psychiatric comorbidity; on the other one, alcohol habit could favor the disease, promoting the positive feedback with inflammatory mediators and oxidative stress.
As already mentioned, our group previously found a significant positive association between alcohol consumption and endometriosis risk20. Our goal was to corroborate this result, updating data with the recent literature.
Excluding the two papers where alcohol was expressed in grams44,45, among the newly selected papers we could observe contrasting results. While Ricci et al. 47 reported an increased, although not significant, endometriosis risk according to alcohol intake (OR 1.48, 95%CI 0.68–2.79), Ek et al.48, Schink aet al.49 and da Silva et al.50 agreed that unaffected patients tended to be more likely alcohol users. Hemmert et al.46 and Saha et al.51 found no association between this habit and endometriosis risk.
Overall, in our meta-analysis considering aggregated data to date, although an increased risk of endometriosis was confirmed among any alcohol users, the finding was only of borderline statistical significance and the OR estimate was lower than in our previous analysis.
To explain these not totally consistent findings, some differences with the previous results should be considered. In the present meta-analysis, a higher number of prospective studies have been included. Only one of the selected recent papers was a cross-sectional study51. Thus, the possible bias derived by ascertaining exposure and outcome at the same time was reduced. In the previous review, the higher proportion of retrospective case–control studies could favor the introduction of selection and recall bias. Collecting lifestyle information before outcome assessment could limit recall bias. Hemmert et al.46 used this approach, denying any association between alcohol and endometriosis occurrence. Indeed, going deeper into the concept of exposure, Wolff et al. assessed in utero exposures and the risk of endometriosis diagnosis. Intriguingly, though not significantly, affected women were less likely to have been exposed to alcohol during pregnancy10.
Interestingly, we confirmed a significant relation between a moderate/regular alcohol intake and endometriosis, with a significant OR of 1.22 (95% CI: 1.03–1.45, p = 0.02). This finding could be in line with the possible double role of alcohol in the natural history of the disease, as described before. Furthermore, it is reasonable to infer that this result is still related to results from the studies included in the previous meta-analysis20. Indeed, we have added only 3 papers to the other 11 previous works reporting data for the subgroup of “moderate intake”. In other words, findings reported before 2012 could have been robust enough to provide still significant results for the subgroup of “moderate intake”, while they could have been diluted in the overall group of “any intake” by the addition of the more recent findings.
We recognize that our study has some limitations that must be addressed. In some papers, endometriosis diagnosis was not confirmed by surgery. This choice may have allowed the inclusion of affected women in the control group; however, the general prevalence of the disease is less than 5%2 and this ascertainment bias cannot be expected to have mainly distorted the results. Moreover, self-reported alcohol intake may have introduced a further bias, especially in the evaluation of number of usual drinks. On the other hand, the funnel plot and the Egger test for funnel plot asymmetry did not show evidence of publication bias.
The difficulty in establishing whether alcohol exposure precedes endometriosis represents the biggest limitation for drawing definitive conclusions and still constitutes the real challenge. This issue was similarly reported in several works, concerning other modifiable factors. In two independent reviews, both Parazzini et al.19 and later Osmanlioglu et al.22 agreed that evidence supporting a significant association between diet and endometriosis is equivocal. Polak et al.21 could not take out any significant conclusions about the relation between environment and endometriosis risk. On the other hand, higher concentrations of trans-nonachlor, and dioxin-like toxic equivalents, together with an increased inflammatory profile have been associated with higher risk of endometrioma65. Environmental exposure remains a major and unsolved issue66.
In line with these papers, our work reflects the tough challenge of isolating the role of specific factors in the natural history of multifactorial diseases, such as endometriosis. Nevertheless, never as now the investigation of modifiable lifestyle factors is urgent for a new integrated therapeutic approach.
From our meta-analysis, we could confirm a significant association between moderate alcohol intake and endometriosis but the strength of our previous results could not be proved considering the other categories. Despite this could be due to some methodological differences (i.e. the nature of published studies), establishing the role of alcohol in the pathogenesis and progression of disease remains an undisputed need.
Data availability
All data generated or analyzed during this study are included in this published article and in its supplementary information file.
References
Kennedy, S. et al. ESHRE special interest group for endometriosis and endometrium guideline development group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 20(10), 2698–704 (2005).
Vercellini, P., Viganò, P., Somigliana, E. & Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 10(5), 261–275 (2014).
Laganà, A. S. et al. The pathogenesis of endometriosis: Molecular and cell biology insights. Int. J. Mol. Sci. 20(22), 5615 (2019).
Ottolina, J. et al. Early-life factors, in-utero exposures and endometriosis risk: A meta-analysis. Reprod. Biomed. Online. 41(2), 279–289 (2020).
Somigliana, E. et al. Natural pregnancy seeking in subfertile women with endometriosis. Reprod. Sci. 27(1), 389–394 (2020).
Ma, H. & Li, J. The ginger extract could improve diabetic retinopathy by inhibiting the expression of e/iNOS and G6PDH, apoptosis, inflammation, and angiogenesis. J. Food Biochem. 20, e14084 (2022).
Hua, F., Shi, L. & Zhou, P. Phenols and terpenoids: Natural products as inhibitors of NLRP3 inflammasome in cardiovascular diseases. Inflammopharmacology 1, 137–147 (2022).
Giacomini, E. et al. Genetics and inflammation in endometriosis: Improving knowledge for development of new pharmacological strategies. Int. J. Mol. Sci. 22(16), 9033 (2021).
Toth, B. Stress, inflammation and endometriosis: Are patients stuck between a rock and a hard place?. J. Mol. Med. (Berl.) 88(3), 223–225 (2010).
Wolff, E. F. et al. In utero exposures and endometriosis: The endometriosis, natural history, disease, outcome (ENDO) study. Fertil. Steril. 99(3), 790–795 (2013).
Reis, F. M., Coutinho, L. M., Vannuccini, S., Luisi, S. & Petraglia, F. Is stress a cause or a consequence of endometriosis?. Reprod. Sci. 27(1), 39–45 (2020).
Cuevas, M. et al. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod. Sci. 19(8), 851–862 (2012).
Kokot, I., Piwowar, A., Jędryka, M., Sołkiewicz, K. & Kratz, E. M. Diagnostic significance of selected serum inflammatory markers in women with advanced endometriosis. Int. J. Mol. Sci. 22, 2295 (2021).
Fan, Y. Y. et al. Expression of inflammatory cytokines in serum and peritoneal fluid from patients with different stages of endometriosis. Gynecol. Endocrinol. 34(6), 507–512 (2018).
Zhou, J. et al. Peritoneal fluid cytokines reveal new insights of endometriosis subphenotypes. Int. J. Mol. Sci. 21(10), 3515 (2020).
Missmer, S. A. et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 160(8), 784–796 (2004).
Sasamoto, N. et al. In utero and early life exposures in relation to endometriosis in adolescents and young adults. Eur. J. Obstet. Gynecol. Reprod. Biol. 252, 393–398 (2020).
Afrin, S. et al. Diet and nutrition in gynecological disorders: A focus on clinical studies. Nutrients 13(6), 1747 (2021).
Parazzini, F., Viganò, P., Candiani, M. & Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 26(4), 323–336 (2013).
Parazzini, F. et al. A metaanalysis on alcohol consumption and risk of endometriosis. Am. J. Obstet. Gynecol. 209(2), 106 (2013).
Polak, G., Banaszewska, B., Filip, M., Radwan, M. & Wdowiak, A. Environmental factors and endometriosis. Int. J. Environ. Res. Public Health 18(21), 11025 (2021).
Osmanlıoğlu, Ş & Sanlier, N. The relationship between endometriosis and diet. Hum. Fertil. (Camb.) 27, 1–16 (2021).
Chiaffarino, F. et al. Coffee and caffeine intake and risk of endometriosis: A meta-analysis. Eur. J. Nutr. 53(7), 1573–1579 (2014).
Sahin Ersoy, G. et al. Cigarette smoking affects uterine receptivity markers. Reprod. Sci. 24(7), 989–995 (2017).
Berube, S., Marcoux, S. & Maheux, R. Characteristics related to the prevalence of minimal or mild endometriosis in infertile women: Canadian collaborative group on endometriosis. Epidemiology 9, 504–510 (1998).
Buck Louis, G. M., Hediger, M. L. & Pena, J. B. Intrauterine exposures and risk of endometriosis. Hum. Reprod. 22, 3232–3236 (2007).
Eskenazi, B. et al. Serum dioxin concentrations and endometriosis: A cohort study in Seveso, Italy. Environ. Health Perspect. 110, 629–634 (2002).
Grodstein, F. et al. Relation of female infertility to consumption of caffeinated beverages. Am. J. Epidemiol. 137, 1353–1360 (1993).
Heilier, J. F. et al. Environmental and host-associated risk factors in endometriosis and deep endometriotic nodules: A matched case-control study. Environ. Res. 103, 121 (2007).
Hemmings, R. et al. Evaluation of risk factors associated with endometriosis. Fertil. Steril. 81, 1513–1521 (2004).
Huang, P. C. et al. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum. Reprod. 25, 986–994 (2010).
Marino, J. L., Holt, V. L., Chen, C. & Davis, S. Lifetime occupational history and risk of endometriosis. Scand. J. Work Environ. Health 35, 233–240 (2009).
Matalliotakis, I. M. et al. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch. Gynecol. Obstet. 277, 389–393 (2008).
Nagle, C. M. et al. Relative weight at ages 10 and 16 years and risk of endometriosis: A case-control analysis. Hum. Reprod. 24, 1501–1506 (2009).
Parazzini, F. et al. Selected food intake and risk of endometriosis. Hum. Reprod. 19, 1755–1759 (2004).
Pauwels, A. et al. The risk of endometriosis and exposure to dioxins and polychlorinated biphenyls: A casecontrol study of infertile women. Hum. Reprod. 16, 2050–2055 (2001).
Signorello, L. B. et al. Epidemiologic determinants of endometriosis: A hospital-based case-control study. Ann. Epidemiol. 7, 267–741 (1997).
Trabert, B. et al. Diet and risk of endometriosis in a population-based case-control study. Br. J. Nutr. 105, 459–467 (2011).
Tsukino, H. et al. Associations between serum levels of selected organochlorine compounds and endometriosis in infertile Japanese women. Environ. Res. 99, 118–125 (2005).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Thornton, A. & Lee, P. Publication bias in meta-analysis: Its causes and consequences. J Clin Epidemiol. 53(2), 207–216 (2000).
Wells, G.A. et al. In Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomized STUDIES in Meta-Analysis (2019)
Upson, K. et al. Phthalates and risk of endometriosis. Environ. Res. 126, 91–97 (2013).
Prescott, J. et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 31(7), 1475–1482 (2016).
Hemmert, R. et al. Modifiable life-style factors and risk for incident endometriosis. Paediatr. Perinat. Epidemiol. 33(1), 19–25 (2019).
Ricci, E. et al. Wine, spirits and beer intake and endometriosis risk among infertile women: Results from a case-control study. Clin. Exp. Obstet. Gynecol. XLIV, 547–550 (2017).
Ek, M., Roth, B., Nilsson, P. M. & Ohlsson, B. Characteristics of endometriosis: A case-cohort study showing elevated IgG titers against the TSH receptor (TRAb) and mental comorbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 231, 8–14 (2018).
Demézio-da-Silva, C. V. et al. Dietary inflammatory index score and risk of developing endometriosis: A case–control study. J.. Endometriosis Pelvic Pain Disord. 13(1), 32–39 (2021).
Schink, M. et al. Different nutrient intake and prevalence of gastrointestinal comorbidities in women with endometriosis. J. Physiol. Pharmacol. 70, 2 (2019).
Saha, R., Kuja-Halkola, R., Tornvall, P. & Marions, L. Reproductive and lifestyle factors associated with endometriosis in a large cross-sectional population sample. J. Womens Health (Larchmt.) 26(2), 152–158 (2017).
Hankinson, S. E. et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J. Natl. Cancer Inst. 87(17), 1297–1302 (1995).
Fernandez, S. V. Estrogen, alcohol consumption, and breast cancer. Alcohol Clin. Exp. Res. 35(3), 389–391 (2011).
Jiang, L., Yan, Y., Liu, Z. & Wang, Y. Inflammation and endometriosis. Front. Biosci. (Landmark Ed). 21, 941–948 (2016).
Ramos-Echevarría, P. M. et al. Impact of the early COVID-19 era on endometriosis patients: Symptoms, stress, and access to care. J. Endometriosis Pelvic Pain Disord. 13(2), 111–121 (2021).
Gao, M. et al. Psychiatric comorbidity among women with endometriosis: Nationwide cohort study in Sweden. Am. J. Obstet. Gynecol. 223(3), 415.e1-415.e16 (2020).
Chen, L. C. et al. Risk of developing major depression and anxiety disorders among women with endometriosis: A longitudinal follow-up study. J. Affect. Disord. 190, 282–285 (2016).
Ek, M. et al. Gastrointestinal symptoms among endometriosis patients—a case-cohort study. BMC Womens Health. 15, 59 (2015).
Lagan, A. S. et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 194, 30–33 (2015).
Pope, C. J., Sharma, V., Sharma, S. & Mazmanian, D. A systematic review of the association between psychiatric disturbances and endometriosis. J. Obstet. Gynaecol. Can. 37(11), 1006–1015 (2015).
Maleki, N. & Oscar-Berman, M. Chronic pain in relation to depressive disorders and alcohol abuse. Brain Sci. 10(11), 826 (2020).
Crews, F. T. et al. Cytokines and alcohol. Alcohol Clin. Exp. Res. 30(4), 720–730 (2006).
Kawaratani, H. et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013, 495156 (2013).
Van-de-Loo, A. J. A. E. et al. The inflammatory response to alcohol consumption and its role in the pathology of alcohol hangover. J. Clin. Med. 9(7), 2081 (2020).
Matta, K. et al. Associations between persistent organic pollutants and endometriosis: A multiblock approach integrating metabolic and cytokine profiling. Environ. Int. 158, 106926 (2021).
Caporossi, L., Capanna, S., Viganò, P., Alteri, A. & Papaleo, B. From environmental to possible occupational exposure to risk factors: What role do they play in the etiology of endometriosis?. Int. J. Environ. Res. Public Health. 18(2), 532 (2021).
Funding
This study was (partially) funded by Italian Ministry of Health- Current research IRCCS.
Author information
Authors and Affiliations
Contributions
F.P. designed the study, L.L.P. and F.C. collected the data, F.C. and S.C. performed the analysis and prepared Figs. 2, 3 and 4, L.L.P wrote the first draft of the manuscript and prepared Fig. 1, Tables 1, 2 and 3, supplementary files. P.V. and E.S. revised and corrected the manuscript. All the authors revised the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
E. Somigliana receveid personal honoraria to give talks at international meetings from Theramex and Merck-Serono, received a donation (US machine) from Merck-Serono for the ART unit and handled grants of research from Ferring and Theramex. The remaining authors report no financial or commercial conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li Piani, L., Chiaffarino, F., Cipriani, S. et al. A systematic review and meta-analysis on alcohol consumption and risk of endometriosis: an update from 2012. Sci Rep 12, 19122 (2022). https://doi.org/10.1038/s41598-022-21173-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21173-9
This article is cited by
-
Diet in Prevention and Treatment of Endometriosis: Current State of Knowledge
Current Nutrition Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.